Short-term effects in canopy gap area on the recovery of compacted soil caused by forest harvesting in old-growth Oriental beech (Fagus orientalis Lipsky) stands
iForest - Biogeosciences and Forestry, Volume 14, Issue 4, Pages 370-377 (2021)
doi: https://doi.org/10.3832/ifor3432-014
Published: Aug 10, 2021 - Copyright © 2021 SISEF
Research Articles
Abstract
Natural treefall gaps have a substantial role in maintaining soil and plant diversity in old-growth forests. However, the amount of information on the effects of gaps on the recovery of physical and chemical properties of compacted soils is scarce. We tested the hypothesis that natural treefall gaps accelerate the restoration of compacted soil by enhancing biological and microbial activity in the topsoil after a period of five years. Five years after a ground-based skidding operation in the Hyrcanian forest, the recovery levels of soil properties were compared among different treatments including natural canopy gaps with an area of 200 m2 (NCG), clear-cuts with an area of 1600 m2 (CC), disturbed trails under a dense canopy (DDC), and an undisturbed area (UND) as control. The lowest soil bulk density (1.07 g cm-3), penetration resistance (1.11 MPa), and the highest macroporosity (36.3%), and sand content (14.4%) among treatments were recorded for the NCG followed by DDC and CC treatments. Significantly lower values of soil pH, and electric conductivity and the highest values of soil organic C, total N, available P, K, Ca, and Mg were detected under the NCG followed by the DDC and CC treatments, as compared to the UND area. The highest values of earthworm density and dry mass, and soil microbial respiration were found in the NCG followed by the DDC and CC treatments. Fine root biomass was significantly higher in the UND area (92.27 g m-2) followed by the DDC, NCG and CC treatments. We can conclude that the effects of gap size on the recovery values of compacted soil were significant in terms of greater nutrient availability and higher earthworm density and dry mass, suggesting that mimicking natural canopy gap was more effective than the clear-cut gap (CC) for the resilience of the forest stand in the restoration of soil quality.
Keywords
Canopy Gap Area, Timber Extraction, Skid Trails, Soil Compaction, Forest Soil Recovery, Earthworm, Hyrcanian Forest
Introduction
Forest soil is an important component of forest ecosystems. It has a remarkable capacity for regulating organic matter and nutrient cycling. Minerals, organic substances, and well aerated porous structures are the promoting factors ([23]). The wide range of macro- and micro-organisms in forest soil with these promoting factors leads to a higher recycling rate of nutrients and better functioning of the ecosystem, which results in an increase in forest productivity ([2], [16]).
The use of heavy machinery in logging operations can potentially lead to soil disturbance and compaction, hence, efforts must be made to mitigate the negative ecological effects on forest soil from the point of view of sustainable forest operation (SFO) and sustainable forest management (SFM - [23]). Air-filled pores in the surface soil were found to be destroyed after machine traffic on the skid trails due to ground-based logging operations ([24], [30]), leading to an increase in soil bulk density ([2], [16], [31]), decreasing total porosity ([25], [18]), and mixing of the mineral and organic horizons ([16]). Moreover, both abiotic and biotic components such as soil microbial populations of forest soil are affected by soil compaction caused by machinery traffic after forest harvesting operations ([23], [16], [31], [32]).
Soil recovery is defined as the ability of impacted soil parameters (i.e., physical, chemical, biological) to restore or return to any former state or condition in response to the effects of disturbance ([34]). Previous studies have proved that the natural recovery of soil properties from compaction following machine traffic is a long-term process, which can take from a few years to several decades ([11], [18], [32]). Nevertheless, the biotic and abiotic mechanisms such as interactions between roots and soil, clay particle expansion and retraction, water freezing and thawing, and macro- and micro-fauna activities can accelerate the recovery processes of soil properties under natural conditions ([6]). In the case of the Hyrcanian forests, Jourgholami et al. ([15]) reported that the physical, chemical, and biological properties of forest soil compacted after mechanized logging operations were significantly restored by the application of mulch with high-quality litter (i.e., hornbeam and maple), in comparison to the untreated trails. However, a full recovery of soil properties did not occur within a 5-year period after forest harvesting operations. Soil compaction has a major impact on the growth and/or mortality rates of forest tree seedlings ([31]). For example, Picchio et al. ([31]) monitored and evaluated soil physical properties (i.e., bulk density, penetration resistance, and total porosity) and their effects on maple and beech seedlings on 10-year-old skid trails in the Iranian Caspian forests. Their results indicated soil bulk density increased by 12.6% on the skid trails (between two skidder tires on each skid trail) and 36.1% on tire tracks, compared to non-skid trails (1.19 g cm-3), penetration resistance increased by 68% on skid trails and 220% on tire tracks, compared to non-skid trails (0.25 MPa), total porosity decreased by 12.8% on skid trails and 30.9% on tire tracks, compared to non-skid trails (54%).
In recent years, several approaches focused on the soil restoration after harvesting through a sustainable forest management (SFM) and the application of sustainable forest operations (SFO). Starting from the observation of a natural phenomenon called the “treefall gap” and its influence on the forest soil, it was possible to develop a specific experimental design.
A treefall gap is a canopy opening of forest stand related to the fall or death of one or more trees at the dominant or co-dominant levels ([38], [27], [45], [22]). Canopy gaps play a key role in ecological succession processes of old-growth forests by maintaining structural diversity and canopy dynamics ([13]), increasing light thus favoring soil and plant richness and diversity ([37], [28]), modifying temperature and soil moisture ([20]), altering the nutrient cycling ([26]), and inducing a suitable microclimate ([20]).
Canopy gaps regulate soil temperature and snow cover which influence microbial biomass in the organic layer ([13], [22]). Previous studies reported that large-size gaps caused a decrease in organic matter cycling and in nutrient release due to the lower amount of litter input and higher decomposition rates ([26]). In the hardwood forests in the Upper Michigan Peninsula (USA), there was a significant decrease in soil microbial and endomycorrhizal biomass, as well as soil respiration as gap size increased ([39]). In Pinus tabulaeformis stands in northern China, the highest concentrations of soil microbial communities and enzymatic activity were observed in the canopy gaps ([45]). Accordingly, Scharenbroch & Bockheim ([38]) stated that large-size gaps need more time than small-size ones to recover from perturbation conditions. Also, Sefidi et al. ([40]) found that the higher values were related to the natural canopy gaps with an area less than 200 m2. Moreover, fallen beech trees were the cause of gaps in the most cases in the Hyrcanian beech forests.
A comprehensive review by Coates & Burton ([4]) focused on literature concerning gap dynamics and gap size or position as predictive variables for silvicultural success or maintenance of ecosystem function. However, no data were provided concerning the potential effects of logging operations. According to Sefidi et al. ([40]), close-to-nature forest management based on mimicking natural canopy gaps is a well-known practice in old-growth Hyrcanian beech forests. However, snowfall and wind damage were known as main ecological disturbances, which can create a gap in the canopy cover. This open area forms an appropriate environment for developing the next generation of tree species, which can affect the dynamics and biodiversity of forest stands ([9]).
As reported by several authors ([35], [26], [29]), the harvesting practices in the gaps may cause further disturbance to soil. However, there is a lack of knowledge on the effect of gaps on soil characteristics, in particular, referring to disturbed soil and related recovery capacities.
Concerning ecological processes of above- and below-ground forest ecosystems, there is hardly any information available in the literature about the effects of natural treefall gaps (as well as those of man-made gaps) on the recovery of compacted soil properties and biological activity. In this study, we hypothesized that small-size natural treefall gaps can accelerate the restoration of compacted soil by enhancing the biological and microbial activity in the topsoil. The study was conducted at the end of a five-year period after logging operations. The objectives of this study were: (i) to elucidate the effects of two different sizes of canopy gaps on the recovery of compacted soil physical and chemical properties five years after ground-based skidding operations; and (ii) to determine the responses of soil microbial respiration and biological activity to canopy gaps, as compared to an undisturbed area.
Materials and methods
Site description
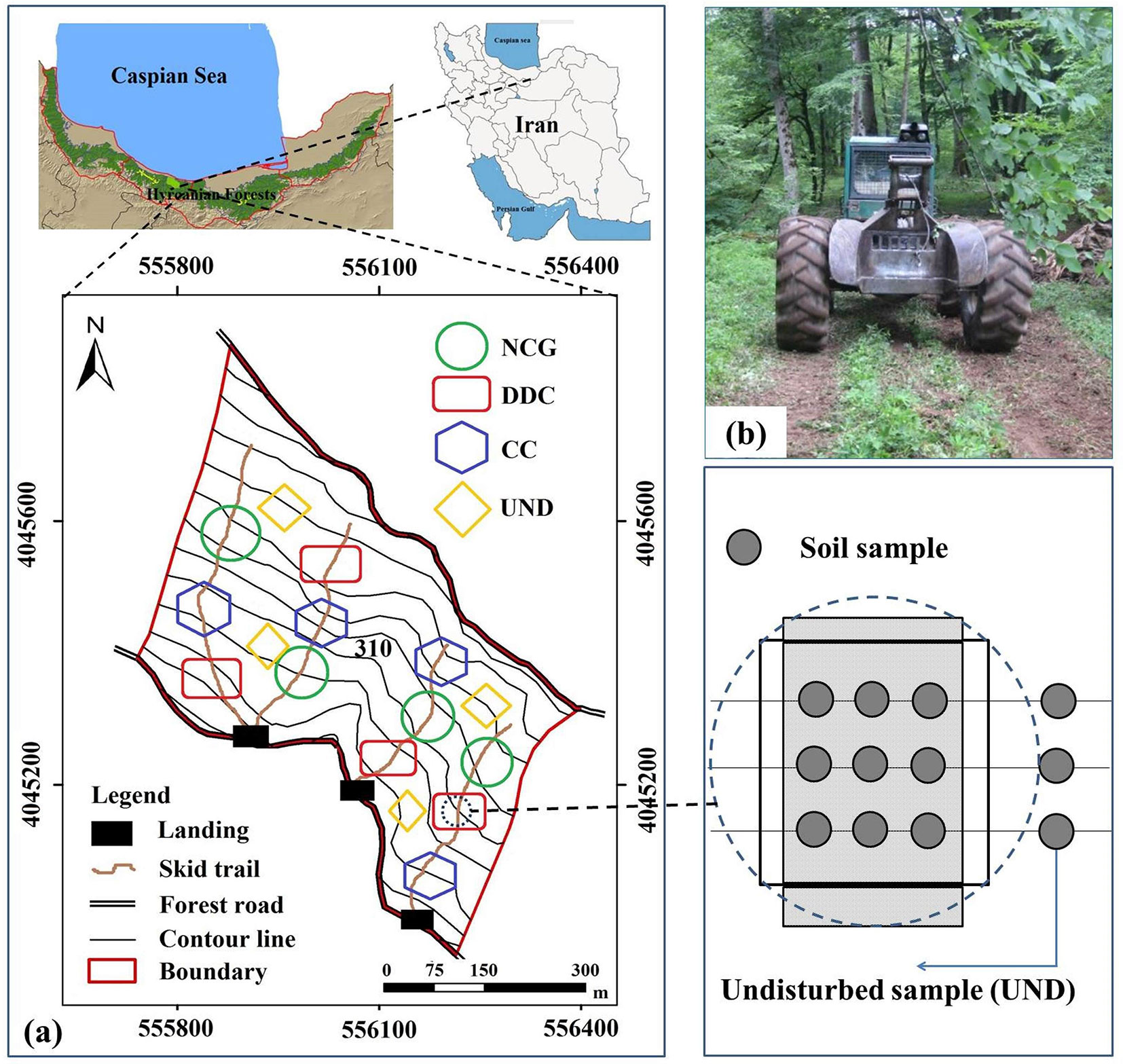
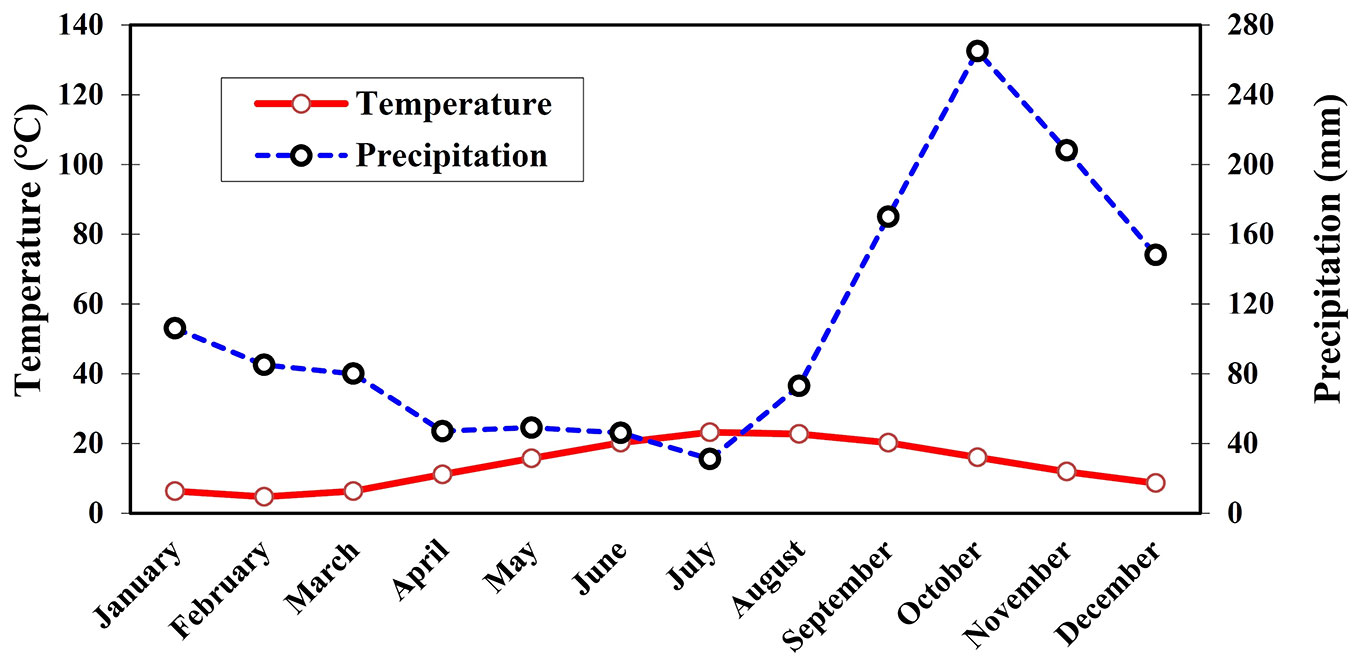
The study areas were established in the compartment no. 310 of the Gorazbon District in the Kheyrud Forest of the greater Hyrcanian forest (northern Iran), at an altitude of 1070-1180 m a.s.l. with a southern aspect (Fig. 1a). The eco-region of the Hyrcanian mixed forests known as old-growth forests are ecosystems characterized by old trees and related structural attributes including large diameter trees, a large proportion of woody debris and dead woody material, multi-layered canopies and canopy gaps, multiple species composition, floristic biodiversity, important natural features, and very high ecological values. Until 2013, it had no history of any previous harvest entries anywhere near the sampling area. The climate of the study area is humid cold (Dsa, based on Köppen-Geiger Climate Classification) with a one-month dry period occurring in July (Fig. 2). The mean annual rainfall is 1308 mm with the highest monthly rainfall occurring in October and the lowest in July. The average annual temperature is 8.5 °C, with July being the hottest month and January the coldest one. The soil in the study area is a deep brown soil (Alfisol, according to the USDA Soil Taxonomy; Calcic Cambisols according to World Reference Base), with silt-loam texture derived from limestone, belonging to the upper Jurassic and lower Cretaceous periods. The dominant tree species in the study area is Oriental beech (Fagus orientalis Lipsky), which is accompanied by other species including hornbeam (Carpinus betulus L.), chestnut-leaved oak (Quercus castaneifolia C. A. Mey), and Caucasian alder (Alnus subcordata C. A. Mey). Tree marking was done in September 2012 and trees were felled by chainsaw in March 2013. A combination of group selection and single-tree selection was applied as the prevalent silvicultural treatment. The average growing stock, total number and volume of removed trees in compartment no. 310 were 477.2 m3 ha-1, 148 trees, and 813.7 m3 (31.8 m3 ha-1), respectively. A Timberjack® 450C four-wheeled skidder with an empty weight of 10.3 t and tires inflated to 220 kPa was used to extract the logs in June 2013 (Fig. 1b); the average load volume was 3.4 cubic meters, and operating trails had a width of 3.5 m. All the skid trails in the different gaps had the same amount of traffic and number of passes.
Fig. 1 - (a) The study area in Kheyrud forests (compartment no. 310 of Gorazbon District) in the Hyrcanian forests (northern Iran) and the the experimental design in relation to the skid trails; the treatments are as follows: natural canopy gap with area of 200 m2 (NCG); clear-cut with area of 1600 m2 (CC); disturbed trails under dense canopy (DDC); and the undisturbed area (UND); (b) the Timberjack® 450C skidder travelling unloaded on the skid trail.
Experimental design and soil sampling
The skid trails were tracked using GPS in 2013 in order to know the exact layout of the trails. Five years after skidding operations, three treatments were randomly selected with four replications in the four skid trails as well as in the undisturbed area to assess the recovery process of soil properties for this study (Fig. 1a). The treatments included were as follows: natural canopy gap with an area of 200 m2 (NCG); clear-cut with an area of 1600 m2 (CC); disturbed trails under a dense canopy (DDC); and the undisturbed area (UND). The natural gaps of the NCG treatment were formed in 2013 due to uprooting (tree throw) and stem breakage following wet snowfall and wind damage ([9]). Hence, all of the four treatments were formed in the same year (2013). These natural canopy gaps were caused by single dead trees and the gap makers were beech trees. To avoid side effects, the plots were located only in areas dominated by beech trees. Clear-cut (CC) plots were contiguously closed canopy forest before being clear-cut. Every small canopy gap (NCG) was perfectly located on the skid-trails center and completely covered the 20×4 m sample section of each skid-trail. The characteristics of each treatment are given in Tab. 1. One replication of each treatment was placed on or near each of the four skid trails and sampling plots were established at an equal distance from landings (50-100 m). Different segments of the trails had the same slope gradients of 15-20%, and were exposed to a high level of machine traffic (ranging 15-20 machine cycles), near the maximum damage level as reported by Cambi et al. ([2]), so as to eliminate the effects of machine traffic intensity and skid trail slope on soil compaction level. The soil sampling plots were oriented and positioned in the skid trail on or near the center of the gaps. In each replication, three plots were randomly established. Then a plot was randomly selected to sample the soil physical, chemical, biological and biochemical properties in June 2018. The sampling plot with a length of 20 m and a width of 4 m was established on the skid trail at each treatment. In each selected sample plot, five transects were established which were perpendicular to the longitudinal axis of the trail at an interval of 4 m, and three of those transects were randomly selected for soil sampling. Soil samples were taken in the undisturbed area (UND) with four replicates adjacent to the skid trails at a distance of 30 m from the trails, to verify the soil properties compared to other treatments. In each treatment, four sample plots were measured, thus providing 36 soil samples. In total, 120 soil samples were collected and analyzed in June 2018, i.e., 4 skid trails × 3 treatments (NCG, CC, and DDC) × 3 randomly selected transects across the sampling plot (out of 5) × 3 soil samples per transect + 12 soil samples in the UND area.
Tab. 1 - The treatments characteristics (mean ± standard deviation) in the study area.
| Treatment | Canopy cover (%) | Gap area (m2) |
|---|---|---|
| UND | 85 ± 9 | 0 |
| NCG | 0 | 200 ± 38 |
| DDC | 81 ± 7 | 0 |
| CC | 0 | 1600 ± 252 |
Some soil characteristics were selected to test the soil disturbance and recovery, according to Picchio et al. ([30]) who found bulk density, total porosity, aggregate stability, and penetration resistance to be good indicators of physical soil quality. Soil samples were collected from the surface soil at less than a depth of 10 cm by using a steel cylinder with a height of 40 mm and a diameter of 56 mm, placed in plastic bags, and shipped to the lab. Soil samples were weighed in a fresh state and then again after oven-drying at 105 °C, upon reaching constant mass for measuring water content and soil bulk density. The following soil physical properties were determined: soil particle size distribution for particles smaller than 0.075 mm by applying the hydrometer method ([10]), soil particle size distribution for larger particles by screening through a series of sieves, macroporosity using the water desorption method ([5]), soil penetration resistance (PR) by using an analog hand-held soil penetrometer (model 06.01.SA, Eijkelkamp, Giesbeek, Netherlands) with a 60° cone and a maximum measuring depth of 1 m, as well as aggregate stability by applying the wet sieving procedure ([17]).
Prior to the soil sampling for determine soil chemical properties the litter layer was removed from an area of 25 × 25 cm within a depth of 10 cm. The collected soil samples were sealed in plastic bags, labeled, transported to the laboratory, air-dried, and then screened through a 2-mm sieve. The following soil chemical properties were determined: soil pH in a solution with a ratio of soil to water of 1:2.5 using the Orion Ionalyzer® (model 901, Orion Corp., Espoo, Finland) pH meter, soil organic C by applying the Walkley-Black technique ([43]), total N by using the Kjeldahl method ([19]), available phosphorous (P) using the Olsen method with a spectrophotometer, and available potassium (K), calcium (Ca) and magnesium (Mg) by ammonium acetate extraction at pH 9 and applying an atomic absorption spectrophotometer ([19]). The number and density of earthworms were determined by manual sampling on the surface soil within an area of 25 × 25 cm and a depth of 10 cm after removing the litter layer. The three main ecological types of earthworms, i.e., epigeic, anecic and endogeic earthworms, were identified based on the identification key of Edwards & Bohlen ([7]). To determine earthworm dry mass, the earthworms were washed, weighed, and oven dried at 60 °C for 24 h ([15]). Soil microbial respiration was determined by measuring the CO2 evolved in a 3-day incubation experiment at 25 °C ([19]). Fine roots with a diameter of less than 2 mm were extracted from each sample and dried at 70 °C to a constant mass for measuring fine root biomass ([16]).
Statistical analyses
A factorial experiment with a complete block design was assigned to the treatments (NCG, CC, DDC, and UND area). The Kolmogorov-Smirnov test (α = 0.05) was used to check the soil properties data for normality. The Levene’s test (α = 0.05) was applied to check the homogeneity of variance among treatments. One-way analysis of variance (ANOVA) was used to relate soil physical, chemical, biological, and biochemical properties recovery in response to treatment. Tukey post-hoc test was used to check the statistically significant differences of the mean values of the treatment group with a 95% confidence level. All statistical tests were performed using the software package SPSS® v. 17.0 (IBM, Armonk, NY, USA). Principal component analysis (PCA) was performed to explore the relationship among soil properties in different treatments using the XLSTAT® 2016 software (Addinsoft, Paris, France).
Results
Soil physical properties
All soil physical properties were significantly different (P < 0.05) among treatments, except for silt content. The highest soil bulk density, penetration resistance, sand content, and the lowest macroporosity were detected for the CC treatment followed by DDC and NCG (Tab. 2). The highest soil moisture (42.91%) was found in the UND area followed by DDC > NCG > CC treatments. Aggregate stability and clay content were highest in the UND area followed by NCG ≈ DDC > CC. Except for soil moisture (Tukey test, P > 0.05), the highest recovery values of all soil physical properties were found in the NCG treatment, while the lowest recovery values were detected for the CC treatment. Nevertheless, the soil physical values did not fully recover among the different types of treatment (i.e., NCG, DDC, and CC) even after five years, and resulted to be even higher than the values of the undisturbed area (UND - Tab. 2).
Tab. 2 - Means (± standard deviation; n=120) of soil physical, chemical, biochemical and biological properties in the four treatments. The treatments are included as follow: natural canopy gap with area of 200 m2 (NCG), clear-cut with area of 1600 m2 (CC), disturbed trails under dense canopy (DDC), and the undisturbed area (UND). Results of the ANOVAs (F test and P value) are given. Different letters within a row indicate significant differences (P < 0.05) between treatment means after Tukey test.
| Soil properties |
Variable | UND | NCG | DDC | CC | F test | P value |
|---|---|---|---|---|---|---|---|
| Physical properties |
Bulk density (g cm-3) | 0.93 ± 0.06 d | 1.07 ± 0.10 c | 1.23 ± 0.13 b | 1.35 ± 0.11 a | 62.08 | <0.001 |
| Macroporosity (%) | 41.27 ± 1.38 d | 36.28 ± 2.35 c | 27.82 ± 2.66 b | 23.47 ± 2.15 a | 287.28 | <0.001 | |
| Penetration resistance (MPa) | 0.92 ± 0.17 d | 1.11 ± 0.18 c | 1.31 ± 0.18 b | 1.55 ± 0.22 a | 46.88 | <0.001 | |
| Soil moisture (%) | 42.91 ± 6.0 a | 32.64 ± 6.34 c | 37.64 ± 5.65 b | 26.46 ± 4.0 d | 38.72 | <0.001 | |
| Aggregate stability (%) | 53.86 ± 6.68 a | 42.27 ± 6.46 b | 38.71 ± 7.41 b | 29.41 ± 6.25 c | 46.61 | <0.001 | |
| Sand (%) | 12.38 ± 1.31 c | 14.41 ± 2.88 b | 13.26 ± 1.88 b | 17.17 ± 1.77 a | 25.80 | <0.001 | |
| Silt (%) | 56.23 ± 2.11 a | 55.46 ± 1.13 a | 56.28 ± 1.98 a | 55.71 ± 1.62 a | 1.75 | 0.16 | |
| Clay (%) | 31.39 ± 3.4 a | 30.13 ± 2.1 b | 30.46 ± 06 b | 27.12 ± 0.7 c | 43.95 | <0.001 | |
| Chemical properties |
pH(1:2.5 H2O) | 5.33 ± 0.23 c | 5.51 ± 0.33 c | 6.01 ± 0.4 b | 6.83 ± 0.42 a | 93.66 | <0.001 |
| EC (ds m-1) | 0.19 ± 0.02 c | 0.22 ± 0.05 c | 0.29 ± 0.05 b | 0.38 ± 0.06 a | 76.87 | <0.001 | |
| C (%) | 4.11 ± 0.43 a | 3.02 ± 0.41 b | 2.93 ± 0.1 b | 1.86 ± 0.38 c | 163.81 | <0.001 | |
| N (%) | 0.53 ± 0.08 a | 0.29 ± 0.04 b | 0.21 ± 0.04 c | 0.11 ± 0.04 d | 303.82 | <0.001 | |
| Available P (mg kg-1) | 26.47 ± 3.88 a | 24.91 ± 3.08 a | 21.62 ± 4.27 b | 14.39 ± 3.58 c | 60.90 | <0.001 | |
| Available K (mg kg-1) | 231.12 ± 15.46 a | 201.47 ± 24.6 b | 196.31 ± 22.11 b | 141.75 ± 21.89 c | 71.88 | <0.001 | |
| Available Ca (mg kg-1) | 177.41 ± 13.62 a | 168.11 ± 26.93 a | 165.93 ± 21.91 a | 108.37 ± 18.48 b | 63.55 | <0.001 | |
| Available Mg (mg kg-1) | 47.16 ± 3.43 a | 41.89 ± 3.6 b | 39.76 ± 4.29 bc | 38.43 ± 4.49 c | 15.38 | <0.001 | |
| Biochemical and biological properties |
Earthworm density (n m-2) | 1.96 ± 0.41 a | 1.14 ± 0.16 b | 0.63 ± 0.11 c | 0.12 ± 0.04 d | 444.73 | <0.001 |
| Earthworm dry mass (mg m-2) | 28.34 ± 4.44 a | 15.65 ± 2.02 b | 8.17 ± 1.4 c | 2.17 ± 1.03 d | 619.26 | <0.001 | |
| Soil microbial respiration (mg CO2-C gsoil-1 day-1) |
0.49 ± 0.08 a | 0.37 ± 0.06 b | 0.33 ± 0.05 b | 0.28 ± 0.05 c | 43.26 | <0.001 | |
| Fine root biomass (g m-2) | 92.27 ± 7.66 a | 74.63 ± 15.1 b | 82.73 ± 18.84 ab | 34.81 ± 8.34 c | 92.16 | <0.001 |
Soil chemical properties
All soil chemical properties were significantly influenced by treatment (Tab. 2). Remarkably, soil pH were higher in the CC followed by DDC > NCG ≈ DDC area (Tab. 2). The highest contents of soil organic C were measured in the UND area (4.11 %) followed by NCG ≈ DDC > CC treatments. The N content was highest for the UND area (0.53 %) followed by NCG > DDC > CC treatments. The values of available P (26.47 mg kg-1), K (231.12 mg kg-1), Ca (177.41 mg kg-1), and Mg (47.16 mg kg-1) were highest for the UND area (Tab. 2). The highest recovery values of chemical properties were observed for the NCG followed by DDC and CC treatments. However, all the measured values continued to be lower than in the UND area 5 years after traffic activity (Tab. 2).
Soil biochemical and biological properties
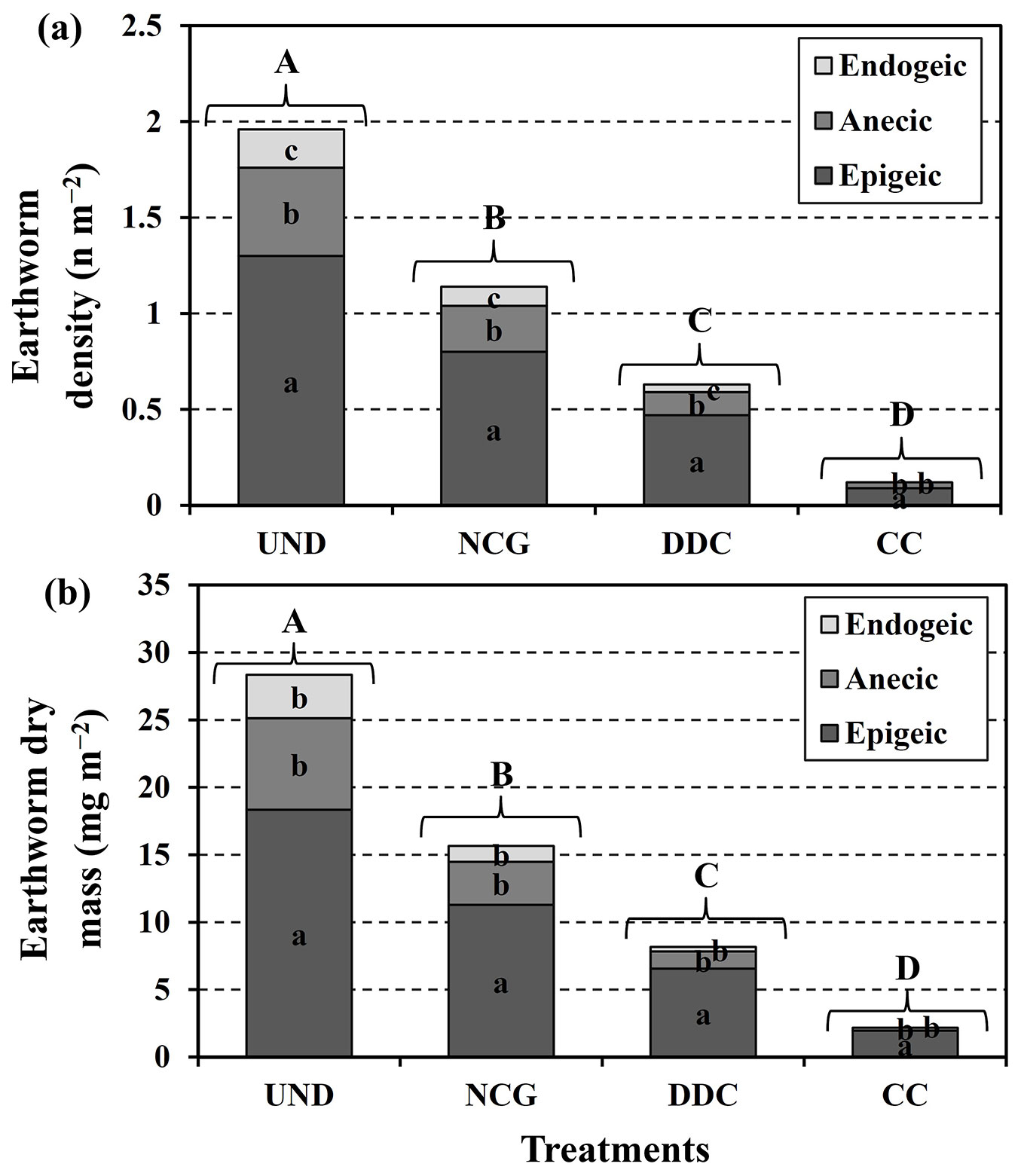
The earthworm density and dry mass, soil microbial respiration, and fine root biomass were significantly different among treatments (Tab. 2). Significantly higher values of earthworm density and dry mass, and soil microbial respiration were found in the UND area followed by NCG > DDC > CC treatments. Fine root biomass was significantly higher in the UND area (92.27 g m-2) followed by DDC > NCG > CC treatments (Tab. 2). Five years after machine traffic on the skid trails, the recovery values of earthworm density and dry mass, and soil microbial respiration were higher in the NCG followed by DDC and CC treatments, all being lower than the values of the UND area, but the recovery values of fine root biomass were highest in the DDC followed by NCG and CC treatments (Tab. 2). In all of the treatments as well as the UND area, density and dry mass of epigeic earthworms were significantly higher than the corresponding values for anecic and endogeic earthworms (Fig. 3a, Fig. 3b).
Fig. 3 - Mean values of epigeic, anecic and endogeic density (a) and dry mass (b) under four treatments. Lowercase letters within the bars and capital letters above bars indicate significant differences (P < 0.01) based on Tukey tests, among earthworm categories and different treatments, respectively.
PCA of the treatments and soil properties
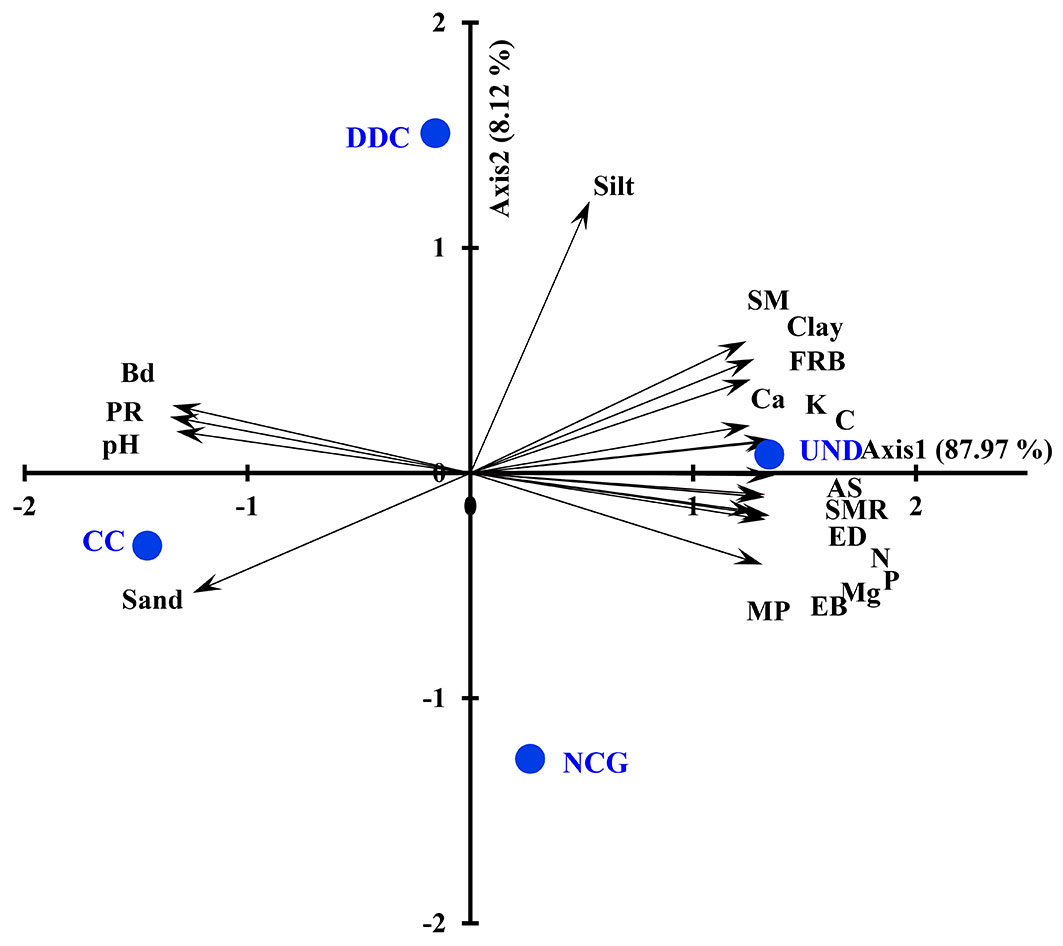
The PCA analysis revealed that the first and second principal components explained 87.97% and 8.1% of the total variance, respectively (Fig. 4). On the positive side of the first principal component, the UND area and the NCG treatment were positively correlated with a favorable soil quality, such as the high values of physical properties which are macroporosity, aggregate stability, silt and clay content, soil moisture, high values of chemical properties such as organic C, total N, available P, K, Ca, and Mg, and high values of biological properties (earthworm density, earthworm dry mass, soil microbial respiration, and fine root density) of soils. The negative side of the first principal component represents an unfavorable soil quality, corresponding to high values of the physical properties bulk density, penetration resistance, sand content and high values of the chemical properties soil pH, and EC. This correlates with the DDC and CC treatments (Fig. 4).
Fig. 4 - PCA biplot showing the principle component scores of the different treatments (NCG: natural canopy gap with area of 200 m2; CC: clear-cut with area of 1600 m2; DDC: disturbed trails under dense canopy; UND: the undisturbed area), and the loadings of the physical variables (BD: bulk density; MP: macroporosity; PR: penetration resistance; MC: moisture content; AS: aggregate stability, sand, silt, clay), chemical variables (pH; EC; C: organic C; N: nitrogen content; P: available phosphorous; K: available potassium; Ca: available calcium; Mg: available magnesium), and biological variables (ED: earthworm density; EB: earthworm dry mass; SMR: soil microbial respiration; FRB: fine root biomass) .
Discussion
Soil physical properties
The recovery values of soil physical properties (i.e., bulk density, macroporosity, penetration resistance, and aggregate stability) were highest in NCG compared to the DDC and CC treatments. However, soil physical properties under the NCG, DDC, and CC treatments did not fully recover within 5 years after gap formation compared to the UND area. The results of the current study revealed that soil physio-chemical and biological properties were restored after creating small-size gaps, following previous literature findings ([27], [39], [13]). In line with the current study, He et al. ([13]) found that soil physical properties are significantly correlated with canopy gap sizes. Several studies have shown significant correlations between gap size and some factors such as soil moisture content and solar radiation ([36], [27]). The received levels of solar radiation logarithmically increased as the gap size increased ([3]). These results are consistent with He et al. ([13]) who reported that soil pore space structure in small gaps shows more enhancement than in the medium and large gaps.
Soil chemical properties
The recovery values of soil chemical properties were highest in the NCG treatment as compared to the DDC and CC treatments, but they were still lower than the values of the UND area even 5 years after machine traffic. The larger gaps can allow for the soil surface to receive more rainfall, thus increasing runoff ([15]) while causing an increase in the loss of nutrients during the leaching process, which leads to a deficit of the inorganic nitrogen levels in soils ([35], [26], [36], [13]). Meanwhile, if the surface of the canopy has large-size openness, the concentration of nutrients due to leaching in the upper layer of soil will show a significant deficit compared to the NCG, as already reported by Schliemann & Bockheim ([39]). Accordingly, the significant decrease in available soil nutrients in the CC treatment can be attributed to the large opening area in the canopy cover. Litter decomposition rates were higher in small-size gaps than in large-size gaps due to the higher microbial activity, which is significantly impacted by soil moisture and the higher inputs of organic matter from neighboring trees in gaps, which is in accordance with the results of Ritter ([35]), Sariyildiz ([36]), and Muscolo et al. ([27]). Consistent with our findings, several studies indicated that the alterations of the microclimate and microbial populations, as well as the reduction of nutrient uptake, are the most important variables causing the high levels of biogeochemical cycling in small-size gaps ([36], [39], [13], [45]). Additionally, Schliemann & Bockheim ([39]) reported a decrease in NH4+ content by increasing gap size due to the lower canopy interception in larger gaps, which resulted in a decrease in the interception of N by canopy cover.
Similar to our findings, Scharenbroch & Bockheim ([37]) demonstrated that the forest floor C and coarse particulate organic matter in soil were higher in the closed canopy than in the treefall gaps. Due to the higher decomposition rate of organic matter, treefall gaps can act as a reductant for organic C in the old-growth beech forests ([37]). After gap formation and subsequent soil disturbance caused by logging operations, the biomass of dead trees is transferred to organic matter and then, the soil organic matter is strongly decomposed by soil microbial activities according to Scharenbroch & Bockheim ([37]). Additionally, the results of this study can be explained based on the “leaky bucket” model ([8]) and therefore newly formed natural canopy gaps are a source of C ([13]), which enhanced the decomposition rate of organic matter over time. Previous studies reported that the increase of soil pH enhances the decomposition rate of litter and nutrient mineralization, which in turn results in an alteration of the soil organisms from fungi to bacteria ([1], [21]). Meanwhile, the decomposition of gap makers in the NCG following microbial activity enhances the organic matter content, which results in an increase of soil total N ([13]). Previous studies found that the favorable conditions of soil temperature and moisture enhanced invertebrate and microbial activities, which lead to a higher degree of humification in gaps than that of the closed canopy ([41], [28]).
Our results revealed that the total N and available nutrients were significantly higher in the NCG than in the DDC and CC treatments. Similarly, Xu et al. ([44]) found that soil N and P were higher in small gaps and under closed canopy than in large gaps. Accordingly, the higher decomposition rate of the litter and the greater leaching of nutrients in large size gaps led to decreased enzyme activity, which resulted in a decrease in soil fertility ([44]). Similarly, Hu et al. ([14]) recommended a gap of 100 to 200 m2 for P restoration in forest soil. Our results also revealed that soil C in the NCG was lower than in the DDC and CC treatments. Similar to our findings, Thiel ([42]) demonstrated that the litter C was significantly lower in gaps than in the stands with a closed canopy. Consistently, with our study, previous investigations suggested that organic matter decomposition rates and nutrient availability were regulated by soil microbial populations and enzyme activity, which in turn were enhanced by canopy gaps ([13], [45]). By increasing the gap size, the microbial biomass decreased, which can have a significant impact on nutrient cycling ([39]).
Soil biochemical and biological properties
Generally, the recovery values of earthworm density and dry biomass were higher in the NCG. However, these values were still lower than the values of the UND area. The accumulation of earthworms under the UND area and small size gaps such as NCG can be attributed to high soil moisture content ([20], [45]). The most favorable conditions, particularly the higher water content in the closed canopy and small-size gap, are the main drivers for the higher accumulation of earthworms ([33], [44]). The accumulation of earthworms showed a positive relationship with the clay content of the soil in both DDC and NCG treatments, which in turn increased the soil moisture content. A similar trend was observed in the effects of canopy gap size on different types of earthworms. On all skid trails, the frequency of epigeic was greater than the anecic and endogeic. These results indicate that epigeic was more effective than the anecic and endogeic in the soil recovery process. Epigeic earthworms live in the soil surface layer and play a crucial role in the formation of humus ([19]). Endogeic was not observed in the CC treatment, giving evidence to its higher level of sensitivity to canopy opening as compared to the anecic and epigeic.
Our results showed that the soil microbial respiration was higher in NCG than in CC. The recovery level of soil microbial properties were higher in light compacted soils than in severe compacted ones ([12]). In line with the current study, Scharenbroch & Bockheim ([37]) found that soil microbial biomass was higher in the small gaps as compared to the closed forests, since the microclimate and substrate conditions in small gaps were optimal for the microbial community. The higher recovery value of soil microbial respiration in NCG than in other treatments contributed to the greater microbial biomass, bacteria as well as fungi, which in turn led to a higher decomposition rate of organic matter and mineralization ([45]). Further, our study is in line with the results from Muscolo et al. ([26]), Xu et al. ([44]), and Yang et al. ([45]), which confirmed that litter inputs with high labile C and N were higher in the large gaps than in the small gaps, as well as the higher leaching, which in turn led to decrease organic matter. Additionally, the lower soil moisture in the large gaps creates unfavorable surrounding conditions, which results in a deceleration of enzyme and microbial activities ([37], [44]).
The greater recovery value of fine root biomass in the DDC and NCG treatments compared to the CC treatment can be attributed to the favorable environment due to the proper conditions of soil physio-chemical properties in small size gaps. Also, the lowest recovery amounts of earthworm density and dry biomass in the CC treatment can be attributed to a larger gap size and higher soil bulk density, which led to lower soil moisture, which in turn resulted in an increase of the sand particles and adverse ecological conditions for earthworms. Similarly, some studies reported that fine root biomass and rates of soil respiration are lower in the canopy gaps than under the closed canopy ([39], [21]). These results are consistent with the findings by He et al. ([13]), stating that the microbial induced decomposition and mineralization rates were higher in the canopy gaps than in the closed canopy due to favorable conditions for microbial activities in the gaps.
One important concern to interpret our results is the differences in soil temperature, solar radiation, and snow cover among the treatments. According to the above mentioned literature, soil temperature can play a crucial role in the recovery processes of soil properties after soil compaction. However, soil moisture was measured in this study, hence the decrease/increase of soil moisture in the soil depth of 10 cm can be attributed to a change in soil temperature. Accordingly, the soil temperature should be measured in a similar circumstance, helping to better interpret the reasons that correspond to any difference among treatments. In particular, the differences during the year in terms of snow cover in different gap size plots, but also the different amount of solar radiation during the summer, represent a key driver for the microbial activity and thus for the soil recovering processes, which should be a topic of research for the future.
One important issue that should be taken into account is that the tree which forms the gap remains for a longer time in the gap of the NCG, which results in a slow decomposition of woody material, leading to an increase of the nourishment of soil biota and available nutrients for macro- and microorganisms. In contrast, the man-made gaps (i.e., the CC treatment) are created by removing one or more trees, however, the stumps and roots remain in the ground ([27]). Previous studies demonstrated that the large-size gaps have different microclimates as compared to the small-size ones, which in turn led to prolong the return period as well as restoring the forest stands to the initial conditions ([26], [13]). The current study hypothesis, stating that an increase in the biological and microbial activities in the topsoil was strongly influenced by the small canopy gaps, is not supported by analyzed data. Hence, full recovery did not take place in any of the treatments. However, the study on the recovery processes of compacted soil physical and chemical properties, due to heterogeneous conditions and miscellaneous successional trends at the treefall gaps in the old-growth forests, requires a longer time span than 5 years.
Conclusion
In this study, the effects of different canopy gaps to accelerate the recovery of soil physio-chemical and biological properties were investigated on compacted soil in the skid trails and compared to levels at the UND area after a five year period since the logging operations. The recovery values of soil physical, chemical, and biological properties were higher in NCG followed by DDC and CC treatments. Nevertheless, five years were not enough to allow for full recovery of soil physical, chemical, and biological properties to restore natural conditions as those in the UND area.
This study showed that the establishment of small-size gaps as a silvicultural treatment results in a faster “recovery” of soil physical, chemical, and ecological properties. Therefore, considering forest management implications based on mimicking the natural treefall gaps, it seems that forest managers should make an effort to apply the single tree selection method in a silvicultural regime, which would not allow the forest canopy to open widely and produce gaps with an area of > 200 m2. However, more studies are needed to understand if gaps with dimensions ranging between the interval of >200 m2 to <1600 m2 have the capacity for soil recovery and to what level of dynamics as compared to the tested gaps of 200 m2.
The remaining dead wood in the natural gaps may affect the organic matter and thus C and N as well as microbiological activity. Hence, further research should be conducted to determine the differences between the gap effect and dead wood effect. Further, simulating small-size gaps allows for a more complicated timber extraction, which can be performed only with a complex and dendritic skid trails network, resulting in highly extraction costs. Moreover, such silvicultural treatment requires high skilled forest operators both for felling and extraction operations. Designated skid trails should be used to at least limit soil compaction, thus preserving the rest of the area. When such a high-impact practice is unavoidable, careful technical surveillance during each phase of work should be carried out by people who are trained and adequately compensated. The application of all of these practices is required to reduce the impact on soil and the whole ecosystem.
Acknowledgements
We thank Dr. Bruno Ferry at AgroParisTech for his review of the draft manuscript and constructive comments and suggestions.
References
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Jahangir Feghhi 0000-0003-2253-5789
Department of Forestry and Forest Economics, Faculty of Natural Resources, University of Tehran, Alborz, Karaj (Iran)
Department of Forestry, Khalkhal Branch, Islamic Azad University (Iran)
Consiglio per la Ricerca in Agricoltura e l’Analisi dell’Economia Agraria - CREA, Centro di Ricerca Ingegneria e Trasformazioni Agroalimentari, v. della Pascolare 16, I-00015 Monterotondo, RM (Italy)
Rodolfo Picchio 0000-0002-9375-7795
Department of Agricultural and Forest Sciences, University of Tuscia, I-01100 Viterbo (Italy)
Corresponding author
Paper Info
Citation
Jourgholami M, Feghhi J, Tavankar F, Latterini F, Venanzi R, Picchio R (2021). Short-term effects in canopy gap area on the recovery of compacted soil caused by forest harvesting in old-growth Oriental beech (Fagus orientalis Lipsky) stands. iForest 14: 370-377. - doi: 10.3832/ifor3432-014
Academic Editor
Angelo Nolè
Paper history
Received: Apr 02, 2020
Accepted: Jun 20, 2021
First online: Aug 10, 2021
Publication Date: Aug 31, 2021
Publication Time: 1.70 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2021
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 34076
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 28658
Abstract Page Views: 2644
PDF Downloads: 2191
Citation/Reference Downloads: 1
XML Downloads: 582
Web Metrics
Days since publication: 1646
Overall contacts: 34076
Avg. contacts per week: 144.92
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2021): 14
Average cites per year: 2.80
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Short-term recovery of fine root carbon stock is inhibited by skid trails in a humid tropical forest
vol. 18, pp. 344-349 (online: 30 November 2025)
Research Articles
Impact of wheeled and tracked tractors on soil physical properties in a mixed conifer stand
vol. 9, pp. 89-94 (online: 22 May 2015)
Research Articles
Potential spread of forest soil-borne fungi through earthworm consumption and casting
vol. 8, pp. 295-301 (online: 26 August 2014)
Research Articles
Assessment of timber extraction distance and skid road network in steep karst terrain
vol. 10, pp. 886-894 (online: 06 November 2017)
Research Articles
Effects on soil physicochemical properties and seedling growth in mixed high forests caused by cable skidder traffic
vol. 16, pp. 127-135 (online: 23 April 2023)
Research Articles
Effects of soil compaction on seedling morphology, growth, and architecture of chestnut-leaved oak (Quercus castaneifolia)
vol. 10, pp. 145-153 (online: 13 June 2016)
Research Articles
Influence of slope on physical soil disturbance due to farm tractor forwarding in a Hyrcanian forest of northern Iran
vol. 7, pp. 342-348 (online: 17 April 2014)
Research Articles
Use of LIDAR-based digital terrain model and single tree segmentation data for optimal forest skid trail network
vol. 8, pp. 661-667 (online: 22 December 2014)
Research Articles
Influences of forest gaps on soil physico-chemical and biological properties in an oriental beech (Fagus orientalis L.) stand of Hyrcanian forest, north of Iran
vol. 13, pp. 124-129 (online: 07 April 2020)
Research Articles
Use of terrestrial laser scanning to evaluate the spatial distribution of soil disturbance by skidding operations
vol. 8, pp. 386-393 (online: 08 October 2014)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword