Short-time effect of harvesting methods on soil respiration dynamics in a beech forest in southern Mediterranean Italy
iForest - Biogeosciences and Forestry, Volume 10, Issue 3, Pages 645-651 (2017)
doi: https://doi.org/10.3832/ifor2032-010
Published: Jun 20, 2017 - Copyright © 2017 SISEF
Research Articles
Abstract
CO2 fluxes from soil, together with soil water content and temperature have been measured over one solar year in an even-aged beech forest (Fagus Sylvatica L.) in southern Italy. We investigated the effects of three different harvested biomass removal treatments (traditional, innovative, unharvested control) on soil respiration (Rs) in three plots from May 2014 to April 2015, with the aim to evaluate the effects of such silvicultural practices on the CO2 respired from the forest floor. The influence of soil temperature and soil moisture on soil respiration was also analysed. Rs showed large variations among the treatments, with the innovative treatment resulting in significantly higher soil respiration than control and traditional treatments. There were no significant differences in soil temperature between the treatments, whereas soil water content was statistically different only in the innovative treatment. The study showed that the mean soil respiration increased with thinning intensity, confirming that after harvesting, residues remaining on the forest floor and decomposing roots may contribute to raise soil respiration, due to the higher microbial activity.
Keywords
Introduction
Respiration is one of the largest fluxes in the global carbon (C) cycle, emitting approximately 10 times more C than that released through fossil fuel combustion and cement manufacturing ([14], [34]). Soil respiration is the soil CO2 efflux representing from 30% to 80% of total forest ecosystem respiration ([8]). It consists of belowground autotrophic (roots and associated mycorrhizae) and heterotrophic (mainly microbes, microfauna, and mesofauna) respiration. It strongly influences net carbon uptake from the atmosphere, more than photosynthesis ([45]).
The factors influencing soil respiration are temperature, soil moisture, photosynthetic supply to roots, substrate quantity and availability ([23]). Moreover, there is a direct correlation between plant photosynthetic activity and soil respiration ([38]). Soil temperature can be used to predict rates of soil respiration through a sensitivity coefficient of soil respiration, commonly referred to as Q10 and used to describe the difference in rates of soil respiration over 10 °C temperature intervals ([21]). Variations in soil moisture content are also accounted for, as this last variable is, together with soil temperature, the most important environmental parameter controlling variations in soil CO2 efflux ([36]).
Given the temperature dependence of soil respiration, a potential positive feedback between increasing temperature and enhanced soil respiration may ultimately accelerate global warming ([44], [12], [7], [39], [37]). Scientists are therefore particularly interested in studying forest management strategies aimed at promoting C storage ([19], [26], [27]). Forest management has thus important effects on both autotrophic and heterotrophic soil respiration, contributing to affect local microclimatic properties of the soil, soil microbial communities and litter input ([32]) through biomass removal and the opening of the forest canopy ([29]). Forest management can potentially affect the Q10 of soil respiration by changing substrate availability and photosynthetic activity ([11]), root activity and inputs of labile organic carbon ([20], [46]), and soil temperature and moisture ([8]). The consequences of forestry practices on C balances and CO2 emissions from the soil are still poorly understood in terms of their impact on global C flux ([1]). The effect of thinning on soil respiration largely depends on the type, intensity and timing of the intervention ([3]).
From May 2014 to April 2015, within ManFor.BD Life project, CO2 fluxes from soil, together with soil water content and temperature have been measured over one solar year in an even-aged beech forest (Fagus sylvatica L.) under three different management options in southern Italy. Beech forests are among the most common forest communities in Italy, from the Alps to the Apennines including Sicily. In Calabria they have been, along with chestnut and silver-fir, a fundamental resource for people living in mountain areas, being used mainly for firewood and charcoal as well as timber for building and furniture ([25]) and this intensive use has significantly modified the distribution, composition and structure of beech stands ([30]). The management of these forest stands should be aimed to ensure that the benefits - both material and intangible - meet present needs, while at the same time ensuring their continued availability and contribution to long-term social and economic development (sustainable management). Here, we investigated the effects on soil respiration of three different silvicultural treatments: (i) thinning from below (traditional treatment), which consisted in the elimination of the worst trees in the dominated layer; (ii) tree-oriented silviculture (innovative thinning sensu [16]) which aimed to diversify forest structure through the creation of heterogeneous gaps; and (iii) no thinning (control).
This experimental study was conceived to evaluate the ecological effects of management, with a focus on the influence on carbon uptake/loss from forest soil. We investigated the hypothesis that an innovative silviculture based on tree-oriented silviculture, in addition to being a sustainable treatment which modifies growing spaces and spatial niches to increase heterogeneity of the stand structure, can also reduce C outputs from soil.
The main aim of this study was to evaluate the effect of such silvicultural practices on the CO2 respired from the forest floor. A secondary objective was to analyse the influence of soil temperature and soil moisture on soil respiration.
Material and methods
Site description and experimental design
The study area is located in the Marchesale Biogenetic Reserve (a Natura 2000 site) in Mongiana, the highest side of calabrian “Serre” mountains, within the province of Vibo Valentia (38° 30′ N, 16° 14′ E). The whole reserve is 1257 ha wide, with altitude ranging between 750 and 1170 m a.s.l. and it is managed by the National Forest Service of Italy (CFS). The forest types are beech forest managed as high forest and chestnut stands managed as coppice. In the area there is a small fraction of mixed beech-fir high forest (5%). The understorey is mainly composed of penciled geranium (Geranium versicolor L.) and Caucasian leopard’s bane (Doronicum orientale Hoffm.). The climate is typical upland Mediterranean (Csb, sensu [17]) with a long-term (1928-2012) mean annual temperature and precipitation of 10.1 °C and 1880 mm, respectively. The warmest month is July (18.4 °C), the coldest month is February (2.2 °C - average of 30 years measurements).
The study area is characterized by Palaeozoic granitoid rocks deeply fractured, the morphology is dominated by a mountains landscape with deep, V-shaped valleys ([5]). According to USDA soil classification ([41]), the most frequent soils are Inceptisols and Entisols, with soil from shallow to moderatly deep (0.20-1.0 m), coarse-textured and acidic pH (3.7-5.8 - [4]).
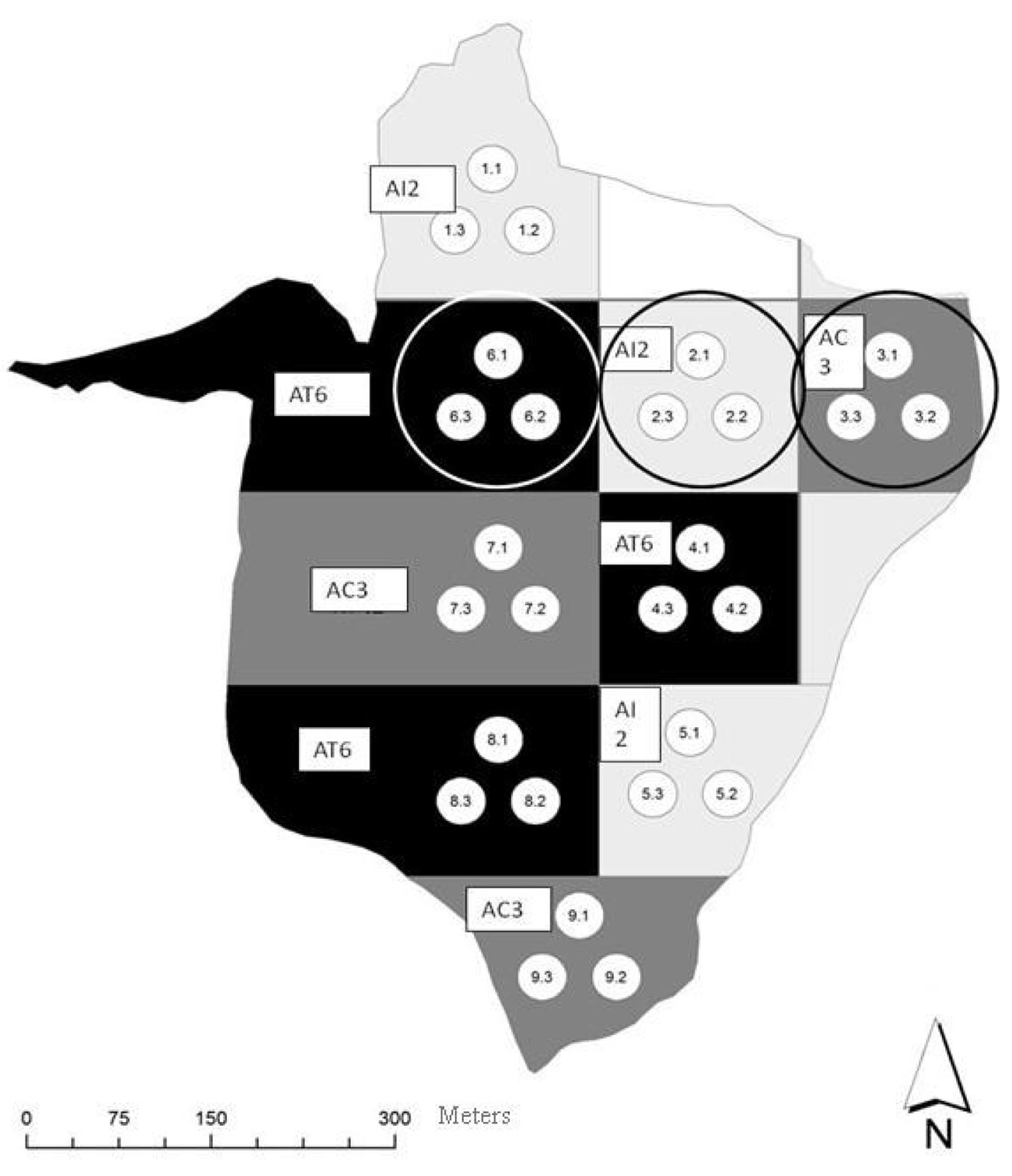
Within the ManFor C.BD. project, a 30 ha stand of 75-year-old high forest of beech at 1100 m elevation was selected and nine areas from 2.8 to 3.5 ha were subjected to three silvicultural thinnings (3 treatments × 3 replicates; 2012-2013 - Fig. 1). Before carrying out silvicultural treatments, statistical differences between dendrometric parameters were analyzed, resulting not significant ([35]).
Fig. 1 - Representation of the nine areas managed with three different treatments (AI2 innovative, AC3 control and AT6 traditional) and the sampling circular plots. Areas where soil respiration measurements were carried out are within the circles.
The options were traditional treatments (thinning from below - AT6), innovative treatments based on the tree-oriented silviculture (AI2) and unharvested control (AC3 - Tab. 1). Traditional treatment was a thinning from below with a moderate intensity, which removed all the dominated trees and the worst dominant trees competing with the best dominant ones (on average 45% trees cut). Innovative treatment was aimed at favouring the 50 best tress per hectare and enhancing structural biodiversity, by harvesting 5 or 6 closest competitor trees of the target trees, irrespective of their social position. Within each treatment, 3 randomly selected circular plots with a 17 m radius were chosen. Soil respiration measurements were conducted for one year within each circular plot at six PVC collars (5 cm height, 10 cm diameter) inserted into the soil to a depth of 5 cm. Once inserted, rings were left in place in the field throughout the year. They were spaced according to a “spiral arrangement” inscribed in a 13 m radius circle. The first one was randomly placed close to the plot centre, the second one at three meters from the centre along the line of maximum slope and the other four were placed at an increasing distance from the centre of 6, 9, 12, and 12 m respectively, with an angle of 90 degrees from the imaginary line connecting the centre to the collar inserted beforehand. The last collar at the 12 m distance from the centre was inserted according to the maximum slope. A portable chamber (SRC-1 soil respiration chamber, stainless steel, 15 cm height, 10 cm diameter) was used to measure CO2 efflux by connecting it to a portable gas-analyzer EGM-4 (Environmental Gas Monitor - PP-systems, Hitchin, Hertfordshire, UK). Fluxes of CO2 were measured by circulating air between a vented, chamber placed over the PVC ring and the portable battery operated EGM-4. The experiment commenced in May 2014, with biweekly measurements until May 2015, apart from the months of December and January, when measurements were suspended due to snowpack. Flux measurements were made between 11:00 and 15:00 hours at each of the 9 circular plots.
Tab. 1 - Main dendrometric characteristics (± standard deviation) of AI2 (innovative), AT6 (traditional), AC3 (control) treatments.
| Treatment | Volume (m3 ha-1) |
Basal area (m2 ha-1) |
No. tree (N ha-1) |
|
|---|---|---|---|---|
| AI2 (innovative) | Before | 338.1 ± 27.7 | 33.9 ± 1.9 | 631 ± 104.4 |
| After | 246.1 ± 21.3 | 26.6 ± 1.6 | 533 ± 94.3 | |
| AT6 (traditional) | Before | 345.7 ± 19.8 | 34.0 ± 1.2 | 557 ± 138.7 |
| After | 301.6 ± 15.6 | 29.7 ± 1.0 | 501 ± 111.5 | |
| AC3 (control) | - | 432.9 ± 174.1 | 38.5 ± 11.4 | 334 ± 201.2 |
Soil temperature was measured adjacent to each PVC collar at the time of the flux measurement, with a digital soil thermometer (Spectrum Technologies, Aurora, Illinois, USA). The depth of the measurement was 10 cm below the top of the litter layer. Soil water content was measured by time domain reflectometry (TDR) with FieldScout TDR 100 Soil Moisture Meter (Spectrum Technologies, Aurora, IL, USA), with two-rod probes 12 cm long placed vertically into the soil in a randomly selected spot around each collar during each CO2 measurement. The output of the soil moisture meter was the percentage volume water content. Daily precipitation were obtained from a weather station installed at the launch of the project in the core of the high forest beech stand under consideration, at a distance of less than 1 kilometer from plots.
Respiration rate (Rs, µmol C m-2 s-1) in each collar was calculated using the following equation (eqn. 1):
where ΔC (ppm) is the CO2 differential between the inlet and outlet, P is the atmospheric pressure, V is the volume of the headspace gas within the chamber, A is the area of soil enclosed by the chamber, T is the soil temperature (K) and R is the universal gas constant (8.314 J mol-1 K-1).
Relationship between soil respiration, temperature and moisture content
The relationship between soil temperature and soil respiration rate was fitted with an exponential model (eqn. 2):
where Rs is the measured soil respiration rate, T is the measured soil temperature (°C), a and b are fitted parameters obtained using a least square non-linear regression. The temperature sensitivity index of soil respiration (Q10), representing the relative increase of Rs when temperature increases by 10 °C within feasible range, was calculated by exponential curve using the following equation (eqn. 3):
In accordance with eqn. 2 and the method of Tang & Baldocchi ([43]), the following equation was used to describe the combined effect of temperature and moisture on soil respiration (eqn. 4):
where SoilT is the Soil Temperature (°C), SWC is the soil water content (%), b1, b2, b3, and b4 are the parameters of the non-linear regression.
Statistical analyses
One-way ANOVA test was used to compare b values among treatments. The standard error (SE) of Q10 was calculated as follows ([32] - eqn. 5):
After determination of the coefficient of variation (cv%) between measurements at the six collars positions in each circular plot, the mean of respiration, temperature and moisture of the six collars were used to represent every plot.
The effect of treatments on soil temperature and soil water content was analyzed with a one-way ANOVA.
A repeated measures ANOVA, with significant differences determined at α<0.05, was used to test the differences of soil respiration between treatments. A Mauchly’s test of sphericity was carried out to test homogeneity of variances.
The model was set up with the respiration rate measurements (average of the 18 measurements from collars for any treatment) as the dependent variable, the treatments as a “between-subjects” factor, soil temperature and soil moisture as covariates. The interaction between treatment and date was also considered in the model. A post-hoc comparison was made using the Tukey’s HSD test.
A non-linear regression analysis was used to evaluate the combined effect of soil temperature and soil water content on soil respiration for each treatment. The estimate of the parameters of the nonlinear regression model was based on the Gauss-Newton method ([40]). Standard errors of the parameters were estimated by a bootstrapping algorithm ([9]). The statistics RSME (root mean squared error), R2 (coefficient of determination) and SEE (Standard Error of Estimate) were used for model evaluation. The regression model was applied to each treatment separately.
Statistical analyses were conducted using SPSS statistics ver. 21 for Windows (IBM Corp., Armonk, NY, USA).
Results
Seasonal variations and effects of soil temperature and soil moisture content on Rs
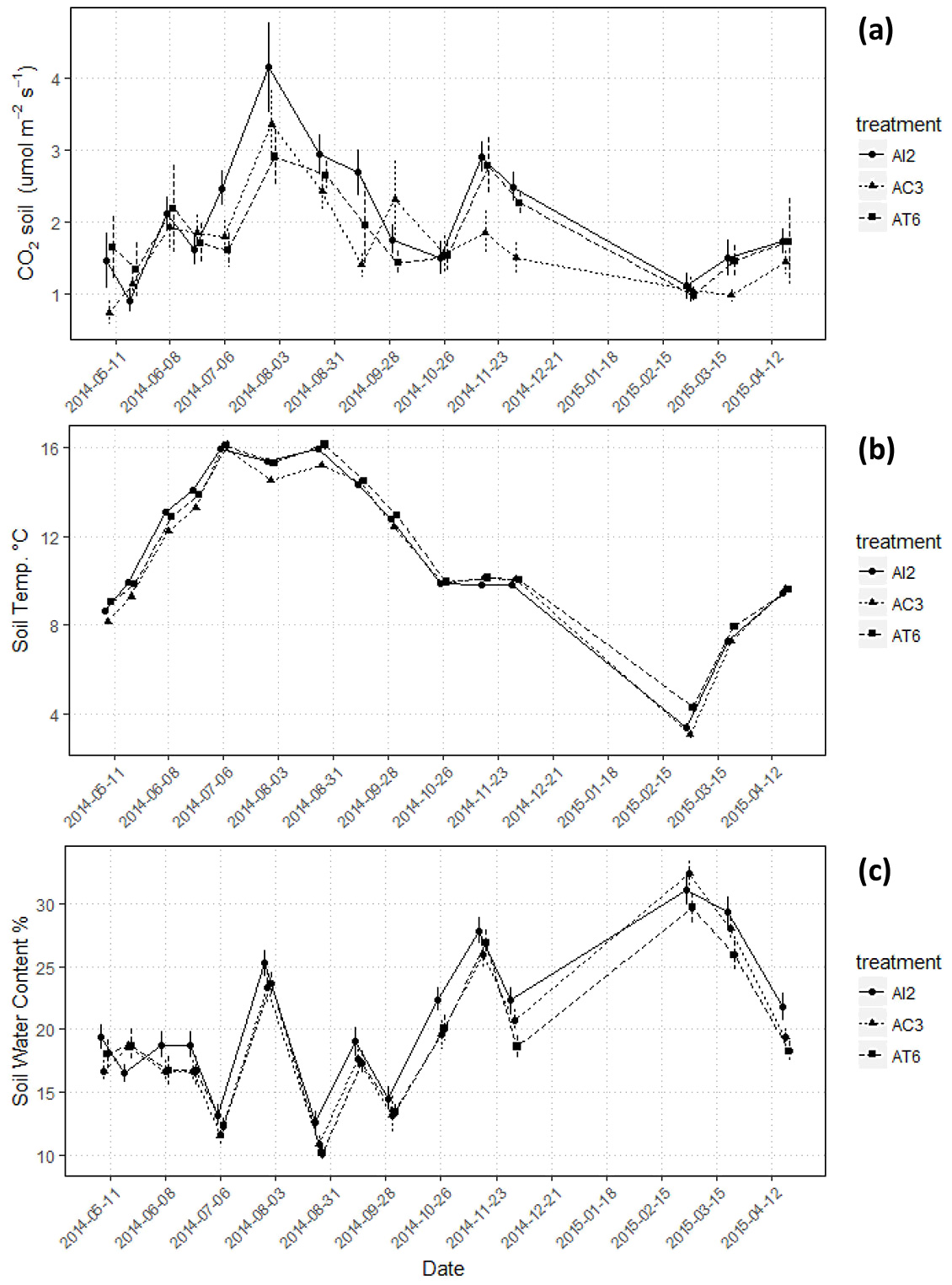
Fig. 2 shows the averaged daily measurements of Rs (a), soil temperature (b) and soil water content (c) for the whole campaign. Each value represents the mean of measurements taken at three plots per treatment (generally between 11:00 and 15:00 h) in every measurement day.
Fig. 2 - (a) Averaged daily measurements of soil respiration (µmol CO2 m2 s-1), (b) soil temperature, (c) soil water content with standard errors (error bars) in AI2 (innovative), AT6 (traditional), AC3 (control) treatments.
Soil respiration varied significantly over the year (p<0.001) for all treatments (AI2: from 0.91 to 4.2 ± 0.85 µmol CO2 m-2 s-1; AC3: from 0.73 to 3.36 ± 0.66 µmol CO2 m-2 s-1; AT6: from 0.98 to 2.90 ± 0.57 µmol CO2 m-2 s-1). Soil temperature and soil moisture also showed a significant seasonal variability (p<0.001).
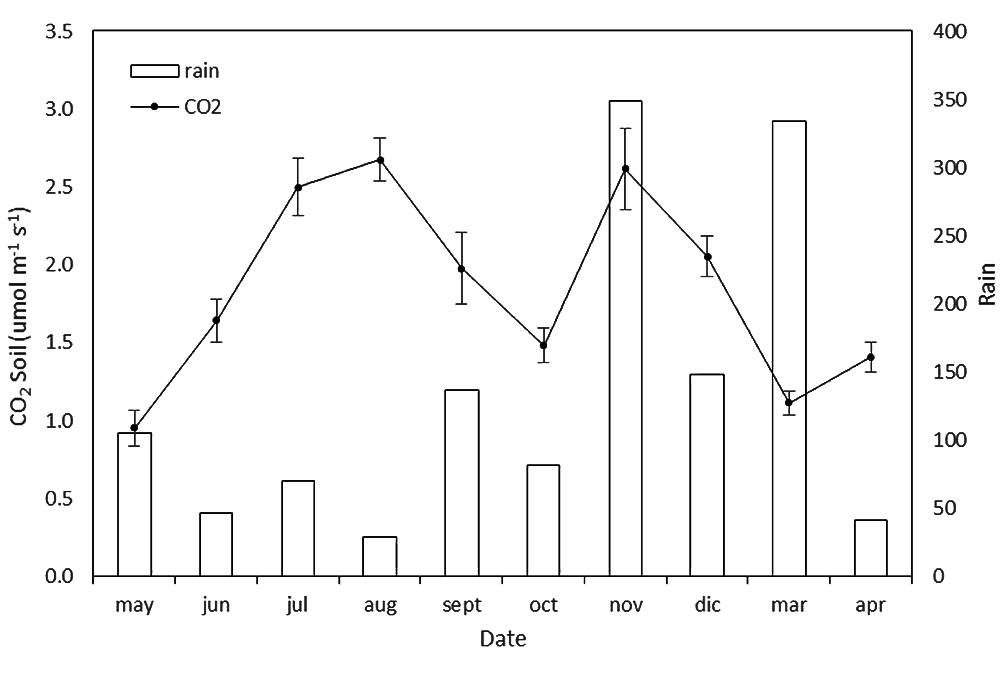
Rs peaked during July to September and then dropped (Fig. 2a) in May (AI2 and AC3) and in March (AT6). AI2 had the highest averaged annual CO2 efflux from soil (2.09 ± 0.85 µmol CO2 m-2 s-1; p<0.001), AC3 the lowest (1.68 ± 0.66 µmol CO2 m-2 s-1; p<0.001). In the warmest and driest period (August 2014 - Fig. 2a) AT6 showed the lowest emission from soil. Without considering the treatments, Fig. 3 shows that the month of July was less dry and warm than the rest of the summer months, resulting in a lower Rs. November was a rainy month (348 mm - Fig. 3) and an increase of soil respiration occurred when soil water content raised.
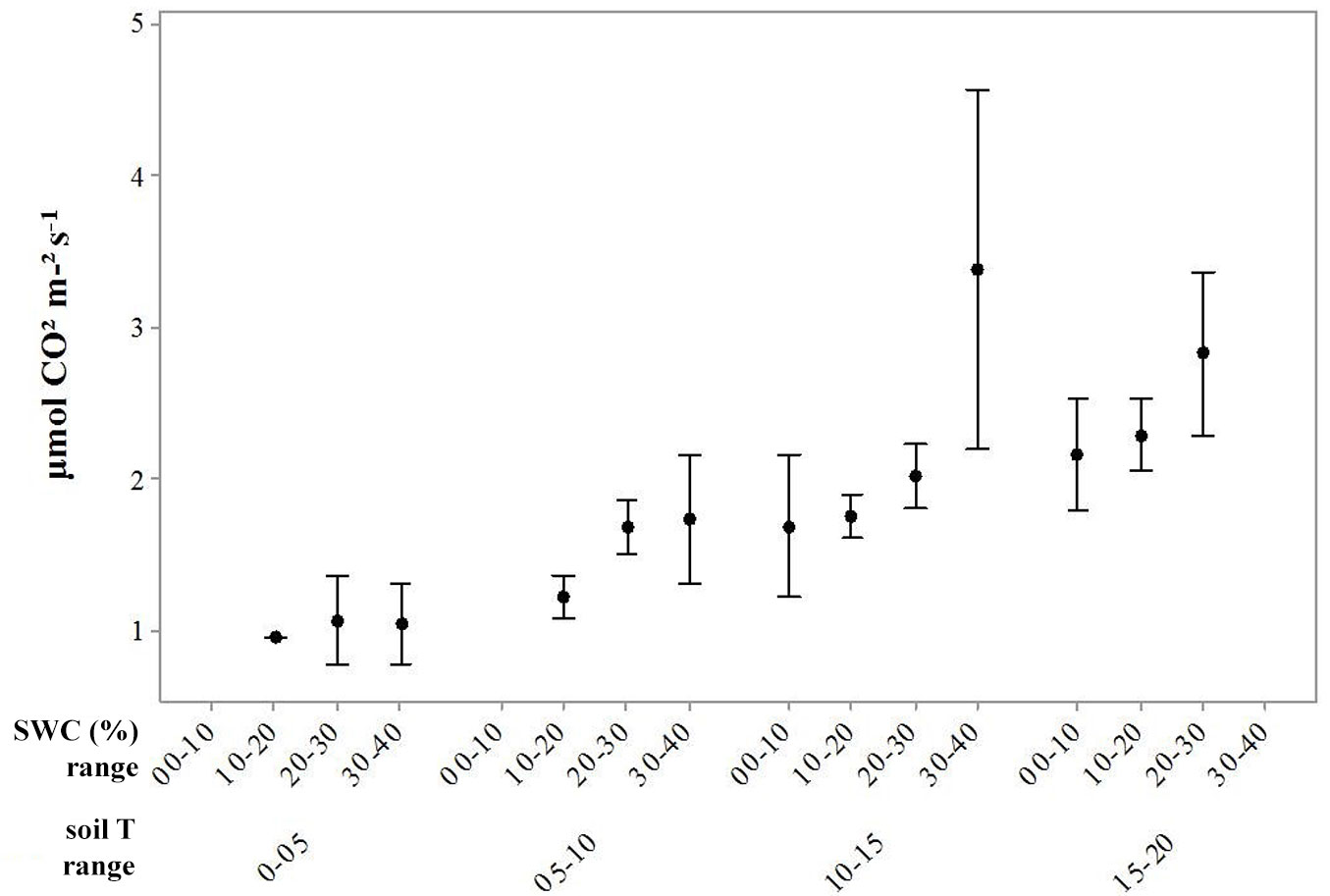
Respiration was inhibited when soil temperature ranged between 0 and 5 °C with a soil moisture ranging between 10 and 20%. The lowest efflux occurred when soil temperature was under 10 °C and soil water content was about 16% in AI2 and AC3. In AT6 the lowest Rs was recorded when soil temperature was 4 °C and soil water content was 29%. Without considering the treatments, Fig. 4 shows that the highest soil emission occurred when soil temperature was recorded ranging from 10 and 15 °C and a soil water content was between 30 and 40%.
Fig. 4 - CO2 (µmol CO2 m2 s-1) efflux with standard errors (error bars), recorded when soil water content (SWC) ranged from 0 to 40 % and soil temperature (Soil T) ranged from 0 to 20 °C.
Q10 was 2.06 ± 0.39 in AC3, 1.94 ± 0.41 in AI2 and 1.74 ± 0.38 in AT6. No significant differences between Q10 values were found (p=0.262).
The one-way ANOVA showed that no significant effect of treatments on soil temperature was found (F2;708=0.537; p=0.585), but treatments had a significant effect on soil water content (F2;708=3.670; p = 0.026).
Repeated measures ANOVA analysis showed that there were no significant differences in soil temperature between the treatments (p > 0.05), whereas soil water content was statistically different only in AI2 (p<0.001).
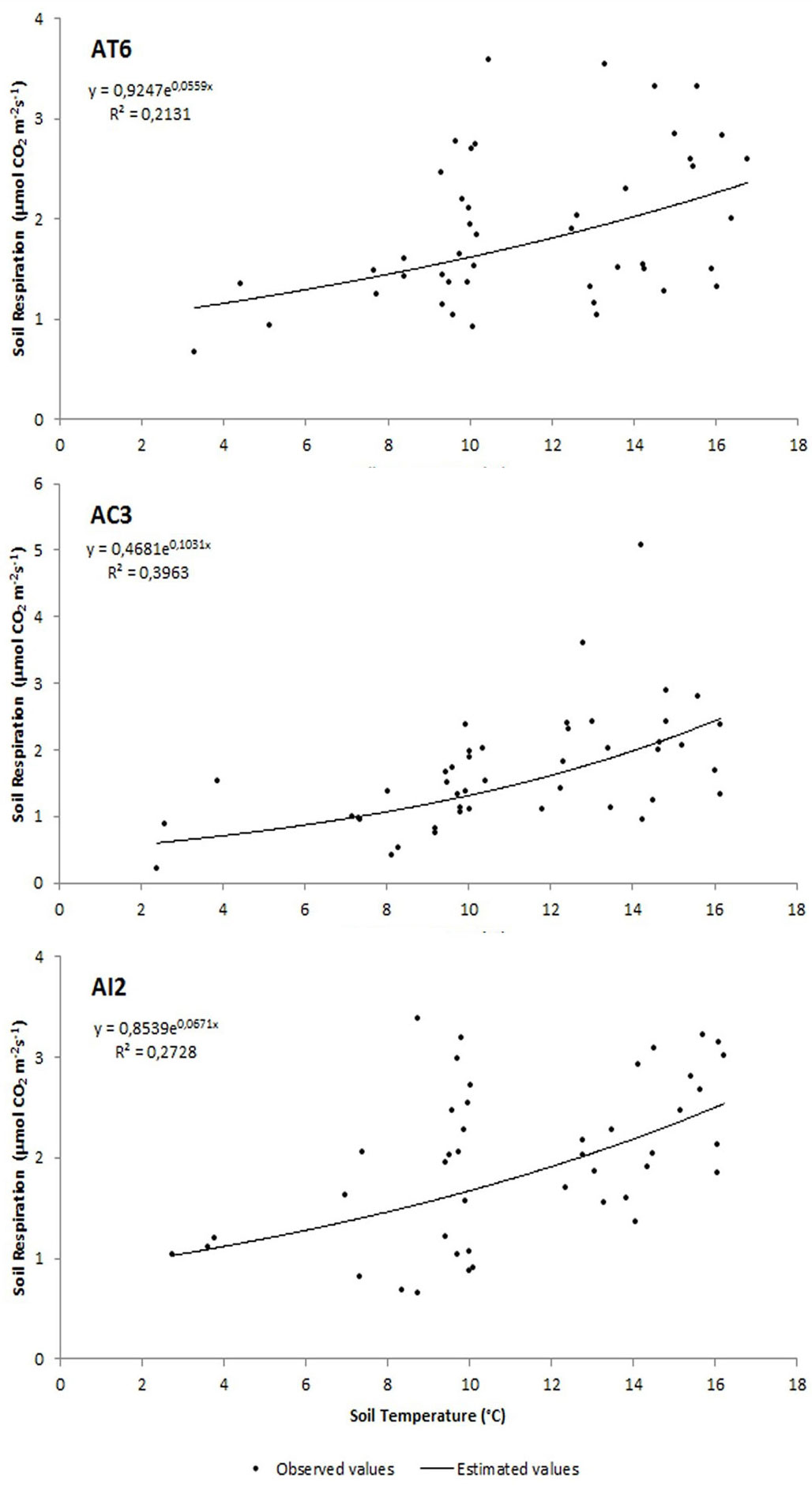
During the experimental period Rs showed a significant positive correlation to soil temperature in all the treatments (p<0.0001). The exponential regression gave low R2 values when considering averaged values for any day of measurements in each sub-plot (R2 = 0.21 for AT6; R2 = 0.40 for AC3; R2 = 0.27 for AI2 - Fig. 5).
Fig. 5 - Relationship between soil respiration and soil temperature in AT6 (traditional), AC3 (control), AI2 (innovative) treatments. The points represent the averaged measures taken at the collars for each sub-plot during sampling days.
The combined effect of soil temperature and water content gave a better description of the variability in soil respiration in treatment AI2 (R2 = 0.48), compared to AC3 (R2 = 0.43) and AT6 (R2 = 0.38). For each treatment, regression analysis resulted significant, and the parameters of the regressions were all significant (Tab. 2).
Tab. 2 - Parameters and coefficients of the regression analysis (eqn. 4) carried out to evaluate the combined effect of soil temperature and soil water content on soil respiration in each treatment (AC3): control; (AI2): innovative treatment; (AT6): traditional treatment; (SE): standard error of the estimated parameters; (SEE): standard error estimate; (RMSE): root mean square error; (R2): coefficient of determination.
| Treatments | Coefficient | Estimate | SE | SEE | RMSE | R2 |
|---|---|---|---|---|---|---|
| AC3 | b 1 |
0.6413 | 0.2073 | 0.896 | 0.846 | 0.429 |
b 2 |
0.0802 | 0.0127 | ||||
b 3 |
-0.0147 | 0.0025 | ||||
b 4 |
0.0007 | <0.0001 | ||||
| AI2 | b 1 |
0.5049 | 0.1539 | 0.957 | 0.958 | 0.485 |
b 2 |
0.0887 | 0.0112 | ||||
b 3 |
0.0051 | 0.0013 | ||||
b 4 |
0.0005 | <0.0001 | ||||
| AT6 | b 1 |
0.6784 | 0.2137 | 0.886 | 0.812 | 0.383 |
b 2 |
0.0706 | 0.0160 | ||||
b 3 |
-0.0152 | 0.0024 | ||||
b 4 |
0.0009 | <0.0001 |
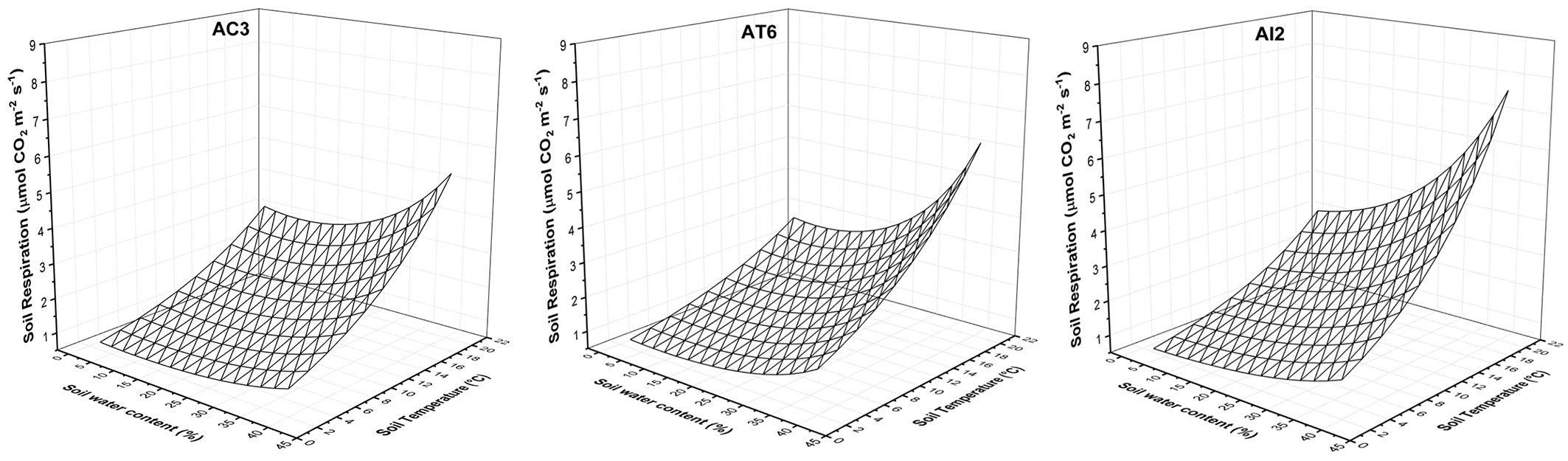
The developed models are shown in Fig. 6. The soil respiration increased with increasing of soil temperature and soil water content. When modeling AI2, it resulted that the increase of soil temperature and soil water content caused a higher raise in Rs compared to the other treatments.
Fig. 6 - The combined effect of soil water content and soil temperature on the soil respiration in AC3 (control) AT6 (traditional), AI2 (innovative) treatments.
The effects of treatments on Rs
Sphericity was assumed since Mauchly’s test gave a p-value of 0.396. Rs showed large variations among the treatments (F2;36= 6.130, p < 0.001). Tukey’s test showed that soil respiration in AI2 was significantly higher than in AC3 and AT6. AC3 and AT6 were not significantly different.
Discussion
High temporal and spatial variation in soil CO2 efflux is a common phenomenon in forests ([6]). In this study, soil respiration rates peaked in the summer (4.14 µmol CO2 m-2 s-1) and were lowest during spring (0.72-0.88 µmol CO2 m-2 s-1), when soil temperature reached the lowest recorded values. We may hypothesize that lower soil respiration rate could result from microbial activity becoming dormant after the decline of soil temperature ([15], [2]). In our study, the effect of soil moisture increased the rate of respiration for the site, when reaching 30-40% level (Fig. 4). Soil moisture directly affects microbial activity and several physical processes, such as water movement, and gas or solute diffusion ([6]). Soil respiration never appeared to be inhibited by near saturated water content. These soils have high sand content, with a good structure and porosity and high infiltration rates, which result in infrequent saturated conditions. The thinning changed the moisture dynamics in AI2, thus making the response of soil respiration to changes in soil moisture more evident, especially when high temperatures occurred together with high percentage of soil water content. This is also evident from the analysis of the combined effect of soil temperature and water content on soil respiration (Fig. 6). We observed that in spring soil temperature was slightly higher in AT6 than in AI2, but this is likely to have had only a minor effect on soil respiration, which was largely constrained by low soil moisture content at that time.
Forest management practices such as thinning affect stand biomass and carbon stored in soil, influencing the interaction between the biotic (roots, invertebrates and microorganisms) and abiotic (temperature and moisture) factors of the forest ecosystem. Both increases and decreases in soil respiration after thinning have been observed in previous studies ([22], [24], [43], [42]), but this is due likely to the timing and duration of the measurement campaigns undertaken following thinning in the different studies.
The increase in soil respiration in the treated areas in this study could be attributed to a greater increase in heterotrophic respiration from the above- and belowground debris than the reduction in autotrophic (root) respiration ([42]). After a short time suspension of root respiration suddenly after thinning ([10]), an enhancement of microbial respiration occurs when the decomposition of dead roots and aboveground residuals under optimum temperatures begins ([7], [13]) and thus offsets the decline in root respiration ([31]). Thinning can also influence site specific microclimatic conditions. For example, the removal of aboveground vegetation is known to increase soil temperature, and a strong positive correlation exists between soil respiration and temperature in thinned forests ([28], [18]). However, in our study soil temperature did not result to be significantly different in the three treatments, therefore we can conclude that the increase of soil respiration in AI2 was mainly caused by the increase of microbial respiration due to detrital decomposition, which possibly peaked when soil water content increased. The increase in soil water content may be attributed to the removal of more trees, which reduced rain interception and evapotranspiration.
Q10 analysis gave an idea of the low influence of temperature on microbial and root respiration. This however proves the low intensity of harvesting interventions, which clearly did not lead to large gaps opening and so they can be defined low disturbance treatments. Consequently, we may expect a change of soil CO2 emission with time, as it can be inferred from the results of the analysis of the interaction between treatment and date in the ANOVA model. The interaction of treatment and date effects resulted significant (p = 0.035), which means that part of the variability of the data resulted from seasonal variations in the microclimatic and microsite conditions created by treatments and these variation could be detected over the all period of measurements, thus we believe different results will be derived from future measurements campaign in the same plots.
Conclusions
This study showed that the mean soil respiration increased with thinning intensity, confirming that after harvesting, detrital biomass remaining on the forest floor may contribute to raise soil respiration, also preventing evaporation of water from soil, and leading therefore to an higher microbial activity. This information is useful for forest managers in predicting the consequences of forest management given the efflux of CO2 from the soil surface of our beech forest. However, it would be interesting to carry on measurements in these sites to analyse long term post-harvest effects. Forest management practices, together with other achievements, must be oriented to enhance carbon sequestration; it is fundamental to better know the effects of forest management on CO2 cycle, considering all the components and their interactions. Therefore, studies examining patterns and mechanisms of soil CO2 in response to management practices are essential. Such studies are critical to accurately predict effects of forest management on the C cycle and to develop appropriate forest management strategies aimed at reducing atmospheric CO2 concentrations ([33]).
References
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Pasquale A Marziliano
Mediterranean University of Reggio Calabria, Feo di Vito, 89020 Reggio Calabria (Italy)
Raffaele Froio
Giorgio Matteucci
Nicola Ricca
Antonella Veltri
Institute for Agricultural and Forest Systems in the Mediterranean (ISAFOM), National Research Council of Italy, Cosenza (Italy)
Rosario Turco
Mediterranean Forestry Research Unit, (CRA-SAM), Rende, Cosenza (Italy)
Institute of Agro-envinronmental and Forest Biology (IBAF), National Research Council of Italy, Montelibretti (RM, Italy)
Corresponding author
Paper Info
Citation
Coletta V, Pellicone G, Bernardini V, De Cinti B, Froio R, Marziliano PA, Matteucci G, Ricca N, Turco R, Veltri A (2017). Short-time effect of harvesting methods on soil respiration dynamics in a beech forest in southern Mediterranean Italy. iForest 10: 645-651. - doi: 10.3832/ifor2032-010
Academic Editor
Giorgio Alberti
Paper history
Received: Mar 01, 2016
Accepted: Apr 08, 2017
First online: Jun 20, 2017
Publication Date: Jun 30, 2017
Publication Time: 2.43 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2017
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 50833
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 42611
Abstract Page Views: 3308
PDF Downloads: 3759
Citation/Reference Downloads: 17
XML Downloads: 1138
Web Metrics
Days since publication: 3178
Overall contacts: 50833
Avg. contacts per week: 111.97
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2017): 3
Average cites per year: 0.33
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Spatial heterogeneity of soil respiration in a seasonal rainforest with complex terrain
vol. 6, pp. 65-72 (online: 07 February 2013)
Research Articles
Soil respiration along an altitudinal gradient in a subalpine secondary forest in China
vol. 8, pp. 526-532 (online: 01 December 2014)
Research Articles
Seasonal dynamics of soil respiration and nitrification in three subtropical plantations in southern China
vol. 9, pp. 813-821 (online: 29 May 2016)
Research Articles
Soil respiration and carbon balance in a Moso bamboo (Phyllostachys heterocycla (Carr.) Mitford cv. Pubescens) forest in subtropical China
vol. 8, pp. 606-614 (online: 02 February 2015)
Research Articles
Effect of different dolomitic limestone dosages on soil respiration in a mid-altitudinal Norway spruce stand
vol. 12, pp. 357-365 (online: 05 July 2019)
Review Papers
Separating soil respiration components with stable isotopes: natural abundance and labelling approaches
vol. 3, pp. 92-94 (online: 15 July 2010)
Research Articles
Comparison of soil CO2 emissions between short-rotation coppice poplar stands and arable lands
vol. 11, pp. 199-205 (online: 01 March 2018)
Research Articles
Thinning effects on soil and microbial respiration in a coppice-originated Carpinus betulus L. stand in Turkey
vol. 9, pp. 783-790 (online: 29 May 2016)
Research Articles
Wood-soil interactions in soil bioengineering slope stabilization works
vol. 2, pp. 187-191 (online: 15 October 2009)
Research Articles
The manipulation of aboveground litter input affects soil CO2 efflux in a subtropical liquidambar forest in China
vol. 12, pp. 181-186 (online: 10 April 2019)
iForest Database Search
Search By Author
- V Coletta
- G Pellicone
- V Bernardini
- B De Cinti
- R Froio
- PA Marziliano
- G Matteucci
- N Ricca
- R Turco
- A Veltri
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
- V Coletta
- G Pellicone
- V Bernardini
- B De Cinti
- R Froio
- PA Marziliano
- G Matteucci
- N Ricca
- R Turco
- A Veltri
Search By Keywords
PubMed Search
Search By Author
- V Coletta
- G Pellicone
- V Bernardini
- B De Cinti
- R Froio
- PA Marziliano
- G Matteucci
- N Ricca
- R Turco
- A Veltri
Search By Keyword