Relationship between microbiological, physical, and chemical attributes of different soil types under Pinus taeda plantations in southern Brazil
iForest - Biogeosciences and Forestry, Volume 17, Issue 1, Pages 29-35 (2024)
doi: https://doi.org/10.3832/ifor4349-016
Published: Feb 28, 2024 - Copyright © 2024 SISEF
Research Articles
Abstract
Over the last decades, Pinus taeda L. plantations in southern Brazil showed a great increase in average production. However, the gains in productivity obtained by genetic selection and breeding have nowadays stabilized. Research on edaphic factors and silvicultural practices is currently performed with the aim of both increasing the productivity of P. taeda plantations and maintaining the soil quality. To this end, soil microbiological attributes are considered better indicators of soil quality as they are more sensitive than chemical and physical ones. In this study, we aimed to evaluate the relationship between microbial activity and the physical and chemical parameters of different soil types under young Pinus taeda plantations at five different sites in southern Brazil. Soil samples were collected at depths of 0-5 and 5-10 cm. The soil microbiological attributes evaluated were: potentially mineralizable nitrogen (PMN), microbial biomass carbon (MBC), microbial biomass nitrogen (MBN), microbial basal respiration (MBR), and metabolic quotient (qCO2). We also evaluated some physical and chemical soil parameters. Sites with the highest values of C, clay, and nutrients in the soil, showed higher values for the soil microbiological attributes, compared to the other study sites. The previous management with minimal tillage in some sites seems to positively affect soil quality. The MCB and MBR showed better sensitivity in indicating differences between sites and showed a good relationship with clay content, C/N ratio, K, and pH. These results suggest that site-specific characteristics such as soil type or forest management influence soil microbiological attributes in Pinus taeda plantations during initial growth in southern Brazil.
Keywords
Soil Microbial Activity, Microbial Biomass Carbon, Microbial Basal Respiration, Forest Management
Introduction
Pinus taeda L. is one of the main forest species cultivated in Brazil, and plantations of the genus Pinus currently occupy 1.7 million hectares, mostly located in the states of Paraná (43%) and Santa Catarina (24%) ([20], [22]). Recently, the application of silvicultural practices combined with genetic improvement resulted in Pinus taeda plantations with high productivity in southern Brazil ([31]). It is recognized that edaphoclimatic factors are crucial in limiting the growth of Pinus plantations ([43], [10]). However, the relationship between microbiological, physical and chemical attributes in the soil is not well clarified, therefore it has still to be established which attributes better reflect the soil quality affecting the growth of P. taeda stands in southern Brazil ([34], [36]).
In Pinus plantations, the microbial activity of the soil depends, among other factors, on the soil type, the environmental conditions, and the type of silvicultural management ([2], [3]). Soil pedogenesis is responsible for many edaphic parameters in the soil, along with soil and silvicultural management ([13]). In the long term, these factors are decisive for the increased productivity and sustainability of forest plantations ([47]).
After a forest exploitation cycle, tree spacing techniques, use of litterfall from the previous cycle, and addition of fertilizers can contribute to maintaining the supply of nutrients to the soil and guarantee the productivity of the forest site in the long term ([44]). Some researchers have evaluated responses to management practices using improved genetic material, mechanical site preparation, control of competing vegetation, and fertilization in Pinus plantations, and found positive results in the relationship between productivity and soil chemical attributes, significantly improving the growth of species when best tillage practices were recommended ([2], [7]). Other studies have reported that successive cycles of Pinus plantations without nutrient replacement tend to cause a nutritional deficit for the trees, resulting in low productivity in forest plantations ([14], [15]). Therefore, assessing soil microbiological attributes and their relationships with chemical and physical soil characteristics, combined with site-specific silvicultural management practices in Pinus taeda, provides a better understanding of soil nutrient dynamics ([39], [41]). Several studies showed that nitrogen mineralization and microbial biomass carbon are potential indicators of soil quality in forest systems ([25], [26]), as microorganisms are responsible for mineralizing elements in the soil and contribute to the availability of nutrients for plants ([35]).
Silvicultural management in forest plantations can increase tree growth in P. taeda plantations, especially in the long term, through the contribution of forest residues from previous harvest crops ([1]).
We hypothesized that the microbial activity in the soil of P. taeda plantations is correlated to some physical and chemical attributes resulting from the soil pedogenesis as well as from the soil and silvicultural management. Thus, our objective was to evaluate the relationship of soil microbiological attributes with chemical and physical parameters, as well as with the initial growth of the Pinus taeda in southern Brazil.
Material and methods
Study area and experimental design
The experimental sites are part of the Cooperative Program on Pinus Research in Brazil, coordinated by the Forestry Science and Research Institute (IPEF). The predominant climate is humid subtropical, with a dry season in winter and classified by Köppen as Cfb ([4]).
The study was carried out in five experimental plots of Pinus taeda planted in 2019 on wavy soft relief, in Santa Catarina and Paraná states, southern Brazil. The sites were selected because of their differences in soil classification (Tab. 1), i.e., differences in soil physical and chemical attributes, but also in the soil use before current P. taeda plantation and in soil management. Tree growth was monitored and measurements were performed in 2021. Local climate data were obtained by the annual precipitation and mean annual temperature during the period 2019-2021 (Tab. 2). Climate database were obtained from a high spatial resolution global weather ([11]).
Tab. 1 - Location and soil taxonomy of experimental sites of Pinus taeda of the initial growth in southern Brazil. (*): Soil classification according to the USDA Soil Survey ([40]).
| Areas | Municipality/State | Latitude | Longitude | Altitude (m a.s.l.) |
Soil (*) |
|---|---|---|---|---|---|
| Site 1 | Telêmaco Borba/Paraná | 24°22′ 57″ S | 50°56′ 89″ W | 835 | Oxisols |
| Site 2 | Lages/Santa Catarina | 27°79′ 28″ S | 50°50′ 02″ W | 916 | Ultisols |
| Site 3 | Caçador/Santa Catarina | 26°74′ 86″ S | 51°07′ 18″ W | 1030 | Oxisols |
| Site 4 | Vargem Bonita/Santa Catarina | 26°55′ 03″ S | 51°47′ 36″ W | 1088 | Oxisols |
| Site 5 | Campo Belo do Sul/Santa Catarina | 28°00′ 29″ S | 50°51′ 20″ W | 956 | Inceptisols |
Tab. 2 - Characteristics of silvicultural management, climate, and average height of trees in the five experimental plots.
| Areas | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 |
|---|---|---|---|---|---|
| Previous soil use | Eucalyptus urophylla | Pinus taeda | Pinus taeda | Pinus taeda | Pinus taeda |
| Age at harvest (years-old) | 8 | 22 | 38 | 15 | 34 |
| Soil management | Removal of residues / Subsoiling 60 cm | Removal of residues / Subsoiling 50 cm | Minimal tillage / Subsoiling 60 cm | Removal of residues / Subsoiling 35 cm | Minimal tillage / Subsoiling 45 cm |
| Present soil use (since 2019) | Pinus taeda | Pinus taeda | Pinus taeda | Pinus taeda | Pinus taeda |
| Spacing (m × m) | 3.1 × 1.9 | 2.4 × 2.6 | 2.5 × 2.5 | 2.5 × 2.0 | 3.0 × 2.5 |
| Average temperature (°C) | 19.1 | 16.1 | 16.4 | 17.2 | 15.2 |
| Annual precipitation (mm) | 1382 | 1750 | 1653 | 1543 | 1450 |
| Average height (m) | 2.5 | 3.2 | 2.5 | 2.4 | 2.8 |
At each study site, 21 replication plots were established in the field with 128 trees (8 rows × 16 trees), including a double border, thus resulting in a set of 32 measured trees per replication plot and 672 trees per site. The mortality index of the sites was around 3%. Starter fertilization was applied 180 days after planting with 20 kg ha-1 of N, 60 kg ha-1 of P2O5, and 33 kg ha-1 of K2O in small holes 10 cm distant from the stem of each tree. It was also applied 675 kg ha-1 of CaO and 150 kg ha-1 of MgO between rows next to the trees’ lines. Complementary fertilization (40 kg ha-1 of N and 67 kg ha-1 of K) was applied 360 days after planting on the soil surface under the tree crowns.
Soil sampling and analysis
Soil samples were collected 360 days after the last fertilizer application, at depths of 0-5 and 5-10 cm in the rows and between rows. For each site, 21 soil samples were collected at each depth.
Soil samples were air dried, sieved (2.0 mm) and analysed for the following parameters. pH was measured in CaCl2 0.01 mol L-1 solution; exchangeable Al3+, Ca2+, and Mg2+ were extracted by KCl 1 mol L-1; available K+ and P were extracted by Mehlich I; (H+ + Al3+) were extracted by calcium acetate 0.5 mol L-1 at pH 7.0; total carbon and nitrogen were obtained by combustion in a Vario EL III® analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany).
All the microbiological attributes were determined on the air-dried and sieved soil samples after being moistened to field capacity. Potentially mineralizable nitrogen (PMN) was determined by anaerobic incubation with an adaptation of the Waring & Bremner ([45]) method. A first extraction of NH4+-N and NO3-N with KCl (2 mol L-1) was performed and then the same samples were inserted in centrifuge tubes (50 mL) and filled with 30 mL of a nutritive solution (Na3PO4-0.005 mol L-1, MgSO4-0.002 mol L-1, CaCl2-0.005 mol L-1). Samples were then incubated in a BOD chamber in the dark at 30 °C for 15 days until the second KCl extraction. Two more incubation/extraction steps were repeated and in total, we had 4 extractions during 45 days of incubation. Ammonium (NH4+-N) was determined by the phenol method using colorimetric spectrophotometry ([6]). Nitrate (NO3--N) was determined by ultraviolet spectrophotometry ([19], [32]). The sum of nitrate and ammonium was considered the PMN.
Microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) were determined by irradiation method ([30]). Briefly, one of the two sets of soil samples (20 g) was irradiated, and the other set of soil samples was not irradiated. Subsequently, all samples were extracted with a K2SO4 (0.5 mol L-1). The MBC and MBN were determined in a Vario TOC Cube® analyzer (Elementar Analysensysteme GmbH, Germany).
Microbial basal respiration (MBR) was performed with 20 g of soil samples moistened to field capacity and placed in hermetically sealed bottles, together with 20 mL of a NaOH solution (0.5 mol L-1). The bottles were then incubated for 240 hours in the dark in a BOD chamber at 25 °C. After the incubation, 5 mL of BaCl2·2H2O (0.5 mol L-1) was added to the NaOH solution to stop the CO2 capture, and the NaOH was titrated with HCl (0.5 mol L-1), and 2 drops of phenolphthalein indicator (0.1%) until the change from red color to lack of color.
The Metabolic Quotient (qCO2) was calculated by the ratio between MBR and MBC ([5]). The result was expressed in mg CO2-C g of MBC h-1 10-3.
Statistical analysis
Analysis of variance and Tukey’s test (P<0.05) for average comparisons were performed using the statistical package “ExpDes” in R version 1.2.2 ([33]). Pearson correlations and linear analyses were used to assess the relationships between microbiological attributes and other soil attributes. Principal components analysis (PCA) was done using the program Minitab v.19 ([28]). All the diagrams were plotted with SigmaPlot® version 12.0 (Systat Software Inc., Palo Alto, CA, USA).
Results
Soil physical and chemical attributes
Small variations were observed in the physical and chemical attributes of the soil between sites (Tab. 3). Site 1 showed the lowest values of clay, carbon, and macro-nutrients in the surface depth. Sites 3, 4, and 5 had the highest values of clay and nutrients.
Tab. 3 - Soil chemical and physical attributes in the 0-5 and 5-10 cm soil depth of the sites of Pinus taeda of the initial growth in southern Brazil. pH (CaCl2 0.01 mol L-1); Al3+, Ca2+, and Mg2+ (extracted with KCl 1 mol L-1); total carbon (C) and nitrogen total (N) (determined by total combustion method); K+ and P (Mehlich-1 extraction); Base saturation (BS); Al3+ saturation (m); cation exchange capacity (CEC pH 7.0).
| Parameter | Units | Sites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | |||||||
| Depth | cm | 0-5 | 5-10 | 0-5 | 5-10 | 0-5 | 5-10 | 0-5 | 5-10 | 0-5 | 5-10 |
| Clay | g kg-1 | 300 | 313 | 625 | 575 | 750 | 713 | 714 | 711 | 619 | 623 |
| pH | CaCl2 | 3.9 | 4 | 4.1 | 4.1 | 3.9 | 3.9 | 4.4 | 4.4 | 4.4 | 4.4 |
| C | g kg-1 | 21.8 | 19.8 | 32 | 27.5 | 53 | 44.5 | 42.9 | 39.9 | 48.3 | 41.4 |
| N | g kg-1 | 1.2 | 1.2 | 1.9 | 1.7 | 2.5 | 2.1 | 2.4 | 2.3 | 2.7 | 2.4 |
| P | mg dm-3 | 2.8 | 6 | 3.9 | 3.3 | 5.9 | 4.8 | 3.4 | 3 | 6.2 | 4.7 |
| K | cmolc dm-3 | 0.06 | 0.05 | 0.13 | 0.08 | 0.15 | 0.13 | 0.14 | 0.12 | 0.17 | 0.1 |
| Ca | cmolc dm-3 | 1.5 | 1.5 | 2.7 | 2.3 | 5.6 | 5.1 | 1.3 | 1.3 | 2.4 | 2 |
| Mg | cmolc dm-3 | 0.62 | 0.51 | 1.12 | 0.99 | 0.99 | 0.88 | 0.42 | 0.33 | 0.68 | 0.47 |
| Al | cmolc dm-3 | 1.7 | 1.6 | 1.7 | 2 | 3.5 | 3.5 | 3.7 | 3.8 | 3.5 | 3.8 |
| CEC | cmolc dm-3 | 11.1 | 11.1 | 14.4 | 12.8 | 20.8 | 20.3 | 17.1 | 16.7 | 17.3 | 17.3 |
| BS | % | 19.2 | 18.8 | 27.1 | 24.4 | 32.3 | 30.4 | 11 | 10.5 | 18.5 | 15 |
| m | % | 44.7 | 43.6 | 30.5 | 38.4 | 34.1 | 36.1 | 66.4 | 66.2 | 52.3 | 59.4 |
Soil microbiological attributes
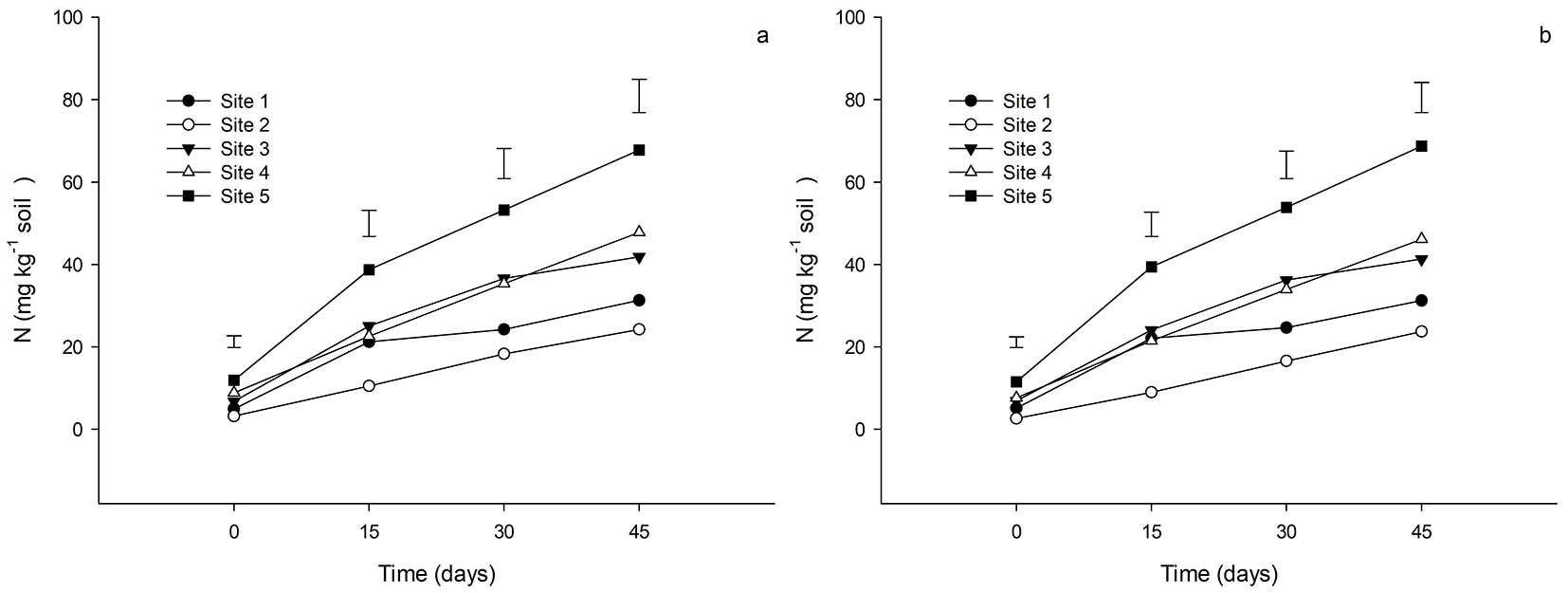
The accumulated potentially mineralizable nitrogen (PMN) along the 45 days of incubation showed a crescent linear behavior which did not reach the stabilization. No identifiable differences were observed between the two soil depths analyzed. Sites 1 and 2 showed the lowest PMN values (less than 32 mg N kg-1 soil), whereas sites 3, 4, and 5 showed the highest PMN values (between 42 and 68 mg N kg-1 soil - Fig. 1a, Fig. 1b).
Fig. 1 - Average of accumulated potentially mineralizable nitrogen (N), as nitrate (NO3-N), and ammonium (NH4-N) during the 45 days of incubation, in the soil of Pinus taeda plantations at different sites in southern Brazil. Depths of 0-5 cm (a) and 5-10 cm (b). The vertical bar represents the least significant difference, according to Tukey’s test (p<0.05).
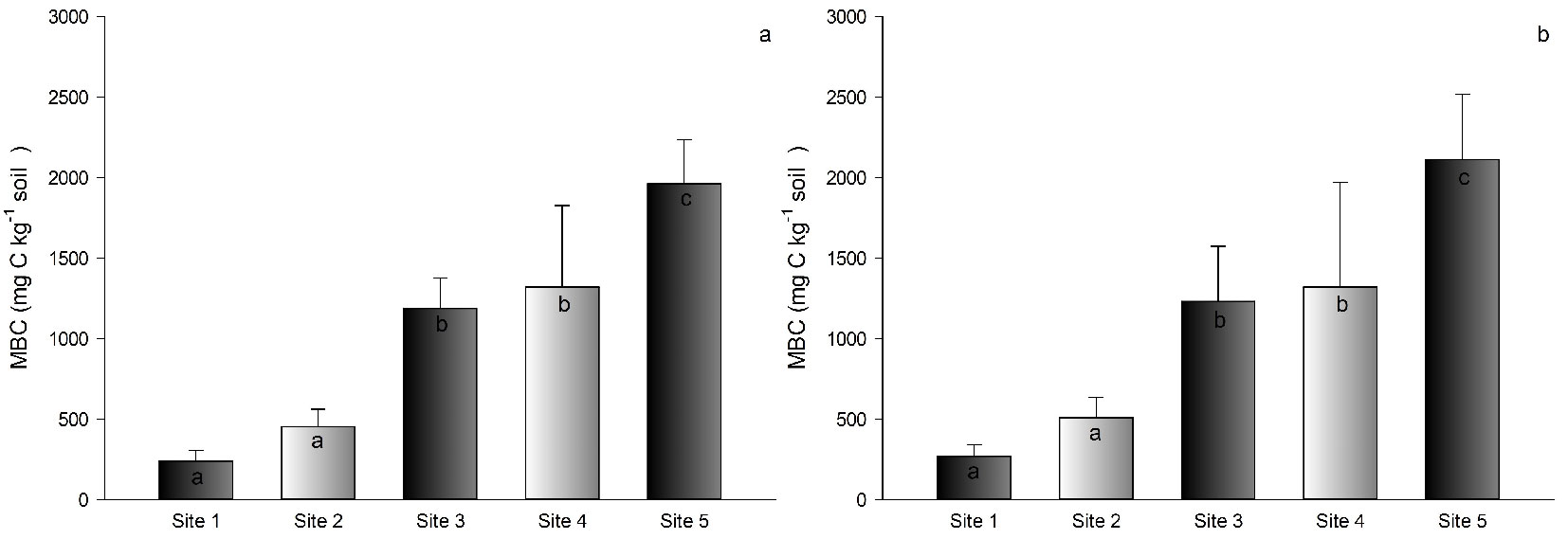
As for MBC, the results were significantly different between the 5 sites (Fig. 2a, Fig. 2b), with site 5 showing the highest values (~2000 mg C kg-1 soil), followed by sites 3 and 4 (~1200 mg C kg-1 soil), and the lowest values of MBC for sites 1 and 2 (~300 mg C kg-1 soil).
Fig. 2 - Average microbial biomass carbon (MBC) in the soil of Pinus taeda plantations at different sites in southern Brazil. Depths of 0-5 cm (a) and 5-10 cm (b). Different letters indicate significant (p<0.05) differences among means after Tukey’s test. Vertical bars represent the standard deviation.
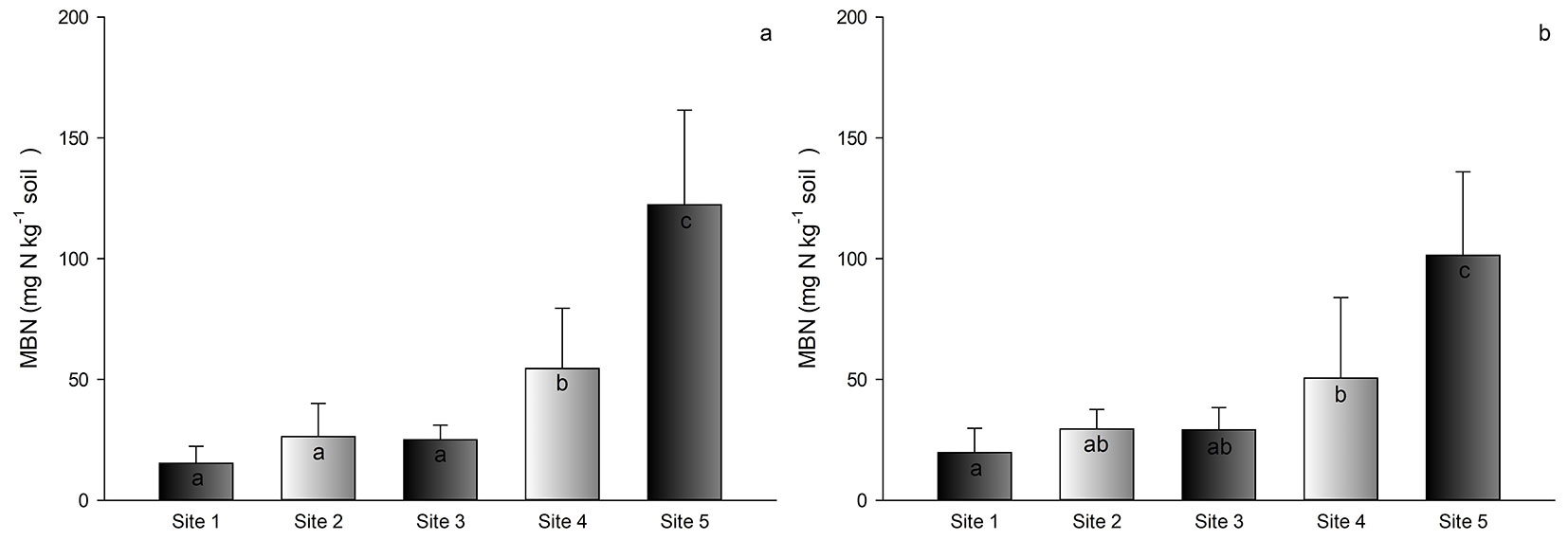
Values of MBN in sites 4 and 5 were higher than those recorded in sites 3, 2, and 1. The lowest values varied between 25 and 29 mg N kg-1 soil, and the highest ones were between 51 and 123 mg N kg-1 soil (Fig. 3a, Fig. 3b).
Fig. 3 - Average microbial biomass nitrogen (MBN) in the soil of Pinus taeda plantations at different sites in southern Brazil. Depths of 0-5 cm (a) and 5-10 cm (b). Different letters indicate significant (p<0.05) differences among means after Tukey’s test. Vertical bars represent the standard deviation.
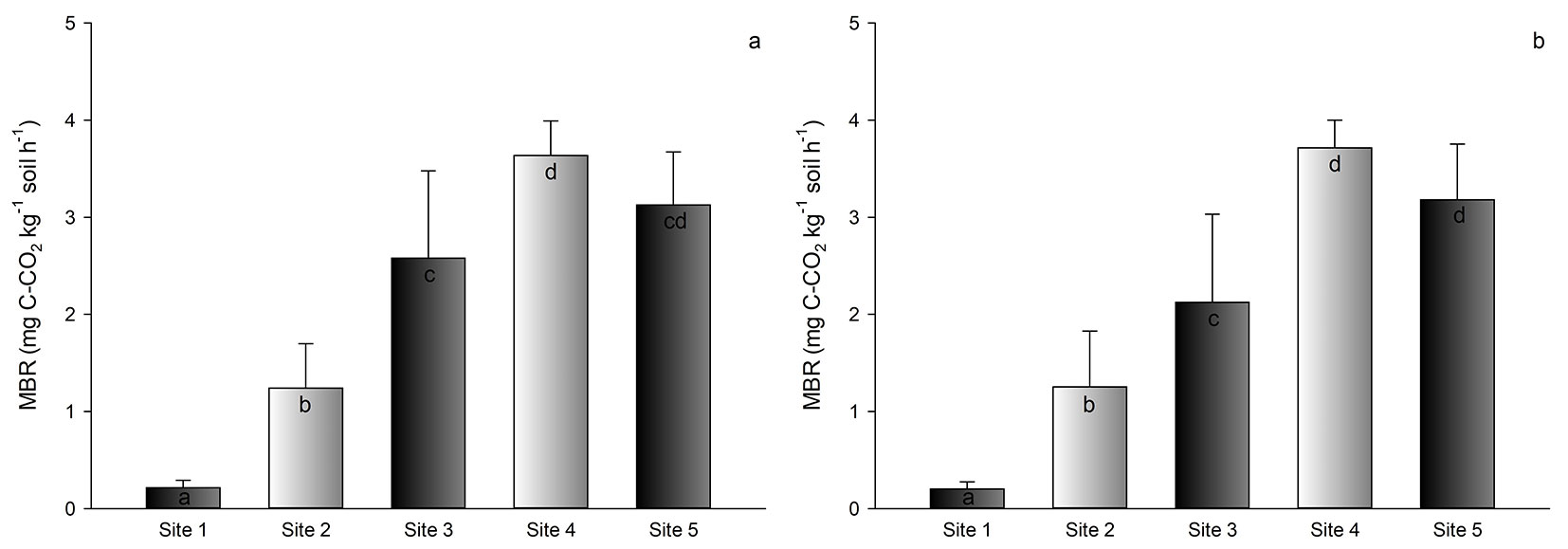
The results for MBR were a little bit different when comparing sites. Site 4 showed the highest value of MBR among all sites, with a value of 3.7 mg CO2-C kg-1 soil h-1). Site 5 showed the second highest value (3.2 mg CO2-C kg-1 soil h-1), followed by sites 3, 2, and 1 (less than 2.0 mg CO2-C kg-1 soil h-1 - Fig. 4a, Fig. 4b).
Fig. 4 - Average microbial basal respiration (MBR) in the soil of Pinus taeda plantations at different sites in southern Brazil. Depths of 0-5 cm (a) and 5-10 cm (b). Different letters indicate significant (p<0.05) differences among means after Tukey’s test. Vertical bars represent the standard deviation.
The lowest values of qCO2 were observed for sites 1 and 5, the highest ones for sites 2 and 4, and the intermediary value for site 3 (Tab. 4).
Tab. 4 - Average of the metabolic quotient (qCO2). Depths of 0-5 cm and 5-10 cm, the ratio between MBR (microbial basal respiration) and MBC (microbial biomass carbon), in the soil of sites of Pinus taeda of the initial growth in southern Brazil. Averages followed by the same letter in the line are not significantly different by Tukey’s test (P<0.05).
| Depth | qCO2 (mg CO2-C g-1 MBC h-1 10-3) | ||||
|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | |
| 0-5 cm | 1.28 a | 3.00 b | 2.32 ab | 3.24 b | 1.63 a |
| 5-10 cm | 0.77 a | 2.84 ab | 1.84 ab | 3.91 c | 1.59 a |
Correlations between soil attributes
Pearson’s correlation between the soil microbiological attributes and the physical and chemical attributes with data from all sites was performed. We observed a tight relationship between some of the microbiological attributes and soil characteristics such as clay, pH, C/N ratio, and potassium (K). However, no significant correlations were found between phosphorus, calcium, and magnesium and basic cations saturation (BS) with the microbiological attributes (Tab. 5).
Tab. 5 - Pearson’s correlation coefficients of microbiological variables (PMN: accumulated potentially mineralizable nitrogen; MBC: microbial biomass carbon; MBR: microbial basal respiration; qCO2: metabolic quotient) with chemical and physical attributes of the soil (pH CaCl2; C/N: ration between total carbon and total nitrogen; P: phosphorous content; Ca, Mg, K: calcium, magnesium, potassium content, respectively; BS: base saturation; m: Al3+ saturation), at depths of 0-5 and 5-10 cm, in Pinus taeda plantations at different sites in southern of Brazil. The pairs of variables showing positive and significant correlation coefficients tend to increase together. (*): p<0.05.
| Soil Variables | PMN | MBC | MBR | qCO2 |
|---|---|---|---|---|
| Clay | 0.371 | 0.514 | 0.299 | 0.826* |
| pH | 0.723* | 0.725* | 0.817* | 0.716* |
| C/N | 0.518 | 0.601* | 0.233 | 0.633* |
| P | 0.316 | 0.269 | 0.235 | -0.015 |
| Ca | -0.052 | 0.092 | -0.2 | 0.073 |
| Mg | -0.472 | -0.306 | -0.342 | -0.333 |
| K | 0.487 | 0.637* | 0.474 | 0.734* |
| BS | -0.447 | -0.316 | 0.136 | -0.38 |
| m | 0.635* | 0.555 | 0.166 | 0.657* |
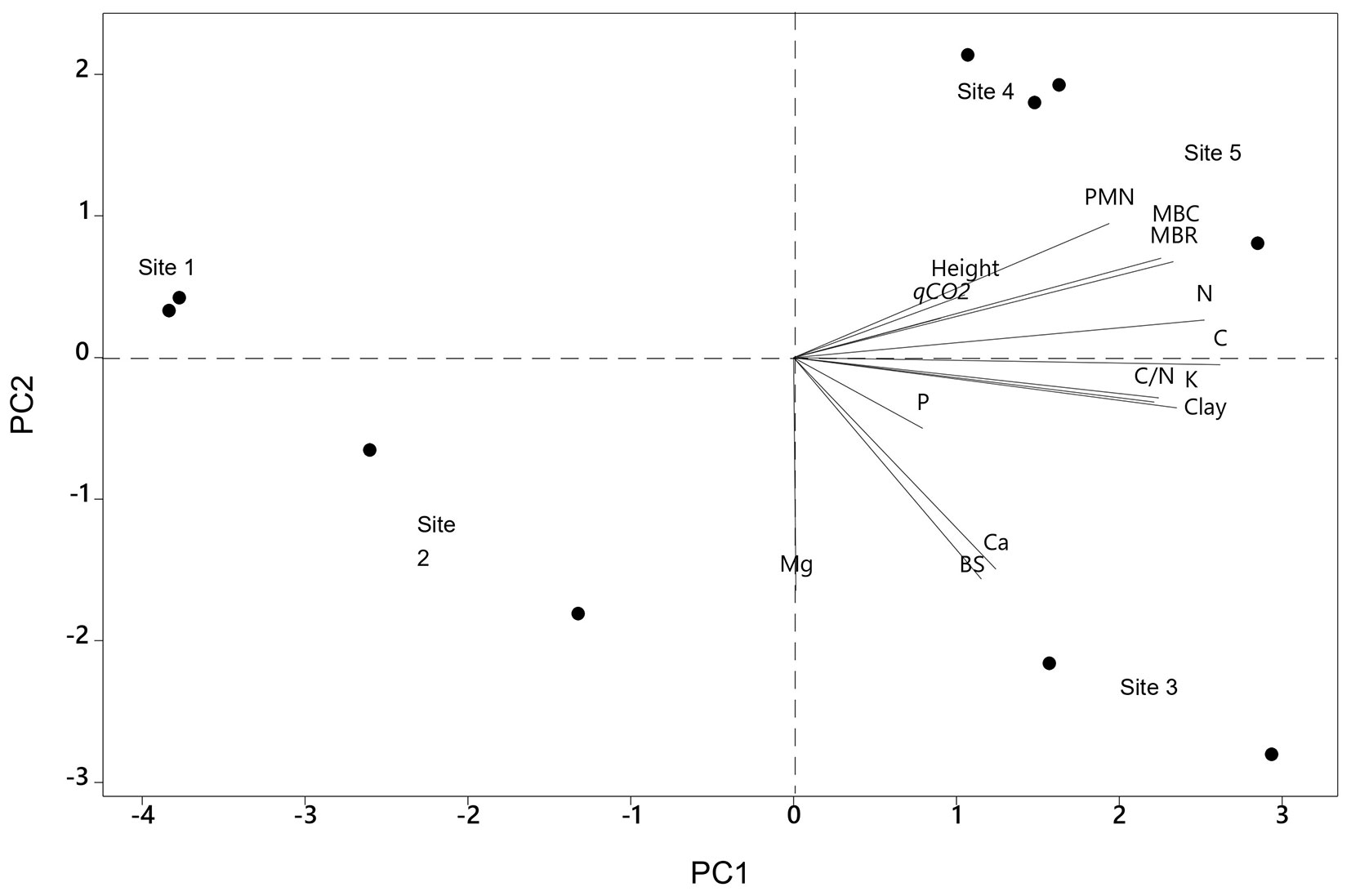
In the PCA, principal components (PC) 1 and 2 accounted for 70% of the variation in the data. PC1 explains 50% of the total variation in the matrix data, and PC2 explains 20% of the variation. The eigenvector matrix represents the weight of each soil attribute factor, in the formation of the principal components. For PC1 the most associated eigenvectors were C, N, K, C/N ratio, MBC, MBR, and PMN, resulting in a direct association. PC2 was influenced by PMN, MBC, and MBR. (Tab. 6, Fig. 5).
Tab. 6 - Results of principal component analysis (PCA) for Pinus taeda plantations at different sites in southern Brazil. (PMN): accumulated potentially mineralizable nitrogen; (MBC): microbial biomass carbon; (MBR): microbial basal respiration; (qCO2): metabolic quotient; (P): phosphorus; (K): potassium; (Ca): calcium; (Mg): magnesium; (C): carbon; (N): nitrogen; (C/N): carbon/nitrogen content ratio; (BS): base saturation.
| - | Principal components | |
|---|---|---|
| PC1 | PC2 | |
| Eigenvalue | 6.9 | 3.2 |
| Proportion | 0.5 | 0.2 |
| Cumulative | 0.5 | 0.7 |
| Variable | Eigenvectors | |
| PMN | 0.28 | 0.30 |
| MBC | 0.32 | 0.22 |
| MBR | 0.32 | 0.21 |
| qCO2 | 0.13 | 0.09 |
| Height | 0.15 | 0.14 |
| P | 0.11 | -0.16 |
| K | 0.34 | -0.11 |
| Ca | 0.18 | -0.47 |
| Mg | 0.00 | -0.52 |
| C | 0.38 | -0.02 |
| N | 0.36 | 0.08 |
| Clay | 0.32 | -0.09 |
| C/N | 0.32 | -0.10 |
| BS | 0.17 | -0.49 |
Fig. 5 - Principal component analysis (PCA) for sites of Pinus taeda of the initial growth in southern Brazil.
Discussion
Effect of soil types on microbiological attributes
In general, the soil microbiological attributes were not influenced by the depth of soil sampling (0-5 and 5-10 cm). The differences identified for the microbiological attributes in the soil were related to site-specific conditions.
Despite all the sites having the same species and with similar ages in the present, previous use of the soil was quite different among sites (Tab. 2). During the initial growth, the effects on soil microbiological attributes are not related to the species planted ([37], [38]), but rather to soil conditions resulting from the history of soil use and the management of forest system of the sites ([44]).
For most of the microbiological attributes evaluated, except for the qCO2, site 5 showed the highest values and site 1 the lowest ones. At site 1, the current P. taeda stand replaced a previous Eucalyptus urophylla plantation, and this could partly explain the lowest soil clay content among all sites. In contrast, at all other sites current Pinus taeda stands replaced previous plantations of the same species. The quality of the remaining organic matter certainly affected the studied microbial attributes ([18], [12]). Most of the residues from tree harvest were removed from site 1 before planting Pinus taeda and we can hypothesize that this has determined the low values of soil microbiological activity recorded. Also, site 1 presented the lowest levels of C, N, K, Ca, and Mg at both soil depths. Therefore, many soil conditions of site 1 could be related to the lowest values of its microbiological attributes.
Biesek ([8]) studied soil microbiological attributes in crop areas, native and planted pasture, and a forest ecosystem, finding that the microbiological characteristics were attributed to the carbon and nitrogen stocks (forest and native pasture) or to the nutrient availability (crop areas) in the soil. Site 5 showed high values for clay, C, N, P, K, Ca, and Mg, likely related to the maintainance of harversting residues of previous P. taeda plantations during the last 34 years ([16]). The combination of all these factors may explain the highest values for the microbiological attributes at this site.
Site 2 had the second lowest values for the microbiological attributes, as well as C, N, P, K, Ca, and Mg in the soil. In this case, most of the vegetal residues were removed when the former P. taeda plantation was harvested. Sites 3 and 4 showed values of clay, C, N, P, K, Ca, and Mg very close to those observed at site 5, but the soil type is an Oxisols, whereas site 5 has an Inceptisols which is less altered when compared to Oxisols. The higher elevation at sites 3 and 4, along with their altered clay content in the soil, may explain the lower values of the microbiological attributes in comparison to site 5.
The levels of C, N, P, K, Ca, and Mg in the soil surface are closely related to the quality of the soil organic matter. Suitable forest management practices, such as soil fertilization and silvicultural techniques, can improve site productivity by incorporating organic matter to the soil, that in turn can improve the soil microbiological quality ([25]).
The C/N ratio is another important factor controlling the rate of mineralization and the microorganism community composition ([23]). Based on C/N ratio, the soil mineralization rate can be estimated, as well as the retention of mineral nitrogen in the soil and the variation in organic matter, which contributes to promoting microbial biomass ([42]). Manirakiza et al. ([27]) evaluated the nitrogen mineralization rates in soil with the application of mineral and organic fertilizer, finding that fertilization improved the C/N ratio to adequate levels (around 14), thus increasing microbial biomass ([24], [9]).
Relationships between soil attributes
The soil pH showed a close relationship with the soil microbiological attributes, mainly with MBC, MBR, and PMN, despite the very small range of variation (3.9 to 4.4) among the study sites. The pH of the soil is an important variable in regulating microbial diversity and the mineralization process ([17]), although it seems unlikely that differences in the microbiological attributes among sites could be explained by the small pH variation observed.
MBC and MBR showed the highest correlations with the C/N ratios, and similar results were found in other studies ([29]). Samuelson et al. ([38]) showed that MBR can be an efficient indicator of soil microbiota in P. taeda plantations, and along with the MBC, they were the most sensitive indicators of the microbiological activity across P. taeda sites. In our study, MBR showed a strong relationship with clay content and was the most sensitive microbiological attribute. Low MBR values can indicate lower substrate availability for soil microorganisms ([42], [46]); therefore, a balanced MBR rate in soils could maintain soil quality.
The qCO2 showed a positive correlation with clay content across the studied sites. Similar qCO2 values for pine plantations, but of different ages and at different soil depths, were found in other studies ([21]). Also, Tulio et al. ([41]) did not find any significant relationships between the metabolic quotient and chemical and physical attributes in the soil of P. taeda plantations. However, in the soil under P. sylvestris stands in northern China, Yao et al. ([46]) found a strong correlation between qCO2 and soil water content, and this allowed to better undestand the soil organic matter (SOM) dynamics in relation to soil physical characteristics ([46]).
Sites 1 and 2 showed lower average values of soil microbiological attributes and a weaker relationship with other soil attributes, while sites 3, 4, and 5 showed the highest average values of microbiological attributes and the strongest correlations with soil attributes. Meanwhile, at sites 4 and 5 microbiological activity was mostly associated with other soil attributes, such as PMN, MBC, MBR, carbon, and nitrogen total, whereas at site 3 it was more related to soil bases, K and Ca. Therefore, we speculate that soil microbiological attributes can be influenced by both the SOM and the nutrient content in the soil.
Conclusion
Our results indicate that physical and chemical attributes intrinsic to the soil types have site-specific effects on soil microbiological attributes. Moreover, previous history of land use at the study sites remarkably affects soil microbiological activity. MBC and MBR were the soil microbiological attributes most sensitive to the differences between sites and showed a close relationship with clay content, pH, C/N ratio, and K in the soil. Considering the sensitivity of microbiological soil attributes to soil management practices, monitoring these attributes is a feasible way to evaluate changes in soil quality.
Acknowledgements
Authors are grateful to the Coordination for the Improvement of Higher Personnel Education (Capes/Brazil) and to National Council for Scientific and Technological Development (Cnpq/Brazil) for the financial support (grants and scholarships), as well as to the Cooperative Program on Pinus in Brazil (PPPIB) and the Brazilian Institute of Forestry Research and Studies (IPEF). We acknowledge the forest companies Klabin S.A., Florestal Gateados, Juliana Florestal Ltda. and Irani Papel e Embalagem S.A., for providing field support and financing the materials used in the experiment.
References
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Renato Marques 0000-0003-3011-6672
Diego Herzog de Carvalho 0000-0001-5431-285x
Janaina Gabriela Larsen 0000-0002-5603-5208
DSEA-UFPR - Department of Soil Science and Agricultural Engineering - Federal University of Paraná, Curitiba, Paraná (Brazil)
Mauro Valdir Schumacher 0000-0003-3277-5671
SCFL-UFSM - Forest Sciences Department - Federal University of Santa Maria, Santa Maria, Rio Grande do Sul (Brazil)
Corresponding author
Paper Info
Citation
Zanon JA, Marques R, Herzog de Carvalho D, Larsen JG, De Souza Kulmann MS, Schumacher MV (2024). Relationship between microbiological, physical, and chemical attributes of different soil types under Pinus taeda plantations in southern Brazil. iForest 17: 29-35. - doi: 10.3832/ifor4349-016
Academic Editor
Maurizio Ventura
Paper history
Received: Mar 14, 2023
Accepted: Nov 03, 2023
First online: Feb 28, 2024
Publication Date: Feb 29, 2024
Publication Time: 3.90 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2024
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 7584
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 2629
Abstract Page Views: 2046
PDF Downloads: 2593
Citation/Reference Downloads: 1
XML Downloads: 315
Web Metrics
Days since publication: 714
Overall contacts: 7584
Avg. contacts per week: 74.35
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effects of altitudinal gradients on leaf area index, soil microbial biomass C and microbial activity in a temperate mixed forest ecosystem of Northwestern Turkey
vol. 10, pp. 334-340 (online: 15 December 2016)
Research Articles
Wood-soil interactions in soil bioengineering slope stabilization works
vol. 2, pp. 187-191 (online: 15 October 2009)
Research Articles
Seasonal dynamics of soil respiration and nitrification in three subtropical plantations in southern China
vol. 9, pp. 813-821 (online: 29 May 2016)
Short Communications
Is microbial biomass measurement by the chloroform fumigation extraction method biased by experimental addition of N and P?
vol. 14, pp. 408-412 (online: 04 September 2021)
Research Articles
Thinning effects on soil and microbial respiration in a coppice-originated Carpinus betulus L. stand in Turkey
vol. 9, pp. 783-790 (online: 29 May 2016)
Research Articles
Effect of different dolomitic limestone dosages on soil respiration in a mid-altitudinal Norway spruce stand
vol. 12, pp. 357-365 (online: 05 July 2019)
Research Articles
Wildfire and harvesting effects on carbon dynamics in an oak-pine mixed forest
vol. 13, pp. 435-440 (online: 16 September 2020)
Research Articles
The effect of clear-cut age on soil organic carbon and nitrogen indices in Scots pine (Pinus sylvestris L.) stands
vol. 18, pp. 146-153 (online: 09 June 2025)
Short Communications
Influences of Black Locust (Robinia pseudoacacia L.) afforestation on soil microbial biomass and activity
vol. 9, pp. 171-177 (online: 16 February 2015)
Research Articles
Soil fauna communities and microbial activities response to litter and soil properties under degraded and restored forests of Hyrcania
vol. 14, pp. 490-498 (online: 11 November 2021)
iForest Database Search
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword