Soil fauna communities and microbial activities response to litter and soil properties under degraded and restored forests of Hyrcania
iForest - Biogeosciences and Forestry, Volume 14, Issue 6, Pages 490-498 (2021)
doi: https://doi.org/10.3832/ifor3583-014
Published: Nov 11, 2021 - Copyright © 2021 SISEF
Research Articles
Abstract
Reforestation has long been the best practice to restore degraded forests due to human interventions. In this paper we investigated the effect of forest degradation (DNF) along with reforestation using 4 endemic species (Alnus subcordata, ASP; Acer velutinum, AVP; Cupressus sempervirens, CSP; Quercus castaneifolia Mey, QCP) on forest’s soil chemical and biological indicators compared to a close-to-virgin natural forest (VNF). For this study, a total of 24 physico-chemical and 25 biological and microbial indicators were measured in soils of all 6 forest stands along with the litter properties. Results showed that the lowest soil quality was observed under DNF, CSP, and QCP which was the result of forest cover degradation in DNF and low litter quality, especially low pH and high C:N, in CSP and QCP. Soil fauna communities were significantly affected by tree species. We found two times higher density of earthworms in VNF compared to ASP, but in DNF the density was 5 times lower than VNF. We found no epigeic earthworms in QCP, CSP and DNF and no endogeic earthworms in DNF. Acarina and Collembola density was high in VNF and ASP, but they showed significant differences (VNF>ASP), and their density sharply decreased in other stands, especially in CSP (3 times lower than VNF) and DNF (8 to 10 times lower than VNF). Nematode density was statistically equal in VNF, ASP, and AVP, but significantly lower in other stands. Protozoa, bacteria and fungi densities were significantly higher in VNF and ASP (VNF>ASP) compared to each other and other forest stands. Basal respiration, substrate induced respiration, microbial biomass N and P, and carbon availability index was also higher in VNF and ASP compared to other stands. Although VNF has the best condition because of old forest cover and high diversity, ASP soil showed significant improvements, demonstrating the importance of litter quality in soil restoration. Restoration effectiveness ranking of the four tested species on soil improvement are therefore ASP>AVP>QCP>CSP. The significant improvement of soil quality under ASP compared to other reforestated stands, only after 3 decades, emphasizes the importance of tree species selection and litter quality on soil chemical and biological restoration.
Keywords
Forest Restoration, Reforestation, Litter Quality, Soil Biological Activity, Soil Chemical Properties, Soil Fauna
Introduction
Reforestation of clear-cut forests and agricultural lands is a well known practice to restore natural forest ecosystems around the world. As soil functional traits (especially carbon sequestration) are affected by forest degradation, the process of restoration can lead to significant increase in the ecological value of the temperate forests and in soil quality ([39]), and improves the microbial community (MC) of the forest soils ([44]). Although reforestation, due to low diversity in tree species, does not lead to the same soil and ecosystem characteristics as of natural forests ([25]), these stands are the most closed ecosystems to the natural ones that human are able to establish, and at some points, the ecosystem and soil characteristics could also be close to natural ones ([39]). Therefore, reforestation has impacts on several aspects, both aboveground and belowground.
At a global scale, reforestation is currently one of the strategies to tackle climate change, as tree plantations can be exploited to sequester atmospheric carbon ([37]). Recent studies found that large scale reforestations, after the bare soil C efflux to the atmosphere, can lead to significant increase in soil carbon pools. This means that the reforestation of clear-cut forests and abandoned agricultural lands are among the best options to increase belowground carbon sequestration ([22]).
At the local scale, forest restoration can lead to significant increase in biodiversity of soil fauna and flora, however, at a lower diversity compared to natural forests. Soil fauna play an important role in soil development and layer formation. Studies have shown that fauna activity have the potential to completely exploit soil organic layer (Oe) and develop a thick organomineral layer (A) in forest soil ([13]). Other studies showed that soil microbial and fauna activities, especially earthworms, can significantly modify chemical and microbial properties of the forest soils through the digestion of organic matter in their guts. Therefore, soil properties can be affected by a combination of tree species and soil fauna activities ([14]). On the other hand, reforestation has significant effects on soil MC structure and consequently, can lead to increased soil C sequestration and soil nitrogen content ([40]). MC of the forest soils is also influenced by the quantity and chemical properties of the input litter. Shao et al. ([40]) found that in the early stages of reforestation, MC have a significant effect on SOC accumulation, however, at the mature stages, these communities will be affected by vegetation diversity and litter quality. Therefore, there is a close mutual relationship between soil MC and tree species characteristics in natural and restored forests ([42]).
Studies also found several influential factors on soil chemical properties under natural restored forests. While soil degradation due to deforestation has significant negative effects on chemical soil properties ([39]), reforestation has a positive effect on soil quality according to the selected tree species ([47]). Tree species not only improve soil chemical quality through litter, but also through root exudates. Several studies have shown that reforestation using coniferous species can significantly increase SOC ([44]), while introducing native broadleaved species will result in higher quality and SOC chemical stability ([47]), with higher enzyme activity in the soil ([10]). Therefore, tree species selection for reforestation and forest restoration is a key influential factor in forest management.
As reforestation using different tree species may not lead to final ecosystem characteristics matching those of the natural ones, monitoring the effect of restoration practices on the ecosystem (especially soil) under different reforestation strategies is crucial. Here we examined soil chemical and biological response to different reforestation species after forest clear-cut. We aimed at determining which of four different tree species can lead to closer soil characteristics to that of a natural forest. The goals of this study are: (i) determining litter quality among different reforestation species; (ii) understanding the relationship between soil quality and biological and microbial soil characteristics in degraded and restored forests; (iii) determining whole species effect on soil chemical and biological characteristics of the restored forest soil; and (iv) disentangling the relationship between soil fauna community changes, soil carbon/nitrogen/phosphorus, and litter quality under different tree species.
Materials and methods
Site description
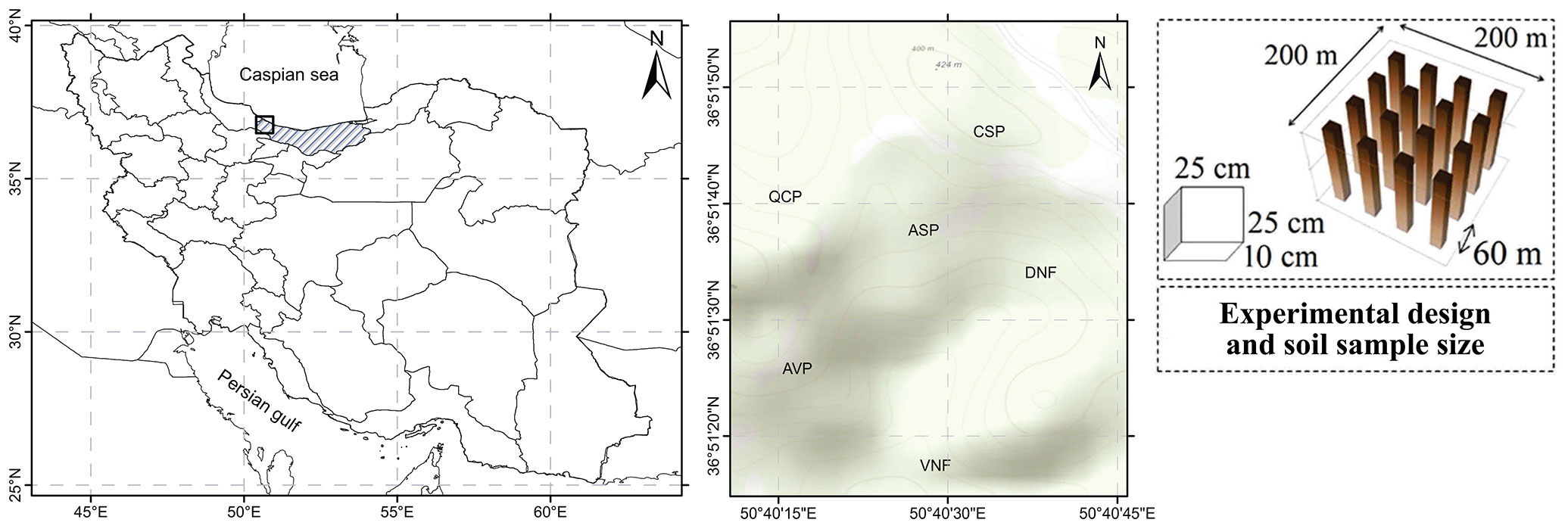
The study site has an area of 1394 ha and is located in the city of Ramsar (Mazandaran province, northern Iran - 36° 48′ 37″ and 36° 51′ 00″ N, 50° 43′ 10″ and 51° 35′ 00″ E). The site has an altitudinal range of 60-2130 m a.s.l. with an average slope of 40%. The climate is classified as seasonal temperate humid with an average annual rainfall of 773 mm and temperature of 12.3 °C. The monthly average temperature ranges from 5.2 in February to 19.5 °C in July, without frost periods. According to the USDA Soil Taxonomy, the forest soils are classified as Loamy-Clay-Sand Alfisols, developed on conglomerate composed of lime stones belonging to the Jurassic period ([41]).
Significant parts of these natural forests have been severely damaged during past decades. After clear cutting in 1988, the Forests and Rangelands Organization of Iran (FROI) performed reforestation practices to restore these habitats. Reforestation were done using Alder (Alnus subcordata C.A. Mey.) in an area of 16 ha, Maple (Acer velutinum Boiss.) 11 ha, Oak (Quercus castaneifolia C.A. Mey.) 4 ha, Mediterranean cedar (Cupressus sempervirens var. horizontalis) 8 ha. Degraded forests dominant species includes hornbeam (Carpinus betulus L.) and ironwood (Parrotia persica C.A. Mey.) species with an area of approximately 6 ha, and close-to-virgin natural forest (VNF) includes Oak (Quercus castaneifolia C.A. Mey.), hornbeam (Carpinus betulus L.) and ironwood (Parrotia persica C.A. Mey.), with an area of approximately 10 ha.
Sampling
Six forest types were selected for the purpose of this study, which includes pure reforestation of Alnus subcordata (ASP), Acer velutinum (AVP), Quercus castaneifolia (QCP), Cupressus sempervirens plantation (CSP), a close-to-virgin natural forest (VNF, oak-hornbeam-ironwood as control) and a degraded forest (DNF, hornbeam and ironwood). All the selected forest types were close to each other with similar soil texture and topographic conditions. A 4 ha area (200 × 200 m) was selected in each site for sampling. In order to reduce the boundary effects, the rows around the sampling area were not considered. Sampling was performed during summer 2019 using four parallel transects (200 m in length and 66 m apart from each other) and five soil and litter samples (25 × 25 cm) were randomly collected at a depth of 0-10 cm (Fig. 1). A total of 60 samples (i.e., 5 litter samples and 5 soil samples type) were transferred to the laboratory for litter and soil analysis ([24]).
Fig. 1 - Locations of the studies forest stands and sampling plots. A 4-ha square was randomly selected in each stand (right panel) and 5 out of 16 subplots were selected at random to measure litter and soil properties.
Laboratory analyses
Litter thickness was measured using a tape, and then transported in bags to the laboratory, washed gently for 30 seconds to remove mineral soil, and dried at 70 °C for 48 h, and then the dry weight measured. Dried litter samples were finely grounded and analyzed. Total C and N, along with nutrient contents in litter samples were determined using dry combustion with an elemental analyzer (Fisons EA1108, Milan, Italy), calibrated by the BBOT [2.5-bis-(5-tert-butyl-benzoxazol-2-yl) -thiophen] standard (ThermoQuest Italia S.p.A., Rodano, MI, Italy). Litter phosphorus (LP) concentration was determined spectrophotometrically. An atomic absorption spectrophotometer was used to determine total litter potassium (LK), calcium (LCa), and magnesium (LMg) concentration by flame emission.
A portion of soil samples was stored in polyethylene bags for biological analysis at 4 °C until processed. Another portion was air-dried and passed through 2-mm sieve (aggregates were broken to pass through a 2 mm sieve). Bulk density (BD) was measured by the clod method ([38]). Soil texture was determined using the Bouyoucos hydrometric method ([4]). Soil water content was measured by drying soil samples at 105 °C for 24 h. Soil pH and electric conductivity (EC) were measured using an Orion Ionalyzer Model 901 pH meter in a 1:2.5 (soil: water) solution ([43]).
Soil organic C was measured using the Walkey-Black method ([2]) and total N using a semi Micro-Kjeldahl method ([5]). Available P was measured by a spectrophotometer using the Olsen method ([18]), and available K, Ca, and Mg were determined using an atomic absorption spectrophotometer (by ammonium acetate extraction at pH 9). Particulate organic matter carbon (POM-C) and nitrogen (POM-N) were determined by physical fractionation ([17]). Fine roots (diameter < 2 mm) were collected from each soil sample and dried at 70 °C. The earthworms were collected simultaneously with the soil sampling by hand sorting, and identified using the Edwards et al. ([11]) key based on ecological categories (i.e., epigeic, anecic and endogeic) by external characteristics. Earthworm biomass was then determined after drying at 60 °C for 24 h and weighing. Soil Acarina and Collembola were extracted with the help of modified Tullgren funnel, as described by Hutson & Veitch ([19]). Nematodes were extracted from 100 g soil sample (fresh weight) by a modified cotton-wool filter method ([29]). Following the extraction method, soil protozoa population densities were counted under a microscope ([33]). Soil total bacteria and fungi were counted following extraction from soil (10 g fresh weight were mixed with 90 ml distilled water) using the homogenization and centrifugation techniques and culturing as described by Wollum ([50]). Soil basal respiration (BR) was determined by measuring the CO2 evolved in a 3-day incubation experiment at 25 °C ([1]). Substrate induced respiration (SIR), was determined using 1% glucose solution as substrate and the evolved CO2 was measured after 72 h incubation. The evolved CO2 was adsorbed in NaOH and measured by HCI titration ([3]). Carbon, nitrogen and phosphorous in the microbial biomass (MBC, MBN, and MBP, respectively) were measured by fumigation-extraction method ([6]). The soil metabolic quotient (qCO2 = BR/MBC - [3]) and carbon availability index (CAI = BR/SIR -[7]) were calculated based on the values of organic C, BR, substrate induced respiration (SIR) and MBC.
Statistical analysis
The normality of the variables was tested by the Kolmogorov-Smirnov test, and Levene’s test was used to examine the homogeneity of variances. Data are presented as mean ± standard error throughout the text, figures and tables. One-way analysis of variance (ANOVA) was used to compare litter and soil features data among the different land covers. Duncan test was further employed to test for mean differences with α = 0.05. Relationships between the measured parameters was also investigated using Pearson’s correlation. All the statistical analyses were done using the statistical software package SPSS® ver. 20 (IBM, Armonk, NT, USA). PCA is frequently used as an ordination and data reduction technique to distinguish treatments and to determine and characterize the most important parameters ([20]). Multivariate correlations and principal components were used to identify significant relationships among the variables using PC-Ord ver. 5.0 ([34]). To better interpret the data, only the first and second components were considered.
Results
Litter and soil properties
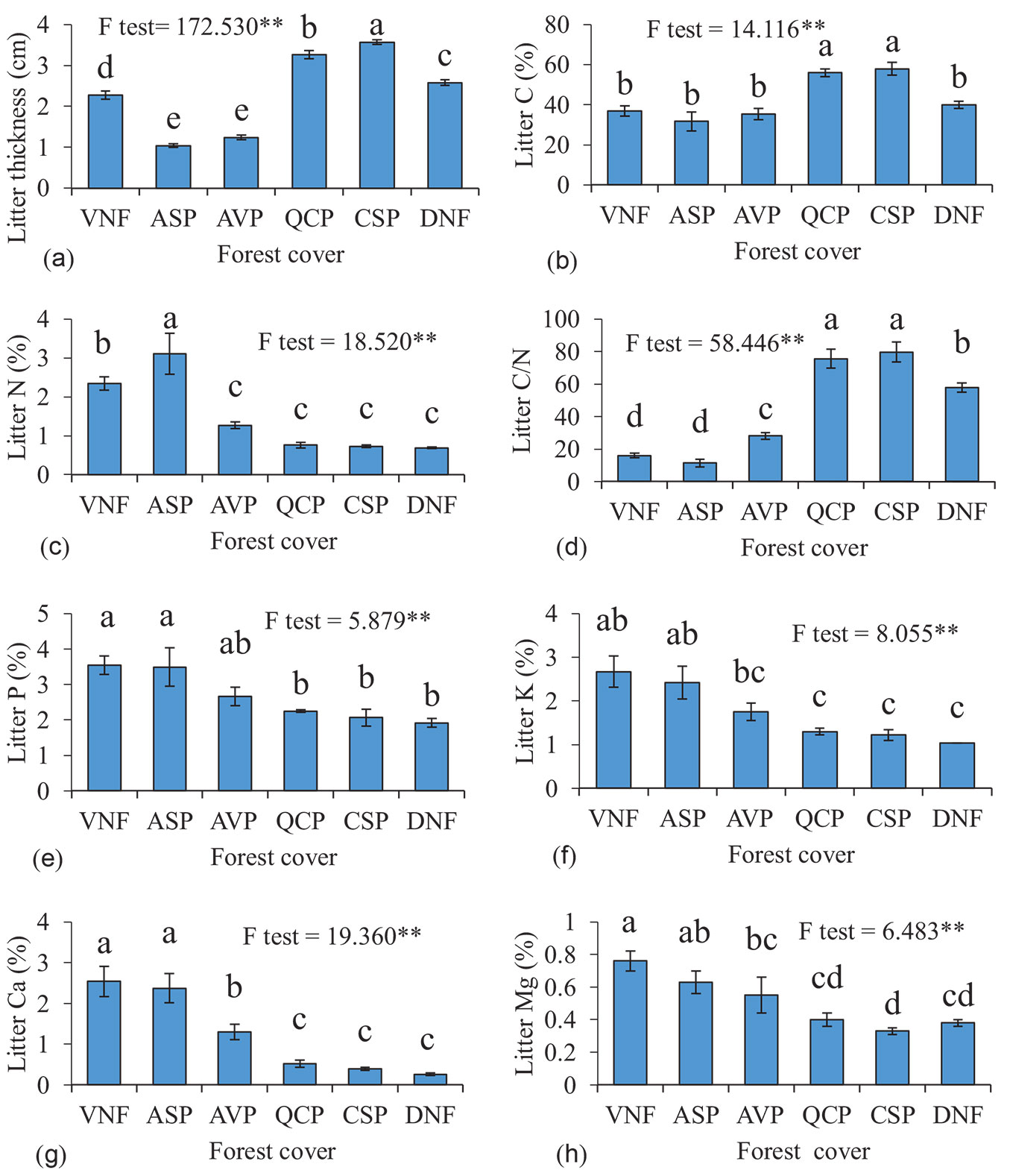
Results showed that there was a significant difference in the characteristics of the litter and soil among different forest covers (Fig. 2, Tab. 1). The mean thickness of litter layer was significantly higher (p < 0.05) in CSP stands (3.57 cm) than in other studied forest covers and was more than 3 times compared to that of ASP (1.04 cm). Litter C was significantly higher in CSP (57%) compared to other forest covers. QCP was the second forest cover with the highest litter C (56%) and the lowest litter C was observed in ASP (31.68%). On the contrary, establishment of ASP led to an increase in the concentration of litter N (3.11%), which was significantly higher than other studied forest covers. Litter C/N ratio in different forest covers was significantly different with the lowest value in ASP, which was the closest one to VNF (11.42 and 15.96, respectively). VNF had the highest concentration of Ca, P, K, and Mg, but no significant difference was observed between VNF and ASP (Fig. 2).
Fig. 2 - Mean values of the litter properties across different forest covers. Different letters indicate significant differences (p<0.05) between the means of forest covers after Duncan test. (VNF): close-to-virgin natural forest; (ASP): Alnus subcordata plantations; (AVP): Acer velutinum; (QCP): Quercus castaneifolia; (CSP): Cupressus sempervirens; (DNF): degraded natural forest. Error bars represent the standard error.
Tab. 1 - Mean (± standard error, SE) of the soil physical, chemical and biological properties analyzed under different forest covers. (VNF): virgin natural forest; (ASP): Alnus subcordata plantation; (AVP:): Acer velutinum; (QCP): Quercus castaneifolia; (CSP): Cupressus sempervirens; (DNF): degraded natural forest. Different letters indicate significant differences (p<0.05) between the means in different forest covers after Duncan test.
| Soil Properties | Forest cover | |||||||
|---|---|---|---|---|---|---|---|---|
| VNF | ASP | AVP | QCP | CSP | DNF | F-test | P-value | |
| Bulk density (g cm-3) | 1.43 ± 0.04b | 1.51 ± 0.07b | 1.46 ± 0.06b | 1.06 ± 0.01c | 1.03 ± 0.00c | 1.73 ± 0.01a | 37.732 | <0.001 |
| Sand (%) | 25.00 ± 2.36bc | 19.80 ± 2.57c | 34.20 ± 8.40ab | 27.40 ± 2.99abc | 29.40 ± 4.61abc | 40.20 ± 2.13a | 2.603 | 0.051 |
| Silt (%) | 39.40 ± 1.77ab | 46.40 ± 4.92a | 30.20 ± 3.77b | 43.60 ± 2.31a | 45.00 ± 4.72a | 36.80 ± 2.35ab | 2.969 | 0.032 |
| Clay (%) | 35.60 ± 1.24a | 33.80 ± 3.39a | 35.60 ± 5.67a | 29.00 ± 2.04ab | 25.60 ± 3.23ab | 23.00 ± 0.63b | 2.892 | 0.035 |
| Water content (%) | 48.48 ± 3.05a | 33.65 ± 3.39bc | 38.61 ± 4.92abc | 44.73 ± 3.91ab | 45.59 ± 3.79a | 30.86 ± 2.47c | 3.714 | 0.012 |
| pH (1:2.5 H2O) | 7.06 ± 0.11a | 7.12 ± 0.06a | 6.88 ± 0.16ab | 6.32 ± 0.09bc | 6.29 ± 0.52bc | 5.77 ± 0.09c | 5.243 | 0.002 |
| EC (ds m-1) | 0.30 ± 0.01a | 0.32 ± 0.03a | 0.24 ± 0.01a | 0.17 ± 0.01b | 0.15 ± 0.04b | 0.12 ± 0.00b | 9.962 | <0.001 |
| Organic C (%) | 4.29 ± 0.45bc | 3.44 ± 0.24c | 3.96 ± 0.43bc | 5.09 ± 0.65b | 6.62 ± 0.26a | 3.91 ± 0.35bc | 7.311 | <0.001 |
| Total N (%) | 0.46 ± 0.05a | 0.56 ± 0.11a | 0.27 ± 0.02b | 0.22 ± 0.03b | 0.17 ± 0.04b | 0.12 ± 0.01b | 9.071 | <0.001 |
| C/N ratio | 9.29 ± 0.13cd | 7.23 ± 1.46d | 14.60 ± 0.66cd | 23.20 ± 2.37bc | 46.48 ± 8.83a | 33.84 ± 7.01ab | 10.388 | <0.001 |
| Available P (mg kg-1) | 29.21 ± 0.86a | 27.15 ± 2.13a | 19.34 ± 3.11b | 13.51 ± 1.67c | 11.03 ± 2.06c | 10.49 ± 0.57c | 17.932 | <0.001 |
| Available K (mg kg-1) | 415.80 ± 16.08a | 405.20 ± 52.78a | 316.60 ± 21.05b | 184.20 ± 13.97c | 166.00 ± 19.86c | 155.60 ± 18.61c | 19.764 | <0.001 |
| Available Ca (mg kg-1) | 293.80 ± 28.35a | 277.20 ± 42.95ab | 214.60 ± 16.33b | 101.40 ± 16.50c | 97.20 ± 7.97c | 89.00 ± 6.72c | 16.342 | <0.001 |
| Available Mg (mg kg-1) | 79.00 ± 1.76a | 71.60 ± 5.83a | 65.60 ± 8.44ab | 50.80 ± 7.35bc | 38.60 ± 3.23c | 42.60 ± 3.35c | 8.780 | <0.001 |
| POM-C (g kg-1) | 3.61 ± 0.20ab | 2.13 ± 0.20c | 2.75 ± 0.51bc | 3.76 ± 0.45a | 4.44 ± 0.18a | 1.02 ± 0.17d | 14.987 | <0.001 |
| POM-N (g kg-1) | 0.50 ± 0.05b | 0.67 ± 0.04a | 0.27 ± 0.02c | 0.15 ± 0.01 | 0.12 ± 0.00d | 0.07 ± 0.01d | 62.340 | <0.001 |
Significant difference was also found between different forest covers in terms of soil physical and chemical properties. Bulk density in DNF (1.73 gr cm-3) was significantly higher compared to all other forest covers, and the lowest bulk density was observed in CSP (1.03 gr cm-3) and QCP (1.06 gr cm-3). Soil texture showed significant difference among some forest covers. ASP had the lowest sand content (19.8%) while DNF had the highest (40.20%). Silt content was only significantly different between ASP and AVP, while clay showed significantly lower content in DNF compared to VNF, ASP, and AVP (Tab. 1).
Soil water content in VNF and CSP (48.48% and 45.59%, respectively) was significantly higher than in DNF. Soil pH also showed significantly low value in DNF (5.77), while ASP and VNF showed the highest values. Soil organic carbon content (SOC) and C/N ratio were significantly different among the sites, with CSP having the highest value (6.62% and 46.48%). Soil total N, and available P, K and Mg were significantly higher in VNF and ASP compared to other forest covers, while the highest amount of available soil Ca (293.80 mg kg-1) was observed under VNF. Lowest soil total N, and available P, and K were observed in DNF.
POM-C and POM-N showed similar pattern to that of soil organic C and total N with the highest average POM-C of 4.44 ± 0.18 in CSP and POM-N of 0.67 ± 0.04 in ASP (Tab. 1).
Soil fauna and microbial biomass
The highest fine root biomass was observed in VNF (62.58 g m-2) while the lowest was measured in DNF (11.9 g m-2) soils. According to our data, degradation of natural forests resulted in a significant decrease in soil fauna, to the extent that reforestation could not restore this community close to that of VNF. VNF showed 7.18 mg m-2 biomass of epigeic earthworms, while no biomass was detected in QCP, CSP, and DNF soils; yet, the differences between the stands were non-significant. Anecic earthworm biomass and density were also lower in CSP and AVP compared to other stands and they were absent in DNF, while we detected 9.7 mg m-2 in VNF. Endogeic earthworm biomass was significantly higher in VNF (24.84 mg m-2) compared to AVP (8.63 mg m-2), QCP (4.49 mg m-2), CSP (4.65 mg m-2), and DNF (1.26 mg m-2). Earthworm density and biomass in VNF (3.20 n m-2, and 41.72 mg m-2, respectively) were significantly higher compared to other stands and more than two folds higher than ASP stand. Earthworm population was close to zero in each DNF and we found only one earthworm in 5 m-2 (at a depth of 10 cm) of forest soils in DNF.
Acarina and Collembola density showed three different levels. Both of these organisms showed significantly higher density in VNF compared to other stands with averages of 47.032 and 37.088 organisms per m2, respectively. Also, in ASP the density of both organisms were significantly higher than the remaining stands (39.560 for Acarina and 26.502 for Collembola per m2). Acarina density was similar in AVP, QCP, CSP, and DNF, while Collembola was the lowest in DNF, with 10 times lower density compared to VNF. Soil nematode community was recovered in ASP (250.6 in 100 g of soil) and AVP (220.4 in 100 g of soil) to a level that is not significantly different from VNF (279 in 100 g of soil), while protozoa density was significantly higher in VNF (646.2 ×102 g soil) and then ASP (468.8 ×102 g soil) compared to other stands. Protozoa community was 7 times higher in VNF compared to DNF (89.4 ×102 g soil).
Total bacteria and total fungi in ASP (4.11 and 2.45 ×107 g soil, respectively) were close to VNF (4.87 and 3.63 ×107 g soil, respectively); however, the differences were significant at p < 0.05. Both communities were more than 10 times higher in VNF compared to DNF (0.41 and 0.33 ×107 g soil, respectively). Bacterial community was statistically equal in AVP (1.95 ×107 g soil) compared to QCP (1.88 ×107 g soil), and CSP (0.67 ×107 g soil) compared to DNF (0.41 ×107 g soil), while fungal community was similar in AVP (0.76 ×107 g soil), QCP (0.72 ×107 g soil), CSP (0.57 ×107 g soil), and DNF (0.33 ×107 g soil - Tab. 2).
Tab. 2 - Mean (± SE) of the soil biological properties analyzed under different forest covers. (VNF): virgin natural forest; (ASP): Alnus subcordata plantation; (AVP:): Acer velutinum; (QCP): Quercus castaneifolia; (CSP): Cupressus sempervirens; (DNF): degraded natural forest. Different letters indicate significant differences (p<0.05) between the means in different forest covers after Duncan test.
| Soil properties | Forest cover | |||||||
|---|---|---|---|---|---|---|---|---|
| VNF | ASP | AVP | QCP | CSP | DNF | F-test | P-value | |
| Fine root biomass (g m-2) | 62.58 ± 5.32a | 42.72 ± 6.07b | 37.57 ± 2.13b | 33.59 ± 5.66b | 29.57 ± 3.42b | 11.09 ± 0.73c | 14.936 | <0.001 |
| Epigeic density (n m-2) | 0.40 ± 0.24a | 0.20 ± 0.20a | 0.20 ± 0.20a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 1.143 | 0.365 |
| Epigeic biomass (mg m-2) | 7.18 ± 4.43a | 2.31 ± 2.31a | 1.93 ± 1.93a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 1.626 | 0.191 |
| Anecic density (n m-2) | 0.80 ± 0.37a | 0.20 ± 0.20a | 0.20 ± 0.20a | 0.40 ± 0.24a | 0.20 ± 0.20a | 0.00 ± 0.00a | 1.425 | 0.251 |
| Anecic biomass (mg m-2) | 9.69 ± 4.86a | 2.65 ± 2.65a | 2.65 ± 2.65a | 7.95 ± 4.87a | 2.33 ± 2.33a | 0.00 ± 0.00a | 1.256 | 0.315 |
| Endogeic density (n m-2) | 2.00 ± 0.31a | 1.00 ± 0.31ab | 0.80 ± 0.37b | 0.60 ± 0.60b | 0.40 ± 0.40b | 0.20 ± 0.20b | 2.711 | 0.044 |
| Endogeic biomass (mg m-2) | 24.84 ± 4.13a | 13.18 ± 4.92ab | 8.63 ± 3.85b | 4.49 ± 4.49b | 4.65 ± 4.65b | 1.26 ± 1.26b | 4.410 | 0.005 |
| Earthworm density (n m-2) | 3.20 ± 0.73a | 1.40 ± 0.40b | 1.20 ± 0.48b | 1.00 ± 0.57b | 0.60 ± 0.60b | 0.20 ± 0.20b | 3.961 | 0.009 |
| Earthworm biomass (mg m-2) |
41.72 ± 9.73a | 18.15 ± 4.79b | 13.22 ± 4.73b | 12.44 ± 5.10b | 6.99 ± 6.99b | 1.26 ± 1.26b | 5.450 | 0.002 |
| Acarina density (n×103 m-2) | 47.03 ± 5.53a | 39.56 ± 0.93b | 13.00 ± 2.10c | 11.46 ± 0.44c | 10.77 ± 0:20c | 5.82 ± 1.35c | 47.780 | <0.001 |
| Collembola density (n×103 m-2) |
37.09 ± 4.03a | 26.50 ± 3.76b | 16.72 ± 1.77c | 13.27 ± 1.30c | 12.19 ± 0.27c | 3.51 ± 0.68d | 23.674 | <0.001 |
| Total nematodes (in 100 g soil) |
279.00 ± 28.82a | 250.60 ± 42.83a | 220.40 ± 31.87a | 102.60 ± 15.69b | 84.00 ± 7.14b | 35.40 ± 9.46b | 14.920 | <0.001 |
| Protozoa density (×102 g soil) |
646.20 ± 123.42a | 468.80 ± 67.10b | 249.80 ± 38.66c | 163.40 ± 15.59c | 110.80 ± 4.65c | 89.40 ± 1.43c | 13.887 | <0.001 |
| Total bacteria (×107 g soil) | 4.87 ± 0.23a | 4.11 ± 0.12b | 1.95 ± 0.19c | 1.88 ± 0.28c | 0.67 ± 0.11d | 0.41 ± 0.09d | 92.089 | <0.001 |
| Total fungi (×107 g soil) | 3.63 ± 0.23a | 2.45 ± 0.22b | 0.76 ± 0.07c | 0.72 ± 0.07c | 0.57 ± 0.07c | 0.33 ± 0.02c | 86.421 | <0.001 |
Soil biological activity
Soil basal and substrate induced respiration (BR and SIR) in VNF and ASP were not statistically different, with averages of 0.76 and 1.64 mg CO2 g-1 day-1 for VNF and 0.73 and 1.49 mg CO2 g-1 day-1 for ASP, respectively, but they were significantly higher than those of other forest covers. Soil carbon microbial biomass showed the highest value (600.61 mg kg-1) in CSP, which was significantly higher than those of ASP, AVP and DNF, but statistically equals to VNF and QCP. Nitrogen microbial biomass was significantly higher in ASP (48.07 mg kg-1) and VNF (41.48 mg kg-1), compared to other forest covers, while phosphorus microbial biomass was the highest in VNF (81.40 mg kg-1). Further, microbial biomass indicators were significantly the lowest in DNF.
The ratios MBC/MBN and MBC/MBP were significantly higher in CSP (33.95 and 22.31) compared to other forest covers, while MBN/MBP was similar for all forest covers (p > 0.05). Metabolic quotient ratio in ASP (1.88 μg CO2 - C mg-1 MBC day-1) was significantly higher than in other sites, and five times higher than that of CSP (0.37 μg CO2 - C mg-1 MBC day-1), but not significantly different from DNF. Finally, ASP and VNF showed the highest carbon availability index (CAI), which was significantly higher compared to other forest stands (0.49 and 0.48, respectively - Tab. 3).
Tab. 3 - Mean (± SE) of the soil microbial properties analyzed under different forest covers. (VNF): virgin natural forest; (ASP): Alnus subcordata plantation; (AVP:): Acer velutinum; (QCP): Quercus castaneifolia; (CSP): Cupressus sempervirens; (DNF): degraded natural forest. Different letters indicate significant differences (p<0.05) between the means in different forest covers after Duncan test.
| Soil properties | Forest cover | |||||||
|---|---|---|---|---|---|---|---|---|
| VNF | ASP | AVP | QCP | CSP | DNF | F test | P-value | |
| Basal respiration (mg co2 g-1 day-1) |
0.76 ± 0.03a | 0.73 ± 0.05a | 0.35 ± 0.02b | 0.26 ± 0.02bc | 0.22 ± 0.01c | 0.12 ± 0.01d | 74.678 | <0.001 |
| Substrate induced respiration (mg CO2 g-1 day-1) | 1.64 ± 0.14a | 1.49 ± 0.10a | 1.10 ± 0.04b | 1.08 ± 0.01b | 1.00 ± 0.05b | 0.84 ± 0.07b | 13.056 | <0.001 |
| Microbial biomass carbon (mg kg-1) | 504.02 ± 72.62ab | 415.93 ± 57.29b | 417.39 ± 54.61b | 519.05 ± 69.75ab | 600.61 ± 14.63a | 111.78 ± 28.7c | 9.913 | <0.001 |
| Microbial biomass nitrogen (mg kg-1) | 41.48 ± 5.66a | 48.07 ± 3.77a | 26.03 ± 2.48b | 21.37 ± 0.66b | 18.22 ± 1.30b | 6.21 ± 1.02c | 25.725 | <0.001 |
| Microbial biomass phosphorous (mg kg-1) | 81.40 ± 0.97a | 60.20 ± 7.78b | 37.00 ± 3.84c | 30.00 ± 3.17c | 27.20 ± 1.35c | 11.20 ± 0.86d | 43.373 | <0.001 |
| MBC/MBN | 12.60 ± 1.81cd | 8.88 ± 1.37d | 16.33 ± 2.22bcd | 24.20 ± 3.20b | 33.95 ± 3.54a | 18.56 ± 4.55bc | 8.969 | <0.001 |
| MBC/MBP | 6.17 ± 0.87c | 7.38 ± 1.21c | 12.25 ± 2.51bc | 17.43 ± 2.23ab | 22.31 ± 1.26a | 10.24 ± 2.81c | 9.978 | <0.001 |
| MBN/MBP | 0.51 ± 0.06a | 0.84 ± 0.11a | 0.77 ± 0.17a | 0.75 ± 0.08a | 0.67 ± 0.05a | 0.54 ± 0.06a | 1.639 | 0.188 |
| Metabolic quotient (μg CO2-C mg-1 MBC day-1) |
1.73 ± 0.41ab | 1.88 ± 0.27a | 0.92 ± 0.16abc | 0.56 ± 0.09bc | 0.37 ± 0.03c | 1.72 ± 0.73ab | 3.152 | 0.025 |
| Carbon availability index | 0.48 ± 0.05a | 0.49 ± 0.02a | 0.32 ± 0.02b | 0.24 ± 0.02b | 0.23 ± 0.01bc | 0.15 ± 0.01c | 20.924 | <0.001 |
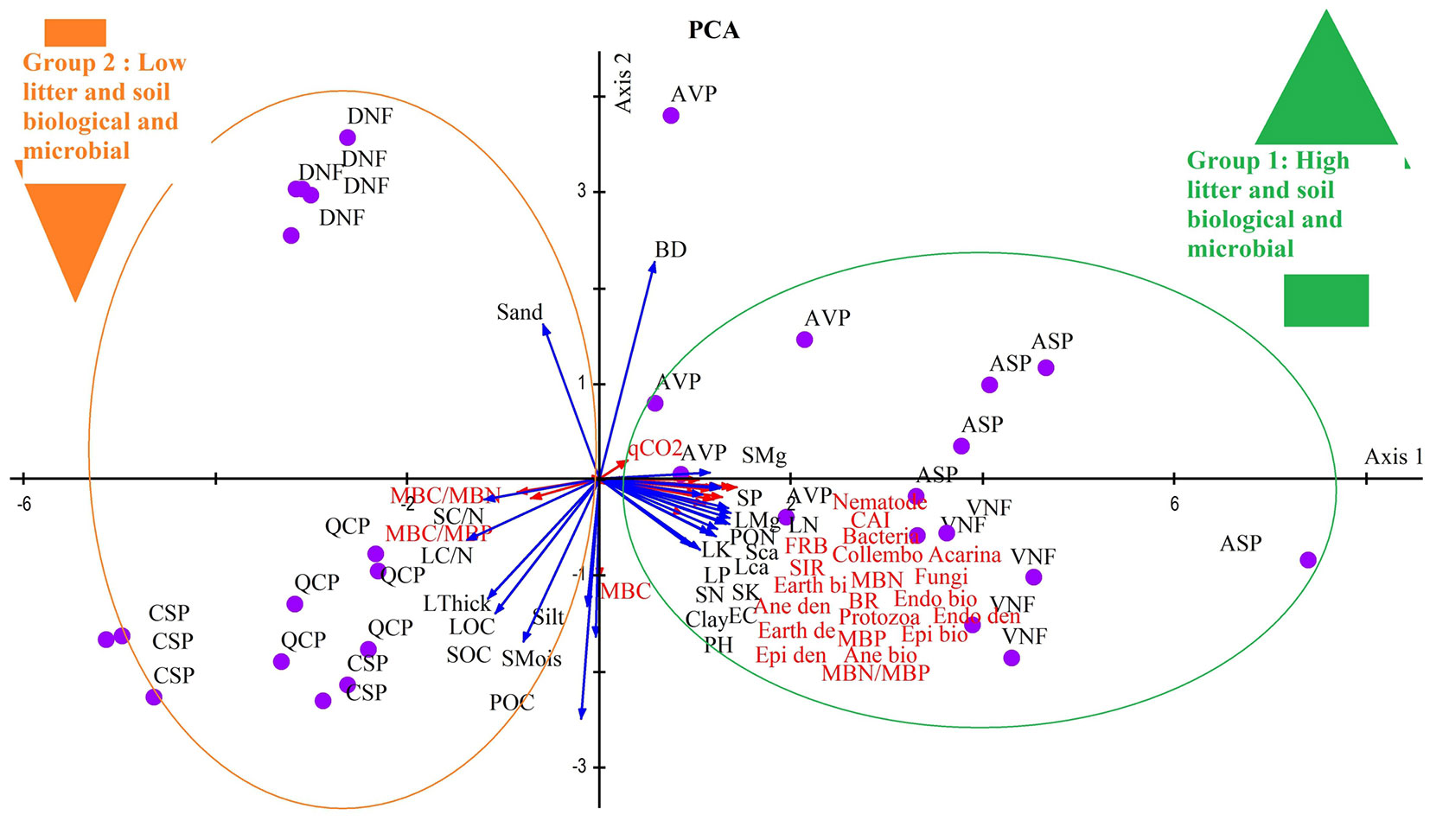
The studied forest sites and their litter and soil characteristics were then analyzed using PCA. The first and second axes accounted for 52.43% and 14.55% of the total variance, respectively. The first PC axis discriminates fairly well two groups of forest covers (sites). The first group reflects good litter quality, accumulation of nutrients, and more biological and microbial activity, and is mainly formed by VNF and ASP sites. While the second group along the PC1 axis shows low quality of litter and soil, low nutrients and low biological activity at DNF, CSP, and QCP sites (Fig. 3).
Fig. 3 - PCA analysis of the measured variables in different forest covers. The first PCA axis accounted for 52.43% of the total variance, while the second axis explained 14.55%.
Relationship between soil properties
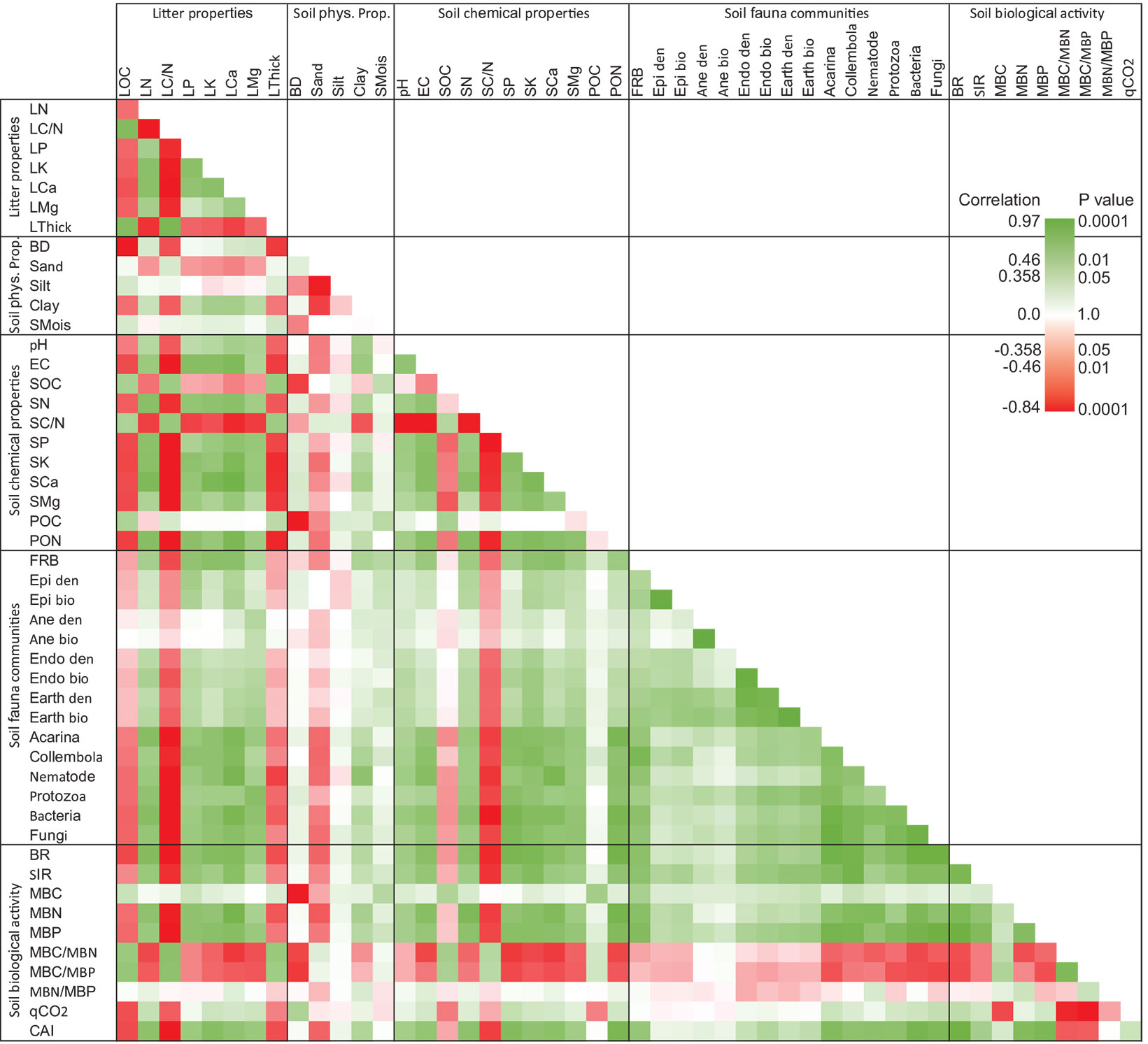
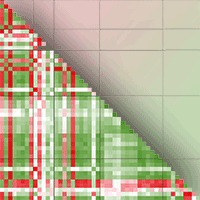
Correlation analysis of the studied soil and litter properties showed that most of the measured variables have positive correlation, however some key variables (e.g., litter C/N ratio and thickness and soil C/N ratio) have significant negative correlation with most of the measured variables. Litter calcium content is likely a key parameter significantly affecting soil chemical properties and fauna communities in a positive way, while soil potassium and phosphorus have significant correlation with soil microfauna communities. Potassium and phosphorus also have significant correlation with soil biological activity. Acarina, Collembola, Nematodes, Protozoa, Bacteria, and Fungi have significant correlation with soil biological properties, especially soil respiration and CAI. It seems that MBC only has significant correlation with soil carbon content (SOC, POM-C, FRB); however, soil bulk density has significant negative relationship with this parameter. Soil metabolic quotient is strongly correlated with the ratios of MBC/MBN and MBC/MBP (Fig. 4).
Fig. 4 - Correlation heatmap of studied soil properties (N=30). Variable labels are reported in the List of Abbreviations (see below).
Discussion
Litter and soil physicochemical properties
Litter production and decomposition are two important processes for the formation of soil organic matter and the nutrient cycle. Also, the ratio between production and decomposition determines the thickness of the substrate layer on the forest floor ([27]). In this study, litter in CSP and QCP stands have a low degradation rate, which was the result of poor litter quality of CSP and high lignin content of QCP litter ([51]), which creates a thicker organic layer under these species compared to ASP, a nitrogen-fixing species with high litter nitrogen content ([30]). However, a faster decomposition under VNF and AVP forests in this study is probably due to a more attractive source for decomposers due to the lower C/N ratio, which resulted in thinner litter layer ([46]).
Vivanco & Austin ([45]) found that litter decomposition rate is significantly controlled by tree species rather than climate, and not only macronutrients can contribute to litter decomposition, but also micronutrients have significant effect on the process. Along with litter quality, soil texture has also a significant role in litter decomposition process by providing moisture and greater microbial biomass due to higher clay content ([36]). This effect can be seen in VNF, AVP, and ASP as the clay content of the forest floor was higher than other stands. Soil pH and EC are also dependent on the vegetation cover of the soil and the decomposition process. Haghdoost et al. ([16]) found that soil EC depends on the type and properties of vegetation litter. On the other hand, lower pH of CSP can be due to the acidification of coniferous litter ([44]). Our study showed similar results to those of abovementioned studies in case of pH, however, low pH in QCP and DNF can be due to C/N ratio of the leaf litters. Kooijman et al. ([26]) found that low litter quality in beech stands resulted in high pH compared to hornbeam stands. Therefore, we can assume that under highly decomposable leaf litter the pH value is significantly higher, which explains why the litter thickness is higher in the stands with low soil pH in our study ([35]).
We observed that VNF and ASP have significantly higher amounts of available P, K, Ca, and Mg. This might be the result of higher soil moisture and higher biological activity of the soil microorganisms derived by higher pH, which have led to faster decomposition of the litter layer, while keeping pH at suitable levels ([31]).
Nitrogen availability in VNF and ASP can be attributed to the presence of nitrogen fixing tree species like A. subcordata. This species can increase soil nitrogen availability by increasing the activity of nitrogen-fixing microorganisms ([47]). On the other hand, higher C/N ratios, lower pH, and lower nutrient content in coniferous needles compared to broadleaves have previously been reported ([44]). POM-C and POM-N provide a sensitive indicator that reflects the impact of short-term changes in soil surface on soil quality. High levels of litter under CSP and QCP lead to significantly higher amounts of POM-C which can be the result of lower decomposition rate in this stands ([44]). On the other hand, higher amounts of nitrogen in ASP and VNF litter led to higher POM-N which is one of the characteristic of these forests ([23]). Studies have shown that soil properties are affected by the interaction effect of substrate and tree species. In a study on 4 different tree species in Poland, Józefowska et al. ([22]) found that soil C:N ratio depends on tree species as well as soil fauna communities. However, as our stands were close to each other, we found that soil properties are affected directly by tree species.
Soil fauna community
Fine root, earthworm, and other soil organism biomass were significantly higher in VNF and ASP compared to other forest covers. Studies on soil respiration showed that basal respiration (BR) significantly depends on soil mineralization process, which mostly results from chemical and biological reactions of the soil and also root respiration of the forest covers ([48]). Our results showed that both BR and SIR significantly increase from DNF< CSP<QCP<AVP<ASP<VNF, which indicates that low forest cover and also conifer forests have lower soil respiration. These results are in line with the results of Jílková ([21]) who showed that a shift in forest cover from conifers to deciduous forests can significantly increase soil respiration. On the other hand, litter properties have significant effects on soil respiration by providing essential nutrients which are needed by soil microorganisms. In other words, in forests with higher litter quality and litter nutrient content, the rate of soil BR and SIR is significantly higher, as recorded in VNF and ASP in this study ([21]). A sharp decline in soil respiration in both SCP and DNF is the result of low biological activity in both forest covers due to lower soil pH and lower water content ([44], [48]).
Forest degradation and the following decline of the canopy created an unfavorable climate for the activity of soil organisms under the degraded forest. The substrate layer is also essential for soil organisms because it acts as both habitat and food source ([42]), which is scarce under DNF cover.
Increase in vegetation diversity, aboveground biomass, and forest structure tends to increase the abundance of soil organisms ([10]), which is the case of VNF in our study. Indeed, higher litter quality and higher soil organism biomass and activity in VNF compared to other forest cover were observed, which could explain the higher SIR. Soils under natural forest with lower bulk density, higher water and nutrients content, and low C:N ratio, with appropriate soil pH has significantly increased the community of soil organisms ([25]). On the other hand, reforestation of the degraded forests with broadleaved species also increased soil organisms ([9]), which is the case of ASP and AVP in this study.
Earthworms, small arthropods (Acarina and Collembola), nematodes, protozoa, bacteria, and fungi in ASP and AVP were all significantly higher than in DNF and CSP. This can imply a higher nutrient availability of the forest soil and thinner litter layer due to higher biological activity in the soil ([12]). Li et al. ([28]) found that a larger community of soil microorganisms can lead to faster decomposition of litter and thus higher availability of nutrients to vegetation roots. Additionally, in forests with high litter quality and low litter C:N ratio, faunal activity (especially earthworms) is higher, which leads to higher bioturbation and thinner litter layer ([13]).
Among the earthworm groups, only endogeic showed a significant increase in the following order: DNF<CSP<QCP<AVP <ASP<VNF, which may be due to the movement of endogeic groups to different layers of soil where they are able to establish in better conditions ([25]). Acarina and Collembola are microphages and indirectly affect the process of the nutrient cycle by controlling the bacteria and fungi in the soil community ([49]). Small arthropods and protozoa are associated with soil pH in forest ecosystems. The amount of nematodes often shows the highest value in soils with pH 7.0 or higher compared to soils with pH between 5.9 and 6.5 ([32]), which can explain our results.
Soil biological activity
MBN is an indicator of soil quality and is used as a criterion for the availability of nitrogen for plants. MBN in VNF and ASP were significantly higher than that of other forest covers, which also confirms that the tree species has affected the soil microbial community ([10]). Although non-significant, MBN was roughly 20% higher in ASP compared to VNF, which indicates the key role of nitrogen-fixing tree species in providing nitrogen for microbial communities, while VNF could provide significantly more P to microbial communities (and higher MBP). This is contrary to what observed for MBC. CSP showed significantly higher MBC compared to ASP, which is an indicator of difference in litter input quality between the two reforested stands ([23]). The microbial indicators MBC/MBN and MBC/MBP differed significantly across different forest covers. According to Kooch et al. ([23]), such significant changes can be attributed to the input of plant residues with different characteristics of understory (i.e., to the annual vegetation cover on the ground) in the studied sites.
Changes in metabolic rate can indicate that different microbial community structures have occurred according to different tree species ([47]). It also can be affected by soil moisture, soil pH, organic carbon, total nitrogen content and nutritional status of the different forest covers in the study area ([8]). In this regard, ASP with higher soil pH and nitrogen content has the highest metabolic rate. To better understand the state of microbial communities, we also calculated CAI ([15]). This parameter which is the ratio between BR and SIR is significantly lower in DNF compared to other forest stands. Such low value for CAI is the result of significantly lower BR in DNF stand. While SIR in VNF is almost two times higher than that of DNF, BR is 6 times higher. This means that significantly low biological activity in DNF soils resulted in low BR ([44]).
Our results showed significant positive correlation between fauna populations, and soil CAI and respiration. This correlation can demonstrate the key role of soil fauna in litter decomposition, as they help distribute organic materials through different soil layers and help leaching and release of POM ([14]).
Conclusion
Our study showed that old grown natural forests are key forests to maintain maximum soil quality and microbial activity, however, some key species like A. subcordata can significantly revive soil quality after a short period of time. In our study, most biological and microbial indicators were significantly higher in close-to-virgin natural forest and A. subcordata plantations (e.g., ecological groups of earthworms, Acarina, Collembola, nematodes, protozoa, bacteria and fungi), along with soil nutrients (N, P, K, Ca, and Mg), which means not only vegetative intactness can contribute to higher soil quality, but also litterfall have a key role in soil activity and fertility. While the activities of soil organisms and soil fertility were at the lowest levels in degraded forests, C. sempervirens plantations, and Q. castaneifolia plantations, they have been significantly increased in A. subcordata plantations only after 3 decades. In addition, soil microbial activities significantly depend on litter N content and C:N ratio along with soil pH, which itself is also a dependent variable to litter quality. A. subcordata, an endemic nitrogen-fixing tree species to these forests, has a good growth rate, and plays an important role in the nutrient cycle and soil-related processes in temperate ecosystems. Therefore, it can be recommended for the restoration of degraded natural forests. Our study clearly shows that the main effect of forest degradation can be directly reflected to soil quality, soil fauna community, and biological activity. On the other hand, species selection for reforestation can significantly contribute to soil restoration, to an extent that planting C. sempervirens, and Q. castaneifolia does not make any difference from a degraded forest.
List of abbreviations
Acarina: Acarina density; Ane bio: Anecic biomass; Ane den: Anecic density; ASP: Alnus subcordata; AVP: Acer velutinum; Bacteria: Total bacteria; BD: Bulk density; BR: Basal respiration; CAI: Carbon availability index; Collembola: Collembola density; CSP: Cupressus sempervirens; DNF: Degraded natural forest; Earth bio: Earthworm biomass; Earth den: Earthworm density; Endo bio: Endogeic biomass; Endo den: Endogeic density; Epi bio: Epigeic biomass; Epi den: Epigeic density; FRB: Fine root biomass; Fungi: Total fungi; LC/N: Litter C/N ratio; LCa: Available litter calcium; LK: Available litter potassium; LMg: Available litter magnesium; LN: Litter nitrogen; LOC: Litter carbon; LP: Available litter phosphorus; LThick: Litter thickness; MBC: Microbial biomass of carbon; MBN: Microbial biomass of nitrogen; MBP: Microbial biomass of phosphorus; Nematode: Total nematode; POM-C: Particulate organic matter carbon; POM-N: Particulate organic matter nitrogen; Protozoa: Protozoa density; qCO2: Metabolic quotient; QCP: Quercus castaneifolia; SCa: Available soil calcium; SC/N: Soil C/N ratio; SIR: Substrate induced respiration; SK: Available soil potassium; SMg: Available soil magnesium; SMois: Soil moisture; SN: Soil total nitrogen; SOC: Soil organic carbon; SP: Available soil phosphorus; VNF: Close-to-virgin natural forest.
Acknowledgments
M.B. and A.S. carried out the field measurements; Y.K. performed the statistical analysis; V.E. and M.B. performed the laboratory analysis; M.B. conceived the study and helped to draft the manuscript. The authors wish to thank Mr. Jahansouz Foroughi for his helps on field sampling and Mr. Ehsan Khedive for his assistance on writing the manuscript. This work was supported by financial supports of the University of Tehran, Iran.
References
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Department of Forestry and Forest Economy, Faculty of Natural Resources, University of Tehran, Tehran (Iran)
Anoushirvan Shirvany 0000-0001-9953-3779
Faculty of Natural Resources, University of Tehran, Tehran (Iran)
Faculty of Natural Resources & Marine Sciences, Tarbiat Modares University, 46417-76489, Noor, Mazandaran (Iran)
Corresponding author
Paper Info
Citation
Bazyari M, Etemad V, Kooch Y, Shirvany A (2021). Soil fauna communities and microbial activities response to litter and soil properties under degraded and restored forests of Hyrcania. iForest 14: 490-498. - doi: 10.3832/ifor3583-014
Academic Editor
Maurizio Ventura
Paper history
Received: Jul 12, 2020
Accepted: Sep 06, 2021
First online: Nov 11, 2021
Publication Date: Dec 31, 2021
Publication Time: 2.20 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2021
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 34540
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 28664
Abstract Page Views: 2804
PDF Downloads: 2325
Citation/Reference Downloads: 9
XML Downloads: 738
Web Metrics
Days since publication: 1553
Overall contacts: 34540
Avg. contacts per week: 155.69
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2021): 3
Average cites per year: 0.60
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Impact of deforestation on the soil physical and chemical attributes, and humic fraction of organic matter in dry environments in Brazil
vol. 15, pp. 465-475 (online: 18 November 2022)
Research Articles
The manipulation of aboveground litter input affects soil CO2 efflux in a subtropical liquidambar forest in China
vol. 12, pp. 181-186 (online: 10 April 2019)
Research Articles
Wood-soil interactions in soil bioengineering slope stabilization works
vol. 2, pp. 187-191 (online: 15 October 2009)
Research Articles
Soil respiration along an altitudinal gradient in a subalpine secondary forest in China
vol. 8, pp. 526-532 (online: 01 December 2014)
Research Articles
Influences of forest gaps on soil physico-chemical and biological properties in an oriental beech (Fagus orientalis L.) stand of Hyrcanian forest, north of Iran
vol. 13, pp. 124-129 (online: 07 April 2020)
Research Articles
Effect of plant species on P cycle-related microorganisms associated with litter decomposition and P soil availability: implications for agroforestry management
vol. 9, pp. 294-302 (online: 05 October 2015)
Research Articles
Relationship between microbiological, physical, and chemical attributes of different soil types under Pinus taeda plantations in southern Brazil
vol. 17, pp. 29-35 (online: 28 February 2024)
Research Articles
Spatial heterogeneity of soil respiration in a seasonal rainforest with complex terrain
vol. 6, pp. 65-72 (online: 07 February 2013)
Research Articles
Changes in the properties of grassland soils as a result of afforestation
vol. 11, pp. 600-608 (online: 25 September 2018)
Research Articles
Soil stoichiometry modulates effects of shrub encroachment on soil carbon concentration and stock in a subalpine grassland
vol. 13, pp. 65-72 (online: 07 February 2020)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword