Wood-soil interactions in soil bioengineering slope stabilization works
iForest - Biogeosciences and Forestry, Volume 2, Issue 5, Pages 187-191 (2009)
doi: https://doi.org/10.3832/ifor0513-002
Published: Oct 15, 2009 - Copyright © 2009 SISEF
Research Articles
Abstract
In this work we propose the use of soil quality indicators with the aim of assessing the environmental impact of soil bioengineering works. This study was carried out in central Italy where soil bioengineering slope stabilization works were established using chestnut wood. In particular the goal of this study was to determine the occurrence of a wood-effect, that is changes of soil properties due to the presence of decomposing logs in two sites characterized by different time span since works setting up. The presence of the logs did not affect soil physico-chemical properties. Conversely, soil biochemical properties such as soil microbial biomass, basal and cumulative respiration activities as well as microbial indexes, were influenced by the presence of the logs confirming the role of these bioindicators as early predictors of changes occurring in soil. Although a general positive trend was observed for the biochemical properties at both sites with respect to the control soils, significant differences were recorded mainly at the site where works were established six years before soil sampling. Soil bioengineering slope stabilization works establish a positive feed-back which ultimately can benefit plants; in fact the increase in microbial mineralization activity can enhance nutrient cycling and thus promote adequate growth conditions for the plant cuttings used in the wooden-work.
Keywords
Wood decomposition, Microbial biomass, Microbial respiration, Soil quality, Soil bioengineering
Introduction
Soil bioengineering uses sound engineering practices in conjunction with integrated ecological principles, using living vegetation and other materials to construct slopes (hillslopes, riverbanks, and lake/shorelines), stabilize slopes, control erosion, protect wildlife habitats and enhance the functioning of ecosystems ([8], [12]). Successes of ecological engineering make it an increasingly attractive alternative to traditional engineering approaches, which are often much more expensive to construct and sustain ([21]). Vegetation can affect the stability of slopes by modifying the hydrological regime in the soil. Species often used in bioengineering applications include willow, poplar, grasses and native shrub species anyway usually fast-growth species.
In the last years wood has been widely used as a suitable natural material to be used in soil bioengineering works ([27]). In particular, among wood characteristics, its mechanical resistance and its duration over time is not always necessary, being its role that of allowing plants colonization in its early stages and so these characteristics are important only in specific situations. Among the various environmental factors influencing the performance of the wooden-work, soil plays an extremely important role. Its properties in fact not only determine wood decay rate, but are in turn influenced by wood decomposition which can deeply affect soil physico-chemical and biochemical features. Another important aspect of soil bioengineering is thus emphasized: soil is an element either active in wood decay or passive, being itself influenced by wood decay. Soil nutrients cycling and availability are indeed deeply affected by wood decay so that decomposing logs have been referred as “slow-release fertilizers” ([6]).
Dead wood is no longer considered as merely debris, and wood decomposition is widely recognized as a key ecological process ([10]). Decaying wood also plays an important role in soil development because the residues from the degradation of wood components (especially lignin) are one of the substrates for humus formation ([32]). Additionally, leaching of dissolved organic matter from decaying wood contributes to the soil organic matter pools and, providing easily available organic substrates, may fuel soil microbial biomass, thus enhancing its size and/or activity ([35], [31]).
However, regarding the effect of decaying wood on the physical, chemical and biochemical properties of the underlying soil, contrasting results were reported and, to our knowledge, not specifically on soil bioengineering works. Some studies found higher C (carbon) and N (nitrogen) percentages in soil under decaying wood, but did not observe changes in the C:N ratios ([17]), while Hafner et al. ([13]) reported higher C:N ratio beneath decaying logs.
Further approaches, aimed to infer effects of decaying wood on nutrient availability, have been focused on soil biological properties. Busse ([5]) found higher microbial biomass and microbial quotient (ratio of microbial C to total organic C) under than away from decaying logs in a pine forest in Oregon and suggested it could be the result of higher mineralizable N. Conversely [17] found the opposite, namely higher mineralizable N away from logs.
As suggested by Klinka et al. ([18]), the effect of decaying wood on soil can be specific for each ecosystem, as it is affected by the characteristics of the physical environment as well as by biotic-mediated processes. Furthermore the wood decay stage and thus residence time are extremely important in determining the amplitude of these effects.
In the last 20 years many properties (physical, chemical and biochemical) have been recognized as indicators of changes occurring within the soil system. In particular, the bioindicators (representing features related to the living component of the soil, mainly the microbial biomass and its metabolic activity) have been considered particularly reliable as early predictors of modifications affecting the soil environment ([11]). Specifically microbial pool and its activity have a central role in the soil biogeochemical cycling, thus determining the potential availability of mineral nutrients for plants growth ([20], [22]).
The aim of this study was to outline a wood-effect, that is changes in soil properties due to wood degradation, that in turn can affect nutrient cycling and soil microrganisms activity and size ([35], [13]). This was studied in two sites characterized by different length of time since works setting up. In particular the effectiveness of the different soil indicators (physico-chemical and biochemical) was evaluated.
Material and methods
Sites description and soil sampling
This study was conducted in central Italy where bioengineering slope stabilization works, using chestnut wood, were established in two sites: Atina, in the province of Frosinone and Barbarano Romano in the province of Viterbo.
Barbarano Romano (340 a.s.l., 42°15’1” N, 12°4’3” E) natural reserve is located in the province of Viterbo, Central Italy. Annual precipitation is 1050 mm and average annual temperature is 13°C.
The typical riparian vegetation is characterized by allochtonous species of Robinia pseudacacia L. e Ailanthus altissima Mill. The double wooden pile-caisson was 1.4 m high and 3 m long. It has been built with debarked chestnut logs (Castanea sativa Mill.) of 14 cm diameter.
Atina, Colle Melfa (450 m a.s.l., 41°37’0” N, 13°48’0” E) is located in the province of Frosinone, Central Italy. Annual precipitation is 1460 mm and average annual temperature is 13.6°C. Dominant tree species include Quercus pubescens Will., Robinia pseudoacacia L., Salix alba L. and Populus alba L. The double wooden pile-caisson was 2.4 m high and 3 m long. It has been built with debarked chestnut logs (Castanea sativa Mill.) of 25 cm diameter. In both wooden-works autochthonous cuttings of Salix purpurea L. and Salixeleagnos L. were used. A schematic drawing of the double wooden pile-caisson realized at Barbarano and Atina and soil sampling scheme is presented in Fig. 1.
Fig. 1 - Drawing of the double wooden pile-caissons realized at Atina and Barbarano. The six grey arrows indicate where soil cores were sampled. Modified from Sauli et al. ([28]).
Soil sampling was carried out at both sitesin November 2007, two and six years later since the double wooden pile-caisson establishment respectively. Six soil samples were collected at 0-10 cm depth directly beneath six logs of the double wooden pile-caisson in both sites (log soils, LS - Fig. 1). In order to account for a wood-effect and to eliminate differences due to other factors such as the pedogenetic characteristics of both sites, six corresponding soils cores, named control soils (CS), were sampled at the same depth at least 5 m away from the double wooden pile-caisson at each site in absence of perennial vegetation, for a total of 12 soils per site. Soil samples were immediately sieved (<2mm) and the moisture content adjusted to 60% of their water holding capacity (WHC). The soil samples were then left to equilibrate at room temperature in the dark for 12 h prior to biochemical analyses.
Soil physico-chemical and biochemical properties
Soil texture was determined following the pipette method ([24]). Organic C (Corg) and total N (Ntot) were determined on 20 mg of oven dry soil. The method was based on dry combustion using an elemental analyser (Thermo Soil NC - Flash EA1112).
Active and exchangeable acidity were measured on sieved soil suspended in a solution of deionised water (active) or in KCl 1N (exchangeable) in 1:2.5 ratio. The pH was measured in the supernatant with a pH meter (pH 211, Hanna Instruments).
Microbial biomass carbon (MBC) was estimated following the Fumigation Extraction (FE) method: two portions of moist soil (20 g oven-dry soil) were weighed, the first one (not fumigated) was immediately extracted with 80 ml of 0.5M K2SO4 for 30 min by oscillating shaking at 200 rpm and filtered (Whatman n. 42); the second one was fumigated for 24h at 25°C with ethanol-free CHCl3 and then extracted as described above. Organic C in the extracts was determined after oxidation with 0.4 N K2Cr2O7 at 100°C for 30 min ([33]). Microbial biomass was calculated as follows (eqn. 1):
where E C is the difference between organic C extracted from fumigated soils and organic C extracted from not fumigated soils and k EC = 0.38. C extracted from not fumigated samples represents the labile pool of K2SO4 extractable C (ExtC).
For measuring microbial respiration 20 g (oven-dry basis) of moist sample were placed in 1L stoppered glass jars. The CO2 evolved was trapped, after 1, 3, 7, 10, 14, 21, 28 days of incubation, in 2 ml 1M NaOH and determined by titration of the excess NaOH with 0.1M HCl ([3]). The potential initial mineralization rate (C0 k) was calculated from the kinetic parameters obtained using the mineralization kinetic model of C ([26] - eqn. 2):
The hourly CO2 evolved after the 10th day of incubation was used as the basal respiration value because, after that period, the soil reached a relatively constant hourly CO2 production rate.
The metabolic quotient (qCO2) is calculated as µg C-CO2 basal h-1 µg Biomass C-1 following Dilly & Munch ([7]) and is a bioindicator used as a measure of disturbance or stress conditions of microbial biomass ([1]).
Considering the differences in soil organic matter content at both sites the biochemical properties have been presented as fractions (%) of total organic C. The use of these quotients avoids the problems of comparing trends in soils with different organic matter content ([30]) and appears to provide more sensitive indications of soil changes than either activity or population measurements alone ([7]).
The wood-effect is presented as the percentage variation of each biochemical parameter measured in the soil beneath the logs (LS) with respect to the relative control soil (CS).
Statistical analysis
Analysis of variance (ANOVA) and Bonferroni post-hoc test were performed to evaluate the main effects of site, logs presence and their interactions on the parameters analysed. Statistical analysis was performed using SYSTAT 11.0, a statistical probability of p<0.05 was considered as significant.
Results
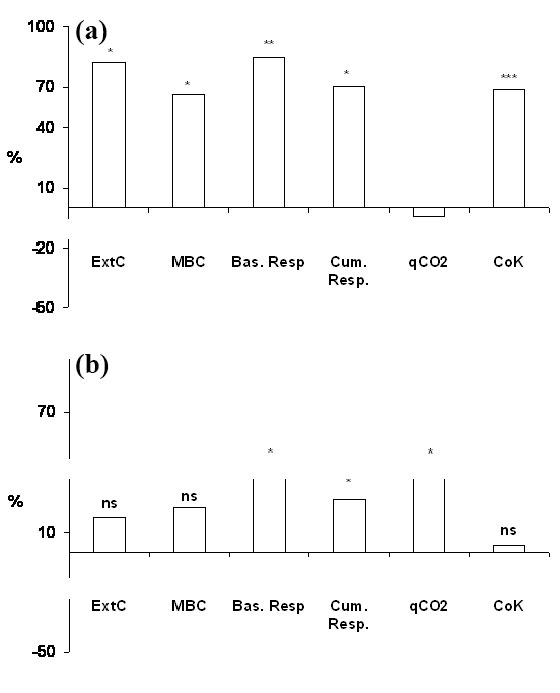
Soil physico-chemical properties of Atina and Barbarano sites are reported in Tab. 1. Atina shows a higher content of soil organic carbon and lower values of soil pH. However, no significant changes of soil physico-chemical properties were detected due to the presence of the wooden-work at both sites (Tab. 1). Soil extractable C, microbial biomass C content, basal and cumulative respiration, expressed as fractions of total organic carbon, metabolic quotient (qCO2) and the potential initial mineralization rate (C0K) are reported in Tab. 2. No significant differences were ascribable to the different sites; however the presence of the logs significantly affected most of these properties although, only in few cases, in interaction with the site (Tab. 2). The wood-effect on extractable C, microbial biomass, basal and cumulative respiration and microbial indexes (qCO2 and C0K) is presented in Fig. 2. Graphs show the percentage variation of each biochemical property in the soil beneath the logs (LS) with respect to the control soil (CS) at Atina (Fig. 2a) and Barbarano Romano (Fig. 2b). At Atina, with the exception of the metabolic quotient, all the parameters show a positive and significant increase due to the presence of the decomposing wood (+75% as an average value). At Barbarano a general positive trend of the wood-effect was also observed, though a significant increase was recorded only for the basal and cumulative respiration and the metabolic quotient (+44, +26 and +40%, respectively).
Tab. 1 - Physico-chemical properties of the soils at Barbarano Romano and Atina measured in control soils (CS) and beneath the logs (LS). Standard error of mean is reported (n=6). (*): p<0.05; (**): p<0.01; (***): p<0.001; (ns): not significant.
| Parameters | Atina | Barbarano Romano | ||
|---|---|---|---|---|
| CS | LS | CS | LS | |
| Texture | Loam, Sandy-loam | Sand, Loamy-sand | ||
| Moisture (%) | 16.28 ± 1.3 | 14.90 ± 0.9 | 17.61 ± 1.8 | 20.69 ± 1.9 |
| TOC (%) | 2.61 ± 0.4 | 2.31 ± 0.2 | 1.82 ± 0.2 | 1.60 ± 0.1 |
| TN (%) | 0.16 ± 0.02 | 0.18 ± 0.01 | 0.22 ± 0.03 | 0.17 ± 0.03 |
| C:N ratio | 12.73 ± 0.9 | 12.96 ± 0.5 | 8.69 ± 1.02 | 10.24 ± 0.89 |
| pHH20 | 7.31 ± 0.01 | 7.34 ± 0.1 | 7.64 ± 0.07 | 7.68 ± 0.06 |
| pHKCl | 7.07 ± 0.04 | 7.03 ± 0.04 | 6.88 ± 0.05 | 6.90 ± 0.06 |
| Analysis of variance | ||||
| Factor | Site | Logs presence | Site × Logs | |
| Moisture | * | ns | ns | |
| TOC | * | ns | ns | |
| TN | ns | ns | ns | |
| C:N ratio | ** | ns | ns | |
| pHH20 | *** | ns | ns | |
| pHKCl | ** | ns | ns | |
Tab. 2 - Biochemical properties of the soils at Atina and Barbarano Romano measured in control soils (CS) and beneath the logs (LS). ExtC, MBC, Basal and cumulative respiration are expressed as fractions of total organic carbon (TOC). Analysis of variance is calculated for the following factors and interactions: site and logs presence. Standard error of mean is reported (n=6).
| Parameters | Atina | Barbarano Romano | ||
|---|---|---|---|---|
| CS | LS | CS | LS | |
| ExtC (μg C μg TOC-1 g-1) 102 |
0.20 ± 0.05 | 0.39 ± 0.1 | 0.36 ± 0.04 | 0.42 ± 0.02 |
| MBC (μg Biomass C μg TOC-1 g-1) 102 |
0.77 ± 0.1 | 1.28 ± 0.2 | 0.85 ± 0.14 | 1.04 ± 0.07 |
| Bas. Resp. (μg C-CO2h-1 μg TOC-1 g-1) 104 |
0.48 ± 0.1 | 0.89 ± 0.1 | 0.64 ± 0.03 | 0.93 ± 0.08 |
| Cum. Resp. (μg C-CO2μgTOC-1 g-1) 102 |
3.44 ± 0.6 | 5.88 ± 0.6 | 4.15 ± 0.23 | 5.23 ± 0.20 |
| qCO2 (μg C-CO2μg Biom. C-1 g-1) 103 |
6.94 ± 0.8 | 6.66 ± 0.4 | 5.72 ± 0.54 | 8.04 ± 0.63 |
| C0K (μg C-CO2g-1 day-1) |
35.0 ± 3.0 | 59.0 ± 3.8 | 44.8 ± 6.1 | 46.2 ± 6.8 |
| Analysis of variance | ||||
| Factor | Site | Logs | Site × Logs | |
| ExtC | ns | * | * | |
| MBC | ns | * | ns | |
| Bas. Resp. | ns | *** | ns | |
| Cum. Resp. | ns | ** | ns | |
| qCO2 | ns | ns | * | |
| C0K | ns | * | * | |
Fig. 2 - Wood-effect calculated as mean percentage variation of extractable C (ExtC), microbial biomass C (MBC), basal respiration, cumulative respiration, metabolic quotient (qCO2) and initial potential mineralization rate (C0K) measured in the soil beneath the logs with respect to control soils at Atina (a) and Barbarano (b). (*): p<0.05; (**): p<0.01; (***): p<0.001; (ns): not significant.
Discussion
The effect of decaying wood on the physical, chemical and biochemical properties of the underlying soil was reported by several studies ([5], [17], [19], [31], [13], [36], [16]) however, to our knowledge, no specific investigations have been done on wooden-work deterioration effects on soil biological features. Thus, our results represent a new field of research aimed to assess the environmental impact of soil bioengineering works.
As regards of soils physico-chemical properties, no significant differences were found at our experimental sites on soil moisture, pH, C and N content in the soil beneath and away from the logs. A lack of effect on soil moisture can be related to the early decay stage of the logs; in fact, [14] reported that a high degree of decay, particularly with degradation of sapwood and heartwood, allows higher infiltration rates of water thus modifying soil humidity.
Kayahara et al. ([17]) and Hafner et al. ([13]) reported higher C:N ratios in the soil under decomposing wood, due to increases in total C coupled with decreases of total N and indicating that decaying wood can represent a source of recalcitrant organic matter. We did not observe significant variations of soil C and N content and of the C:N ratio. However, a positive significant effect on the extractable C (K2SO4-extractable C) at Atina provided evidence of an increased flux of soluble C forms deriving from the logs; this process is probably at its beginning and accumulation in stable C fractions cannot so far be highlighted. We did not register changes of soil pH suggesting that, at this decomposition stage, no leaching of acidic dissolved organic matter from decaying wood - as reported by Klinka et al. ([18]) - is occurring.
Conversely, a general positive wood-effect on soil biochemical properties was found in this study at both sites. Microbial biomass and its mineralization activity are widely considered “early warning” of changes occurring in soil and are thus used as reliable bioindicators of soil quality. Indeed, soil physico-chemical parameters alter only when the soil is subjected to really drastic changes ([9]), while biochemical parameters are more sensitive even to slight modifications of soil environment ([23], [34]).
Decaying wood can affect soil microbiota providing labile C and/or N sources. Several studies report increase of microbial biomass C:N ratio and denitrification activity ([13]), increase of microbial biomass C and of microbial quotient (qmic - [5]), increase of non symbiotic N-fixing bacteria ([15]). Conversely, [37] did not find any difference in soil microbial biomass size in soils under decomposing logs when compared to control soils. However, the substrate induced respiration (SIR) method used in that work does not provide a complete assessment of microbial biomass but only the amount of the glucose responsive microorganisms. Furthermore, decay of wood under aerobic conditions results mainly from the action of fungi, while bacteria are the primary degraders of wood under oxygen limiting conditions ([25]). In our study the microbial biomass increased in LS at both sites; however, this increase was significant only at Atina (+66%) where microbial biomass was positively correlated to ExtC (r=0.536, p<0.01), confirming that the enhanced flux of soluble C forms favoured microbial C immobilization. Furthermore, the ratio of microbial biomass to total organic carbon is an index of substrate availability to the soil microflora ([4]) and can thus predict a future C accumulation trend ([2]).
As for microbial metabolic activity only data on N mineralization were found in the literature: Busse ([5]) reported higher mineralizable N under decaying logs, while Kayahara et al. ([17]) found the opposite, namely higher mineralizable N away from logs. We measured microbial C mineralization activity: higher rates of basal and cumulative respiration were found beneath the logs at Atina and Barbarano promoting at both sites a significant wood-effect. Moreover, at Atina we could observe a significant increase of C0k, an indicator of the degree of availability, as well as differences between the mineralized organic compounds ([26]). This index has been proved to be effective for identifying the relationship between the decomposition kinetics of different types of residues and their chemical composition ([29]).
The metabolic quotient is an ecophysiological index informing on stress conditions of the microbial community: in fact, an increase of qCO2 suggests a disturbance affecting microbial maintenance energy ([1]). qCO2 values were significantly higher in LS only at the Barbarano site, indicating an alteration of microbial ecophysiological performances due to the recent establishment of the wooden works; as for the Atina site the metabolic quotient did not change proving that, after six years, soil microbes shifted the energy supply to biosynthesis processes as the increase of microbial biomass C showed.
Conclusions
The use of soil quality indicators was effective to assess the environmental impact of soil bioengineering works. In particular soil biochemical properties (microbial biomass, respiration and microbial indexes) were more responsive than soil physico-chemical characteristics in outlining effects due to wood decomposition. Increases of microbial mineralization activity can positively affect nutrient cycling and an improvement of soil fertility can promote adequate growth conditions for the plant cuttings used in the wooden-work. However the length of time since the double wooden pile-caisson were established determined the amplitude and thus the significance of the wood-effect recorded at both sites.
Acknowledgements
The authors are grateful to Ing. P. Cornelini for suggesting the case-studies at Atina and Barbarano Romano.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
A Lagomarsino
A Di Tizio
S Grego
Dipartimento di Agrobiologia e Agrochimica, Università della Tuscia, Viterbo (Italy)
S Cenfi
S Spina
Dipartimento di Tecnologie, Ingegneria e Scienze dell’Ambiente e delle Foreste, Università della Tuscia, Viterbo (Italy)
Corresponding author
Paper Info
Citation
Moscatelli MC, Romagnoli M, Cenfi S, Lagomarsino A, Di Tizio A, Spina S, Grego S (2009). Wood-soil interactions in soil bioengineering slope stabilization works. iForest 2: 187-191. - doi: 10.3832/ifor0513-002
Academic Editor
Roberto Tognetti
Paper history
Received: May 07, 2009
Accepted: Aug 18, 2009
First online: Oct 15, 2009
Publication Date: Oct 15, 2009
Publication Time: 1.93 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2009
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 63314
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 53368
Abstract Page Views: 4146
PDF Downloads: 4680
Citation/Reference Downloads: 24
XML Downloads: 1096
Web Metrics
Days since publication: 5923
Overall contacts: 63314
Avg. contacts per week: 74.83
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2009): 6
Average cites per year: 0.35
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Seasonal dynamics of soil respiration and nitrification in three subtropical plantations in southern China
vol. 9, pp. 813-821 (online: 29 May 2016)
Research Articles
Relationship between microbiological, physical, and chemical attributes of different soil types under Pinus taeda plantations in southern Brazil
vol. 17, pp. 29-35 (online: 28 February 2024)
Research Articles
Effects of altitudinal gradients on leaf area index, soil microbial biomass C and microbial activity in a temperate mixed forest ecosystem of Northwestern Turkey
vol. 10, pp. 334-340 (online: 15 December 2016)
Research Articles
Thinning effects on soil and microbial respiration in a coppice-originated Carpinus betulus L. stand in Turkey
vol. 9, pp. 783-790 (online: 29 May 2016)
Research Articles
Soil fauna communities and microbial activities response to litter and soil properties under degraded and restored forests of Hyrcania
vol. 14, pp. 490-498 (online: 11 November 2021)
Research Articles
The effect of clear-cut age on soil organic carbon and nitrogen indices in Scots pine (Pinus sylvestris L.) stands
vol. 18, pp. 146-153 (online: 09 June 2025)
Short Communications
Is microbial biomass measurement by the chloroform fumigation extraction method biased by experimental addition of N and P?
vol. 14, pp. 408-412 (online: 04 September 2021)
Research Articles
Effect of different dolomitic limestone dosages on soil respiration in a mid-altitudinal Norway spruce stand
vol. 12, pp. 357-365 (online: 05 July 2019)
Research Articles
Soil respiration along an altitudinal gradient in a subalpine secondary forest in China
vol. 8, pp. 526-532 (online: 01 December 2014)
Research Articles
Short-time effect of harvesting methods on soil respiration dynamics in a beech forest in southern Mediterranean Italy
vol. 10, pp. 645-651 (online: 20 June 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword