Is microbial biomass measurement by the chloroform fumigation extraction method biased by experimental addition of N and P?
iForest - Biogeosciences and Forestry, Volume 14, Issue 5, Pages 408-412 (2021)
doi: https://doi.org/10.3832/ifor3374-014
Published: Sep 04, 2021 - Copyright © 2021 SISEF
Short Communications
Abstract
The chloroform fumigation extraction (CFE) method determines microbial biomass carbon (MBC) or nitrogen (MBN) by calculating the increase in extractable carbon (C) or nitrogen (N) due to microbial lysis during chloroform fumigation. In China, many studies have focused on the impacts of N and phosphorus (P) addition on soil MBC and MBN in forest ecosystems, where substantial atmospheric N deposition has strongly acidified soils. The addition of nutrients may alter the extraction process applied in the CFE method, potentially influencing the MBC and MBN determined by the CFE method independently of the actual microbial biomass. In this study, we tested whether the MBC and MBN determined by the CFE method were biased by the experimental addition of N and P in strongly acidified Chinese forest soils by adding N and P to the soils immediately before chloroform fumigation, which should not affect the actual microbial biomass. P addition significantly elevated the dissolved organic carbon (DOC) content, especially after fumigation, while N addition significantly reduced the dissolved nitrogen (DN) content. The added N was subtracted using blank samples without soil. However, the altered DOC and DN contents did not affect the MBC and MBN contents determined by the CFE method. In conclusion, our study suggests that the CFE is a relatively robust method to test the impacts of nutrient addition on microbial biomass in the strongly acidified soils of Chinese forests. We also suggested that: (i) even if a fertilization experiment results in an elevated DOC content following P addition, it does not necessarily indicate a stimulation of DOC production by microbes; and (ii) the soil adsorption capacity or the strength of microbial N uptake during the extraction procedure applied in the CFE method may affect the determination of MBN by influencing the DN extraction efficiency.
Keywords
Chloroform Fumigation Extraction, Microbial Biomass, Nitrogen, Phosphorus, Soil, Tropical Forest
Introduction
The chloroform fumigation extraction (CFE) method ([2], [36]) is widely used in ecological studies ([37]) to determine microbial biomass via elements such as microbial biomass carbon (MBC) or microbial biomass nitrogen (MBN). The CFE method determines microbial biomass by calculating the increase in soil extractable fractions, such as dissolved organic carbon (DOC) or dissolved nitrogen (DN) extracted by 0.5 M K2SO4, during chloroform fumigation. During fumigation, microbial cells lyse and a portion of the dead microbial constituents are transformed into extractable components through enzymatic autolysis. The CFE method assumes that the increases in DOC or DN are in proportion to the soil microbial biomass, and therefore the microbial biomass can be calculated using conversion factors ([14]). This method enables a relatively accurate and quick determination of soil microbial biomass.
Researchers have investigated the impacts of nutrient addition, such as nitrogen (N) and phosphorus (P), on soil microbial biomass. This has enabled an evaluation of the impacts of anthropogenic nutrient loading into soils, such as atmospheric nutrient deposition or nutrient fertilization, on soil microbial biomass ([34], [16]). Additionally, microbial nutrient limitations have been determined ([17], [18], [35], [27]). In China, many studies have focused on the impacts of the substantial increases in atmospheric N deposition, which have strongly acidified forest soils ([20]), and the subsequent imbalanced input of N and P into ecosystems that is consequently occurring ([4]). The CFE method has been generally used to examine the effects of N and P addition on soil microbial biomass ([34], [16], [17], [35], [6], [26]).
Several studies have reported methodological weaknesses of the CFE method. For example, Alessi et al. ([1]) suggested that chloroform can adsorb onto the soil during fumigation, especially onto the clay minerals, causing an increase in DOC and hence an overestimation of the MBC in clay-rich soils ([31]). It has also been demonstrated that soil moisture can affect the determination of MBC ([30]). Despite these methodological problems can under- or overestimate soil MBC and MBN, it is assumed that the impacts of nutrient addition on soil microbial biomass can be tested relatively robustly, because both nutrient-amended soils and a non-amended control are similarly influenced by these methodological problems.
However, the robustness of the CFE method for testing the impacts of nutrient addition on soil microbial biomass has not been fully examined. Added nutrients may alter the extraction process applied in the CFE method, potentially influencing the MBC and MBN determined by the method independently of the actual microbial biomass. It is possible that the changes in soil pH through nutrient addition affect the microbial biomass determined by the CFE method, because soil pH can affect the extraction efficiency of DOC by K2SO4 ([9], [10]). Müller et al. ([28]) reported that NH4+ immobilization may occur during fumigation. The determination of MBN could then be affected by nutrient addition through changes in DN immobilization ([7], [8]) during fumigation. Changes in soil adsorption capacity may also affect the determination of MBC or MBN. It has been reported that the extracted DOC content can be physico-chemically affected by P fertilization ([27]), because P has a higher affinity with soil surfaces than DOC ([15]) and P can desorb DOC at the mineral surfaces ([11]). If a portion of the DOC flushed out by chloroform fumigation (i.e., increase in DOC during fumigation) is adsorbed by soil (and 0.5 M K2SO4 does not extract all of the adsorbed DOC), P addition could cause larger MBC values by desorbing the adsorbed DOC.
The aim of this study was to test whether the MBC and MBN determined by the CFE method are biased by the experimental addition of N and P in strongly acidified Chinese forest soils. By adding N and P immediately before fumigation, we evaluated the CFE method-dependent impacts of nutrient addition on microbial biomass determination. Because the addition of N and P immediately before fumigation should not affect the actual microbial biomass (i.e., microbes have no time to change their biomass), any changes in the MBC and MBN should be caused by the impacts of N and P addition on the CFE method (e.g., alterations to the DOC and DN extraction process).
Materials and methods
Study sites
Soil samples for the experiment were collected from six forests in China. Three of the six forest sites were located in the Dinghushan Biosphere Reserve (DHS; 23° 10′ N, 112° 10′ E - [22], [23]): a primary monsoon evergreen broadleaf forest (BF), a secondary mixed pine/broadleaf forest (MF), and a planted Pinus massoniana forest (PM). Two forest sites were located in the Heshan National Field Research Station (HS; 22° 34′ N, 112° 50′ E -[38]): a planted Acacia auriculiformis forest (AA) and a planted Eucalyptus urophylla forest (EU). The final site was a mixed deciduous forest (MDF) located in the Jigongshan National Nature Reserve (JGS; 31° 46′ - 31° 52′ N, 114° 01′ - 114° 06′ E - [39]). The annual average temperature is 21.0 °C, 22.5 °C, and 15.2°C and the annual average precipitation is 1580, 1927, and 1119 mm in DHS, HS, and JGS, respectively ([12], [39], [33]). The soils in DHS, HS, and JGS are a lateritic red earth formed from sandstone ([22], [42]), an Acrisol ([43]), and a yellow brown soil ([39]), respectively. The basic characteristics of the study sites are summarized in Tab. 1. All six forests have received large amounts of atmospheric N deposition ([23], [38], [39], [21], [42]).
Tab. 1 - Selected basic characteristics (mean ± standard error) of the six forest sites investigated. Data collection and source: (a) measured in 2015 (from [41]); (b) measured in 2015 (from [40]); (c) measured in Dec 2011 (from [43]); (d) measured in Jul 2018 (from [19]); (e-f) measured in the present study. Sample size: (a-d) n = 3; (e) n = 5; (f) n = 1. Sites: (BF) primary monsoon evergreen broadleaf forest; (MF) secondary mixed pine/broadleaf forest; (PM) planted Pinus massoniana forest; (AA) planted Acacia auriculiformis forest; (EU) planted Eucalyptus urophylla forest; (MDF) mixed deciduous forest. Variables: (DOC) dissolved organic carbon; (DN) dissolved nitrogen. Soil samples for chemical analysis were taken from 0-10 cm depths.
| Characteristics | Sites | |||||
|---|---|---|---|---|---|---|
| BF | MF | PM | AA | EU | MDF | |
| Soil organic C (g kg-1) | 40.0 ± 4.2 a | 32.1 ± 4.0 b | 23.3 ± 1.6 b | 23.8 ± 1.7 c | 18.5 ± 0.4 c | 63 ± 13 d |
| Soil total N (g kg-1) | 2.9 ± 0.5 a | 2.0 ± 0.3 b | 1.4 ± 0.2 b | 2.0 ± 0.1 c | 1.5 ± 0.2 c | 3.4 ± 0.5 d |
| Soil available P (mg kg-1) | 2.1 ± 0.4 a | 1.0 ± 0.3 b | 1.5 ± 0.3 b | 2.5 ± 0.2 c | 2.1 ± 0.1 c | 6.9 ± 2.0 d |
| DOC (µg C g-1) | 220.7 ± 3.6 e | 154.4 ± 2.5 e | 208.0 ± 4.4 e | 275.6 ± 3.5 e | 297.9 ± 3.6 e | 135.0 ± 2.0 e |
| DN (µg C g-1) | 29.9 ± 1.9 e | 17.9 ± 1.0 e | 25.9 ± 1.8 e | 38.5 ± 1.1 e | 41.8 ± 2.2 e | 25.4 ± 0.8 e |
| Soil pH (H2O) | 3.53 f | 3.68 f | 3.6 f | 3.4 f | 3.65 f | 4.25 ± 0.10 d |
| Soil water content | 0.24 f | 0.18 f | 0.22 f | 0.23 f | 0.27 f | 0.13 f |
Experimental setup and chloroform fumigation
Surface soil samples (0-10 cm) were collected from subplots at the six forest sites using soil cores (three, three, and four subplots in DHS, HS, and JGS, respectively). Soil samples taken from the same site were combined, and six replicates were prepared for the experiment. We sieved the soil samples through a 2-mm sieve after removing the fine roots and coarse organic matter. Sieved soil samples (6 g) were placed in bottles (glass bottles for fumigated soils and plastic bottles for unfumigated soils) and 1.5 mL N (500 µg N per 1.5 mL solution in the form of NH4NO3) or P (500 µg P per 1.5 mL solution in the form of KH2PO4) were added. The final concentrations of the added N and P (around 100 µg N and P per g soil) were in a similar range to that of previous laboratory experiments where nutrient concentrations were decided based on the nutrient doses in the field ([5], [3], [13], [24], [25]). Controls without N or P addition were prepared in the same manner by adding pure water. Blanks without soils were also prepared for both fumigated and unfumigated samples. The DOC and DN before the fumigation (DOCbef and DNbef) were extracted immediately after the addition of the N or P solutions by shaking the soils with 30 mL 0.5 M K2SO4 for 30 min. Fumigated samples were placed in a vacuum desiccator and exposed to chloroform vapor for 24 h ([36]). The DOC and DN after the fumigation (DOCaft and DNaft) were extracted in the same manner as DOCbef and DNbef. The MBC and MBN were then calculated by subtracting DOCbef from DOCaft and DNbef from DNaft, respectively. A conversion factor of 0.45 was used for calculating both MBC and MBN ([14]). The DOC and DN were measured with a total organic carbon analyzer (TOC-5000®, Shimadzu, Japan). Soil pH(H2O) (soil to water ratio 1:2.5) was measured using unfumigated soils. We had only five replicates for the pH analysis because we failed to analyze JGS soils.
Statistics
A paired t-test was used to determine the statistical significance of differences between control soils and N- or P-amended soils. This statistical analysis was used because our main purpose was to confirm that N and P addition did not affect the MBC and MBN determination by the CFE method (i.e., repeating a t-test increases type 1 errors, and therefore a more robust result could be obtained if there were no statistical differences). If the paired t-test revealed any significant differences, an additional analysis was performed using a linear mixed effect model. All statistical analyses were performed using R version 4.0.2 ([29]).
Results
Effects of N and P addition on DOC and DN
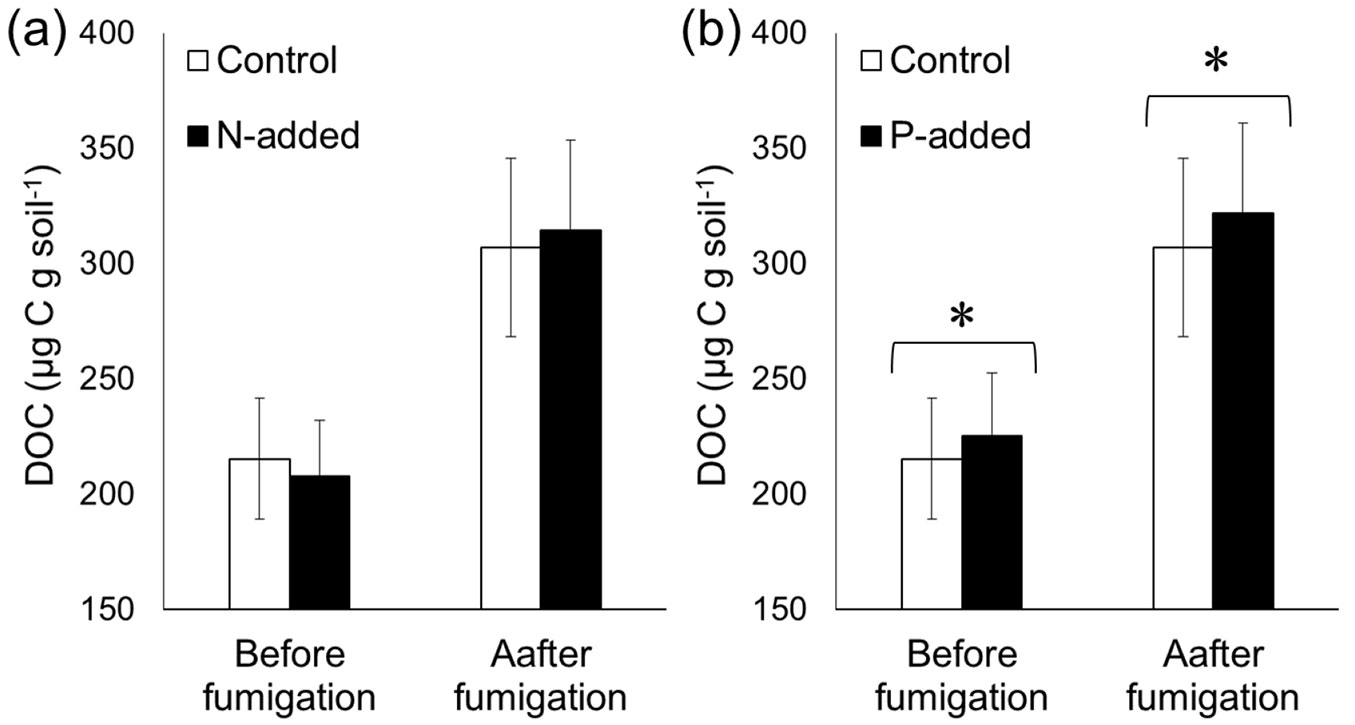
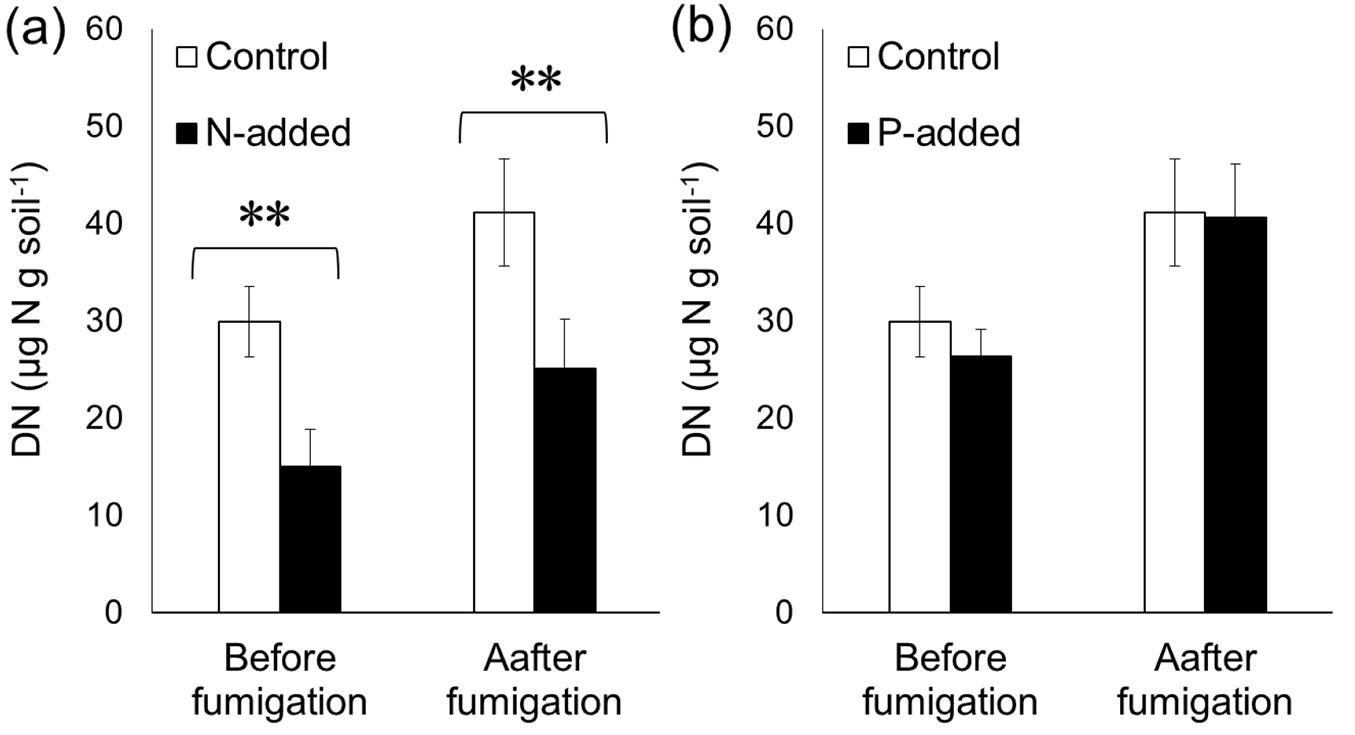
The DOC and DN contents in our study sites ranged from 130 to 300 µg C g soil-1 and from 15 to 45 µg N g soil-1, respectively. Chloroform fumigation caused a large elevation in both the DOC and DN contents (by ~100 µg C g soil-1 and 10 µg N g soil-1, respectively - Fig. 1, Fig. 2). N addition did not show any impact on the DOC content (DOCbef or DOCaft) extracted by 0.5 M K2SO4 (p > 0.05 - Fig. 1a). By contrast, a paired t-test demonstrated that P addition significantly elevated the DOC content (p < 0.05 - Fig. 1b). According to a linear mixed effect model analysis, the impact of P addition was statistically significant on the DOCaft content (p < 0.01), but not on the DOCbef content (p = 0.26). Both the paired t-test and linear mixed effect model analysis demonstrated that N addition significantly decreased both the DNbef and DNaft contents (p < 0.01 - Fig. 2a). Note that the amount of added N was subtracted using the blank samples. P addition did not affect the DN content (p > 0.05 - Fig. 2b).
Fig. 1 - Effects of experimental (a) N addition and (b) P addition on the dissolved organic carbon (DOC) content before and after chloroform fumigation. The DOC was extracted by 0.5 M K2SO4 after 30 min of shaking. Error bars indicate the standard error of data from six sites. Statistical significance was determined by a paired t-test. (*): p < 0.05; (**): p < 0.01.
Fig. 2 - Effects of experimental (a) N addition and (b) P addition on the dissolved nitrogen (DN) content before and after chloroform fumigation. The DN was extracted by 0.5 M K2SO4 after 30 min of shaking. Error bars indicate the standard error of data from six sites. Statistical significance was determined by a paired t-test. (*): p < 0.05; (**): p < 0.01.
Effects of N and P addition on MBC, MBN, and pH
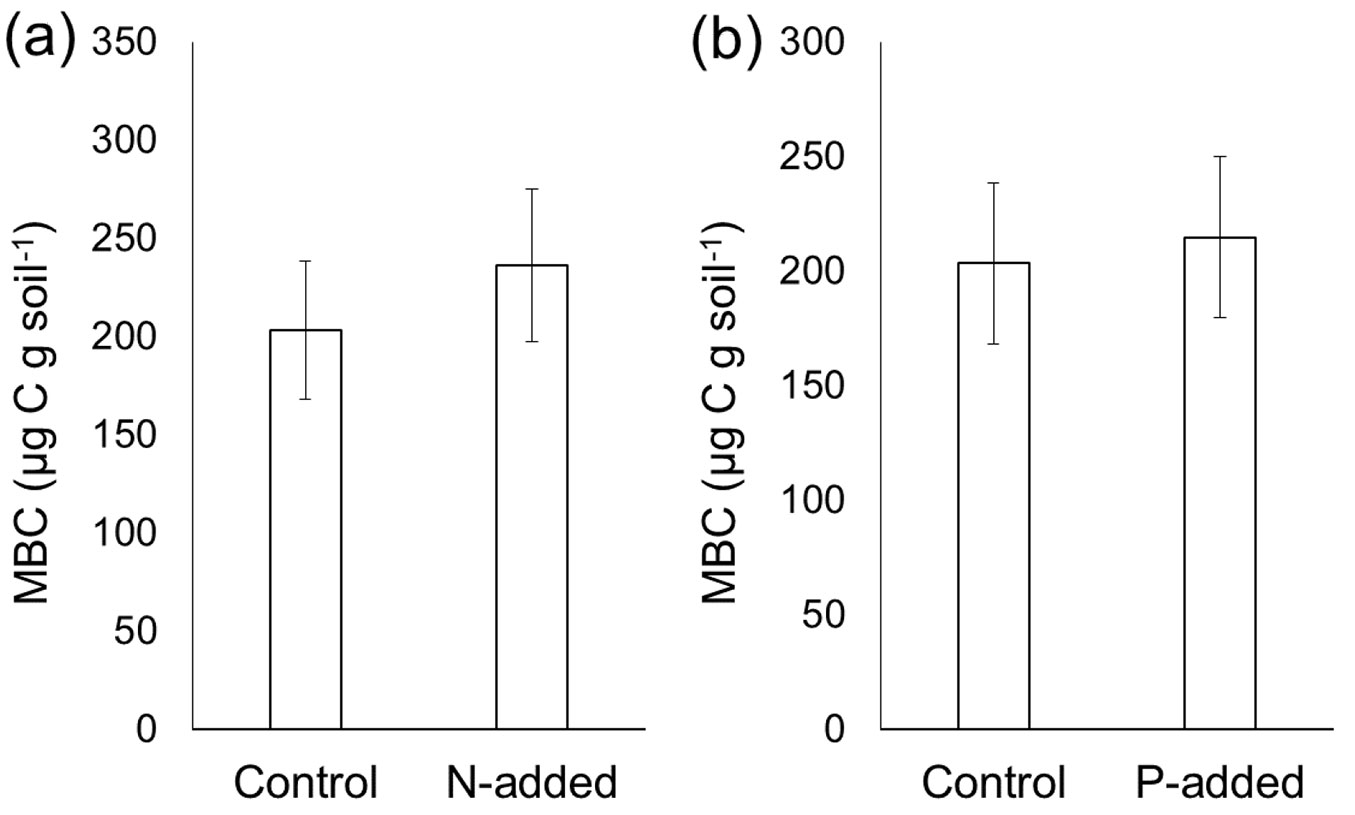
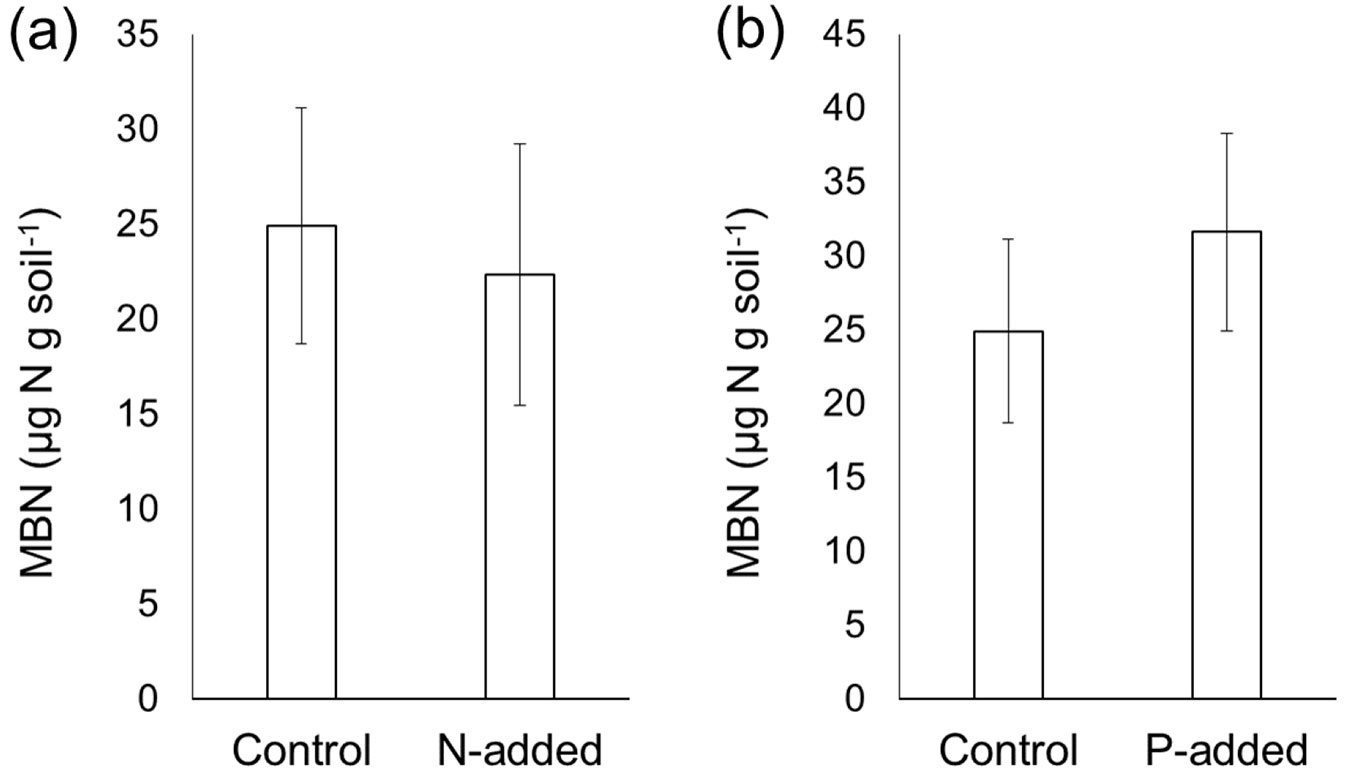
N addition did not affect the MBC content determined by the CFE method (p > 0.05 - Fig. 3a), which was consistent with the fact that neither DOCbef nor DOCaft were affected by N addition. Similarly, the MBN content was not influenced by P addition (p > 0.05 - Fig. 4b). Despite the altered DN content by N addition, the MBN content was not affected by N addition (p > 0.05 - Fig. 4a). P addition did not affect the MBC content (p > 0.05 - Fig. 3b), although P addition significantly increased the DOCaft content.
Fig. 3 - Effects of experimental (a) N addition and (b) P addition on the microbial biomass carbon (MBC) content before and after chloroform fumigation. Error bars indicate the standard error of data from six sites. A paired t-test did not reveal significant differences (p > 0.05).
Fig. 4 - Effects of experimental (a) N addition and (b) P addition on the microbial biomass nitrogen (MBN) content before and after chloroform fumigation. Error bars indicate the standard error of data from six sites. A paired t-test did not reveal significant differences (p > 0.05).
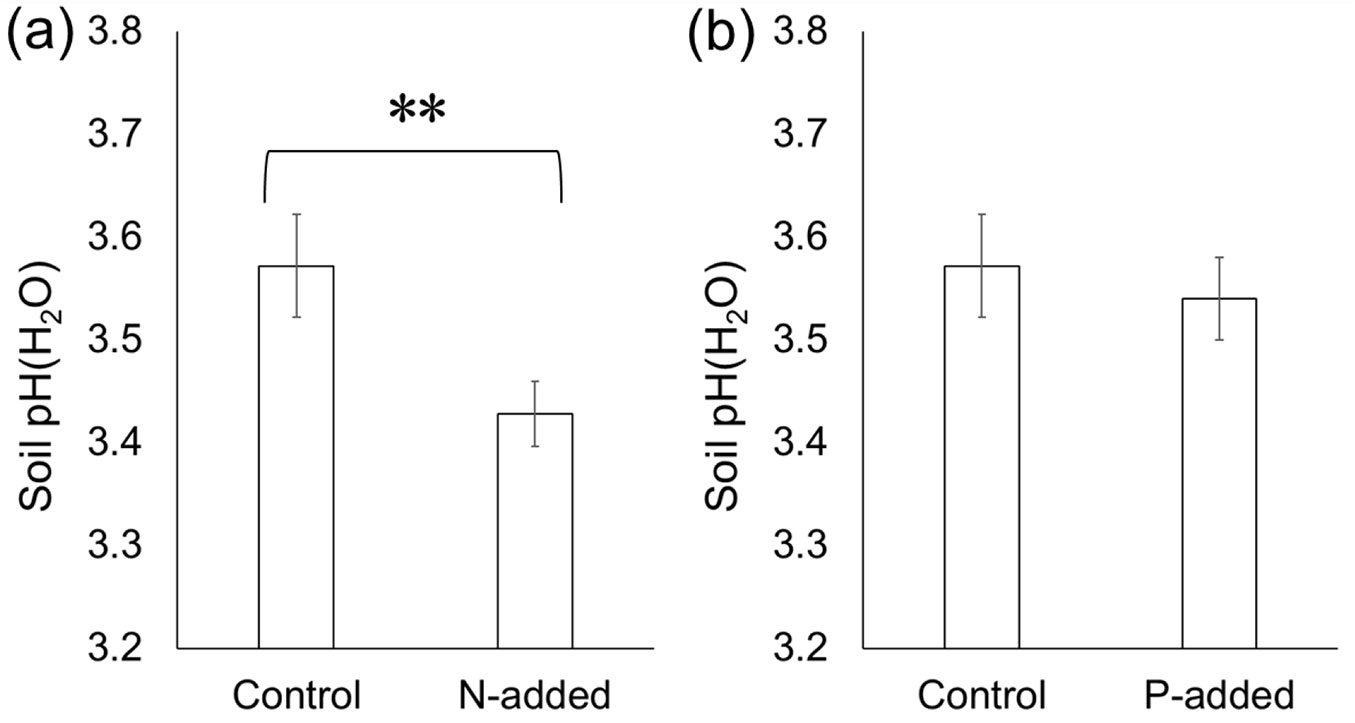
Both the paired t-test and linear mixed effect model analyses demonstrated that N addition significantly decreased soil pH (p < 0.01 - Fig. 5a). Meanwhile, P addition did not have a significant influence (p > 0.05 - Fig. 5b).
Fig. 5 - Effects of experimental (a) N addition and (b) P addition on soil pH. Error bars indicate the standard error of data from five sites (BF, MF, PM, AA, and EU). A paired t-test did not reveal significant differences (p > 0.05).
Discussion
Effects of N and P addition on DOC and DN
The decrease in the DN content following N addition in both the unfumigated and fumigated soils indicated that a portion of the added N (NH4NO3) was adsorbed by the soil or immobilized by microbes (Fig. 2a). It has been suggested that microbes could reduce the DN content during the extraction process ([32]). This is important because it indicates that the soil adsorption capacity or the strength of microbial N immobilization may affect the DN extraction efficiency during the extraction procedure applied in the CFE method, influencing the MBN calculation. If this is the case, the recovery efficiency of the flush of DN during fumigation should be taken into account when calculating the MBN. Further studies are required to investigate this.
We also found that P addition elevated the DOC content (especially DOCaft - Fig. 1b). This was probably because the DOC adsorbed at the mineral surface (part of which was not extracted by 0.5 M K2SO4) was desorbed by P ([27]). Kaiser & Zech ([15]) performed sorption experiments where H2PO4- had a higher affinity to soil than DOC. Hobara et al. ([11]) demonstrated that extracting organic C using a phosphate solution provided around 10 times more C than the use of KCl or water as an extractant, indicating that P has a strong ability to extract organic C from soils. Thus, we attributed the increased DOC content to the desorption of DOC by the added P. Changes in soil pH following P addition may have also caused the higher DOC content in P-amended soils ([10]), but this was less likely because P addition did not change soil pH(H2O) significantly (Fig. 5b), and the decrease in soil pH in N-amended soils (Fig. 5a) did not affect the DOC content (Fig. 1a). The higher DOC content in P-amended soils in our study suggested that the impacts of P fertilization on DOC content should be carefully interpreted in fertilization studies, because the elevated DOC content following P addition does not necessarily indicate stimulated DOC production by microbes.
Effects of N and P addition on MBC and MBN
By adding N and P immediately before fumigation, we conducted tests to determine whether the MBC and MBN measured by the CFE method were biased by the experimental addition of N and P in strongly acidified Chinese forest soils. Our results demonstrated that the MBC and MBN contents were not significantly biased. Despite the increase in the DOC content following P addition (Fig. 1b) and reduced DN content following N addition (Fig. 2a), neither MBC (calculated as the differences between DOCbef and DOCaft) nor MBN (calculated as the differences between DNbef and DNaft) was affected by N or P addition. The reduced DNaft content following N addition did not differ from the reduced DNbef content (Fig. 2a), which consequently caused the MBN content to be unaffected by N addition (Fig. 4a). Similarly, the elevated DOCaft content following P addition did not differ from the elevated DOCbef, resulting in an insignificant difference in the MBC between P-amended soils and the control without P addition (Fig. 3b). Overall, our results suggest that the CFE method is a relatively robust method to test the impacts of nutrient addition on microbial biomass in the strongly acidified soils of Chinese forests. However, to generalize our results, more studies are needed, especially in soils with a high pH.
Conclusions
By testing whether the N or P addition immediately before fumigation affected the results of a microbial biomass determination by the CFE method, we evaluated the robustness of the CFE method for determining the impacts of N and P addition on microbial biomass C and N in the strongly acidified soils of Chinese forests. We found that P addition significantly elevated the DOC content (especially DOCaft); N addition significantly reduced the DN content; and the altered DOC and DN contents did not change the MBC or MBN contents determined by the CFE method. We concluded that CFE is a relatively robust method to determine the impacts of nutrient addition on microbial biomass in the strongly acidified soils of Chinese forests. The results also suggest that even if a fertilization experiment revealed an elevated DOC content after P addition, it does not necessarily indicate DOC production by microbes. The soil adsorption capacity or strength of microbial N uptake during the extraction procedure applied in the CFE method may affect the determination of MBN by influencing the DN extraction efficiency.
Acknowledgements
TM conceived the research and wrote the draft of the manuscript, TM, SW, and CW performed experiment, JM and WZ established research sites, all of the authors joined the discussion of the research. We thank Mr Fu and Ms Hu for their support for our field work. This study was financially supported by National Natural Science Foundation of China (no. 42077311, no. 41731176), Grant-in-Aid for JSPS Postdoctoral Fellowships for Research Abroad (28-601), and a grant from the Sumitomo Foundation (153082).
References
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Senhao Wang 0000-0001-6228-3999
Cong Wang
Jiangming Mo
Wei Zhang 0000-0002-6623-1341
Key Laboratory of Vegetation Restoration and Management of Degraded Ecosystems, South China Botanical Garden and Guangdong Provincial Key Laboratory of Applied Botany, Chinese Academy of Sciences, Guangzhou, 510650 (China)
Kyushu Research Center, Forestry and Forest Products Research Institute, FFPRI, Kurokami 4-11-16, Kumamoto, 860-0862 (Japan)
Cong Wang
University of Chinese Academy of Sciences, Beijing 100049 (China)
Corresponding author
Paper Info
Citation
Mori T, Wang S, Wang C, Mo J, Zhang W (2021). Is microbial biomass measurement by the chloroform fumigation extraction method biased by experimental addition of N and P?. iForest 14: 408-412. - doi: 10.3832/ifor3374-014
Academic Editor
Maurizio Ventura
Paper history
Received: Feb 13, 2020
Accepted: Jul 07, 2021
First online: Sep 04, 2021
Publication Date: Oct 31, 2021
Publication Time: 1.97 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2021
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 43954
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 36360
Abstract Page Views: 3312
PDF Downloads: 3714
Citation/Reference Downloads: 20
XML Downloads: 548
Web Metrics
Days since publication: 1622
Overall contacts: 43954
Avg. contacts per week: 189.69
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2021): 9
Average cites per year: 1.80
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
The effect of clear-cut age on soil organic carbon and nitrogen indices in Scots pine (Pinus sylvestris L.) stands
vol. 18, pp. 146-153 (online: 09 June 2025)
Research Articles
Relationship between microbiological, physical, and chemical attributes of different soil types under Pinus taeda plantations in southern Brazil
vol. 17, pp. 29-35 (online: 28 February 2024)
Research Articles
Wood-soil interactions in soil bioengineering slope stabilization works
vol. 2, pp. 187-191 (online: 15 October 2009)
Research Articles
Response of soil bacterial communities to nitrogen and phosphorus additions in an age-sequence of subtropical forests
vol. 14, pp. 71-79 (online: 11 February 2021)
Research Articles
Effect of exogenous nitrogen and phosphorus inputs on the microbe-soil interaction in the secondary Castanopsis sclerophylla forest in east China
vol. 11, pp. 794-801 (online: 14 December 2018)
Research Articles
Seasonal dynamics of soil respiration and nitrification in three subtropical plantations in southern China
vol. 9, pp. 813-821 (online: 29 May 2016)
Research Articles
Thinning effects on soil and microbial respiration in a coppice-originated Carpinus betulus L. stand in Turkey
vol. 9, pp. 783-790 (online: 29 May 2016)
Research Articles
The responses of soil microbial community and enzyme activities of Phoebe zhennan cultivated under different soil moisture conditions to phosphorus addition
vol. 11, pp. 751-756 (online: 15 November 2018)
Research Articles
Effects of altitudinal gradients on leaf area index, soil microbial biomass C and microbial activity in a temperate mixed forest ecosystem of Northwestern Turkey
vol. 10, pp. 334-340 (online: 15 December 2016)
Research Articles
Dynamics of humus forms and soil characteristics along a forest altitudinal gradient in Hyrcanian forest
vol. 14, pp. 26-33 (online: 10 January 2021)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword