Soil respiration along an altitudinal gradient in a subalpine secondary forest in China
iForest - Biogeosciences and Forestry, Volume 8, Issue 4, Pages 526-532 (2014)
doi: https://doi.org/10.3832/ifor0895-007
Published: Dec 01, 2014 - Copyright © 2014 SISEF
Research Articles
Abstract
The subalpine forest ecosystems in the Miyaluo Forest District in western Sichuan (China) could be very sensitive to global climate change, with important consequences for the regional carbon (C) balance. In a birch secondary forest in this area, we measured plots with (Control) and without (No Litter) leaf litter to explore variation in soil respiration and its relationship with environmental factors along an altitudinal gradient, and to quantify the litter contribution to soil respiration. Soil respiration rate decreased with elevation. The average of soil respiration rates along the elevation gradient during the measurement period was 2.83 ± 0.14 μmol CO2 m-2 s-1 in the Control treatment and 2.35 ± 0.16 μmol CO2 m-2 s-1 in the No Litter treatment, with an average proportion of litter layer contribution to soil respiration of 17%. A significant linear relationship between soil respiration and soil temperature along the altitudinal gradient was found, while soil respiration was not significantly correlated with soil water content in both treatments. Soil temperature accounted for 94.9% and 95.6% of the total variation in soil respiration in Control and No Litter treatments, respectively. At altitudes of 2910 m, 3135 m, 3300 m and 3492 m a.s.l., soil respiration had a significant exponential relationship with soil temperature (p<0.05), but it was not significantly correlated with soil water content in both treatments (p>0.05). Soil temperature accounted for more than 92% and 81% of the total variation in soil respiration in Control and No Litter treatments, respectively, at all altitudes except at 3135 m a.s.l. Our results suggest that the expected temperature increases by global warming might enhance soil respiration in the birch secondary forest.
Keywords
Birch Secondary Forest, Soil Respiration, Soil Temperature, Soil Water Content, Litter
Introduction
Carbon dioxide (CO2) is released from soils in the process referred to as soil respiration (SR), soil-CO2 evolution or soil CO2 efflux. Soil respiration is a major flux in the global carbon cycle, second in magnitude to gross primary productivity and equal to or greater than the estimated global terrestrial net primary productivity ([19]). Atmospheric CO2 concentrations are predicted to double the pre-industrial values by the end of the 21st century. It is likely that increased levels of CO2 and other greenhouse gases will result in a 1.4 °C to 5.8 °C increase in global air temperatures by the middle of this century ([35]). A major concern of scientists is the potential positive feedback between increasing temperature and enhanced soil respiration that may ultimately accelerate global warming ([29], [8], [5], [24], [21]).
Three principal components of soil respiration may be well defined: root respiration, surface-litter respiration, and the respiration of soil organic matter (including root detritus - [19]). The factors influencing soil respiration, such as temperature, soil moisture, photosynthetic supply to roots, substrate quantity and availability, differentially affect the aforementioned three principal components, making the interpretation of soil respiration complex ([18], [10], [22], [2], [30]).
The subalpine forest ecosystems in the Eastern Qinghai-Tibet Plateau could be very sensitive to global climate change, with important consequences for the regional C balance ([34]). The Miyaluo Forest District located in the Eastern Qinghai-Tibet Plateau is characterized by an extremely mountainous relief with relative differences in elevation of about 2000 m ([31]). Recently, several studies on soil respiration have been conducted in this area, mainly focused on the coniferous forests dominated by fir or spruce ([3], [39], [2]). On the contrary, the secondary forests naturally grown after large-scale forest harvesting are scarcely investigated, though they are now widely distributed. More studies on such forests are necessary to produce reliable regional estimates of soil respiration and to predict their responses to climate change.
In this study, we measured soil CO2 efflux with and without litter in a birch secondary forest starting at an altitude of 2910 m and continuing every 200 m to a maximum altitude of 3492 m, along an altitudinal gradient of about 600 m in the Miyaluo Forest District. This altitudinal gradient in the birch secondary forest provided a unique opportunity to evaluate the effects of in situ environmental factors on soil respiration, minimizing differences in forest community as a confounding factor. The objectives of our study were to: (1) explore variation in soil respiration along the altitudinal gradient and its relationship with environmental factors; (2) examine soil respiration variation at each elevation; (3) quantify the litter contribution to soil respiration.
Materials and methods
Site description
The experiment was carried out in the Miyaluo Forest District (31° 24′ 00″ - 31° 55′ 12″ N; 102° 34′ 48″ - 103° 04′ 12″ E), administratively belonging to Miyaluo Town, Li Country, Aba Tibet-Qiang People’s Autonomic District of Sichuan Province, China ([15]). The district is located on the upper reaches of the Zagunao River, a tributary of the Minjiang River, across a transition zone from the Sichuan Plain to the Qinghai-Tibetan Plateau. The area has the typical Qinghai-Tibet Plateau climate with warm humid summers and dry cold winters. The warmest month is July, with a mean temperature of 12.6 °C; the coldest month is January, with a mean temperature of -8 °C. Annual precipitation is 600-1000 mm, with high frequency and low intensity. Annual evaporation and frost-free days are 1000-1900 mm and 200 days, respectively ([2]).
Sample sites were previously dominated by native, dark coniferous forests before large-scale harvest activities started in the early 1950s. The major harvest period was from 1953 to 1978 and the peak years were from 1958 to 1960. Harvest areas were mainly at elevations ranging from 2800 to 3600 m a.s.l. The harvest activities stopped in 1998 when the national key programs of the “Natural Forest Protection Program (NFPP)” and the “Sloping Land Conversion Program (SLCP)” were started. A significant plantation period started from the mid-1950s ([13]). Spruce was used as the major species for plantation on the clear-cut site. Meanwhile, the natural regeneration of birch also generally took place in other harvest residue lands, forming a large area of secondary broadleaved forests where Betula albo-sinensis and B. utilis were the dominant tree species, mixed with Acer spp. and Tilia chinensis, as well as shrubs such as Prunus spp. and Sorbus spp. ([36], [37]).
Experimental design
In June 2010, three 30×10-m plots were randomly selected at 2910 m, 3135 m, 3300 m and 3492 m a.s.l. in the birch secondary forest in the Miyaluo Forest District. Site characteristics and soil properties (0-20 cm) of the experimental sites, as determined in June 2010, are shown in Tab. 1. In order to quantify the litter contribution to soil respiration, two 1×1-m subplots were established in each 30×10-m plot. Control and No Litter treatments were applied to two 1×1-m subplots. In the Control treatment, subplots were left undisturbed. In the No Litter treatment, litter on the ground was removed and new litter was excluded using 2 mm-mesh nylon screens. The fractional litter contribution could then be calculated as the difference between soil respiration (SR) in Control treatment and SR in No Litter treatment divided by SR in Control treatment. In each subplot, three PVC collars, 9 cm in height and 10 cm in diameter, were randomly and permanently inserted 6 cm from ground surface into the soil. Standing vegetation within each collar was clipped at the soil surface to eliminate the effects of photosynthesis.
Tab. 1 - Site characteristics and soil properties of the experimental sites (n=3). (EC): Electrical conductivity; (BD): Bulk density; (TC): Total carbon; (SOC): Soil organic carbon; (TN): Total nitrogen; (P): Phosphorus; (K): Potassium.
| Altitude (m asl) | 2910 | 3135 | 3300 | 3492 | ||||
|---|---|---|---|---|---|---|---|---|
| Aspect | NE | NE | NE | NE | ||||
| Slope (°) | 30 | 15 | 20 | 20 | ||||
| Depth (cm) | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 | 0-10 | 10-20 |
| pH | 6.6 | 6.4 | 5.0 | 5.0 | 4.7 | 4.6 | 4.1 | 4.4 |
| EC | 299 | 158 | 255 | 174 | 207 | 78 | 229 | 107 |
| Clay (%) | 47.1 | 48.8 | 47.4 | 44.7 | 46.2 | 45.6 | 47.5 | 43.0 |
| Silt (%) | 36.1 | 36.6 | 41.1 | 37.2 | 36.6 | 35.0 | 39.1 | 34.7 |
| Sand (%) | 16.9 | 14.6 | 11.5 | 18.1 | 17.2 | 19.4 | 13.4 | 22.3 |
| BD (g cm-3) | 0.34 | 0.61 | 0.48 | 0.79 | 0.74 | 0.72 | 0.44 | 0.70 |
| TC (%) | 9.19 | 5.28 | 15.1 | 10.8 | 7.87 | 3.68 | 7.66 | 4.91 |
| SOC (%) | 8.07 | 4.31 | 12.1 | 8.34 | 6.86 | 3.18 | 6.69 | 4.05 |
| TN (%) | 0.64 | 0.39 | 0.95 | 0.71 | 0.58 | 0.29 | 0.52 | 0.34 |
| P (mg g-1) | 1.26 | 1.11 | 1.08 | 1.04 | 1.01 | 0.83 | 1.07 | 0.87 |
| K (%) | 2.12 | 2.35 | 2.09 | 2.34 | 2.64 | 2.85 | 2.51 | 3.22 |
Field measurements
Soil respiration was measured with a Li-Cor 8100 (Li-Cor, Nebraska, USA) portable infrared gas analyzer equipped with a proprietary 10 cm survey chamber. Measurements were taken in June 2010 and in July and September 2011, once a month. Soil respiration in each collar was measured five or six times from 07:20 to 18:20 on measurement days. Simultaneously with the soil respiration measurements, soil temperature (ST) at a depth of -10 cm was measured using a thermometer attached to the Li-Cor 8100, and volumetric soil water content (SWC) at a depth of -6 cm was measured with the TFR (ML2X, England) in the vicinity of each collar.
Data analysis
All statistical analyses were conducted by using the software SPSS® 16.0. The Kolmogorov-Smirnov test was used to check for normality of variances. All data were normally distributed. Paired-samples t test was used to test the effect of litter exclusion. One-way ANOVA with a post-hoc LSD test was used to test for difference in soil respiration among the different altitudes. Pearson correlation analyses with two-tailed significance tests were used to clarify the relationship between soil respiration, soil temperature and water content. Regression analyses were used to further test the relationship between soil respiration and soil temperature according to eqn. 1 along the altitudinal gradient and to eqn. 2 at each altitude as follows:
where y is soil respiration, x is soil temperature at a depth of -10 cm, a, b, c and d are the fitting parameters.
Results
Soil respiration
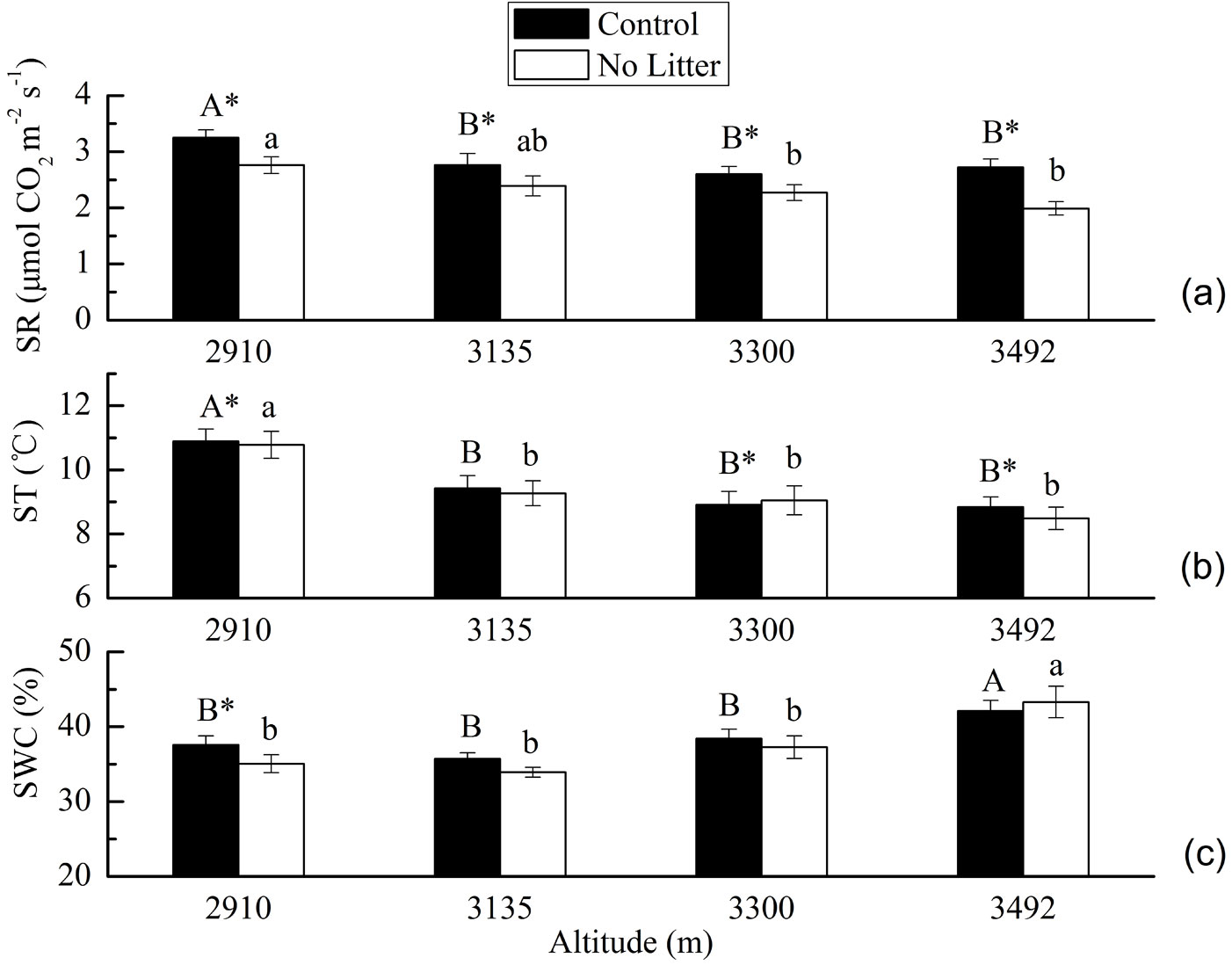
At altitudes of 2910 m, 3135 m, 3300 m and 3492 m, the soil respiration rates (mean ± SE) calculated using the mean of all three replicated plots across all measuring dates were 3.25 ± 0.14, 2.76 ± 0.21, 2.6 ± 0.14 and 2.72 ± 0.15 μmol CO2 m-2 s-1 in the Control treatment, and were 2.76 ± 0.15, 2.39 ± 0.18, 2.27 ± 0.14 and 1.99 ± 0.12 μmol CO2 m-2 s-1 in the No Litter treatment. Compared with the Control treatment, the No Litter treatment reduced soil respiration rates by 5%, 13%, 13% and 27% at altitudes of 2910 m, 3135 m, 3300 m and 3492 m, respectively. The average of soil respiration rates along the elevation gradient during the measurement period was 2.83 ± 0.14 μmol CO2 m-2 s-1 in the Control treatment and 2.35 ± 0.16 μmol CO2 m-2 s-1 in the No Litter treatment, a reduction of 17%.
Paired-samples t-test revealed that, at all altitudes, soil respiration rates in the Control treatment were significantly greater than those in the No Litter treatment (p<0.05) and that they were significantly correlated (p<0.05 - R=0.924, n=16 at 2910 m a.s.l.; R=0.981, n=16 at 3135 m a.s.l.; R=0.989, n=16 at 3300 m a.s.l.; R=0.938, n=17 at 3492 m a.s.l.).
One-way ANOVA showed that soil respiration rates were significantly different among altitudes in the two treatments (p<0.05). The post-hoc LSD test showed that in the Control treatment, the soil respiration rate at the altitude of 2910 m was significantly greater than that at the other three altitudes (p<0.05), while there were no significant differences among soil respiration rates among these other three altitudes (p>0.05). In the No Litter treatment the soil respiration rate at the altitude of 2910 m was statistically equal to that at the altitude of 3135 m, but significantly greater than that at altitudes of 3300 m and 3492 m (p<0.05). Also, no significant differences were found among those at the altitudes of 3135 m, 3300 m and 3492 m (p>0.05 - Fig. 1a).
Fig. 1 - Soil respiration (SR, μmol CO2 m-2 s-1) (a), soil temperature (ST, °C) (b) and soil water content (SWC, %) (c) in the two treatments (Control and No Litter) along the altitudinal gradient. Values are mean ± standard error (n=16 at altitudes of 2910 m, 3135 m, 3300 m and n=17 at altitude of 3492 m a.s.l.). Different capital letters indicate significant differences (p<0.05) among altitudes after post-hoc LSD test in the Control treatment. Different lower case letters indicate significant differences (p<0.05) among altitudes after the post-hoc LSD test in the No Litter treatment. An asterisk indicates significant differences (p<0.05) between Control and No Litter treatments at a specific altitude after paired-samples t-test.
Soil temperature and soil water content
In the Control and No Litter treatments, soil temperature decreased with elevation, ranging from 8.84 ± 0.32 °C to 10.9 ± 0.38 °C and from 8.49 ± 0.35 °C to 10.8 ± 0.42 °C, respectively, while soil water content increased with elevation, ranging from 35.7 ± 0.82% to 42.1 ± 1.45% and from 33.9 ± 0.67% to 43.3 ± 2.09%, respectively (mean ± SE). Moreover, in both treatments soil temperature at the altitude of 2910 m was significantly higher than those at the other three altitudes (p<0.05), and no significant differences were detected among these three altitudes (p>0.05 - Fig. 1b). Also, in the two treatments soil water content at the altitude of 3492 m was significantly greater than those at the other three altitudes (p<0.05), with no significant differences between these other three altitudes (p>0.05 - Fig. 1c).
Relationship of soil respiration with soil temperature and soil water content along the altitudinal gradient
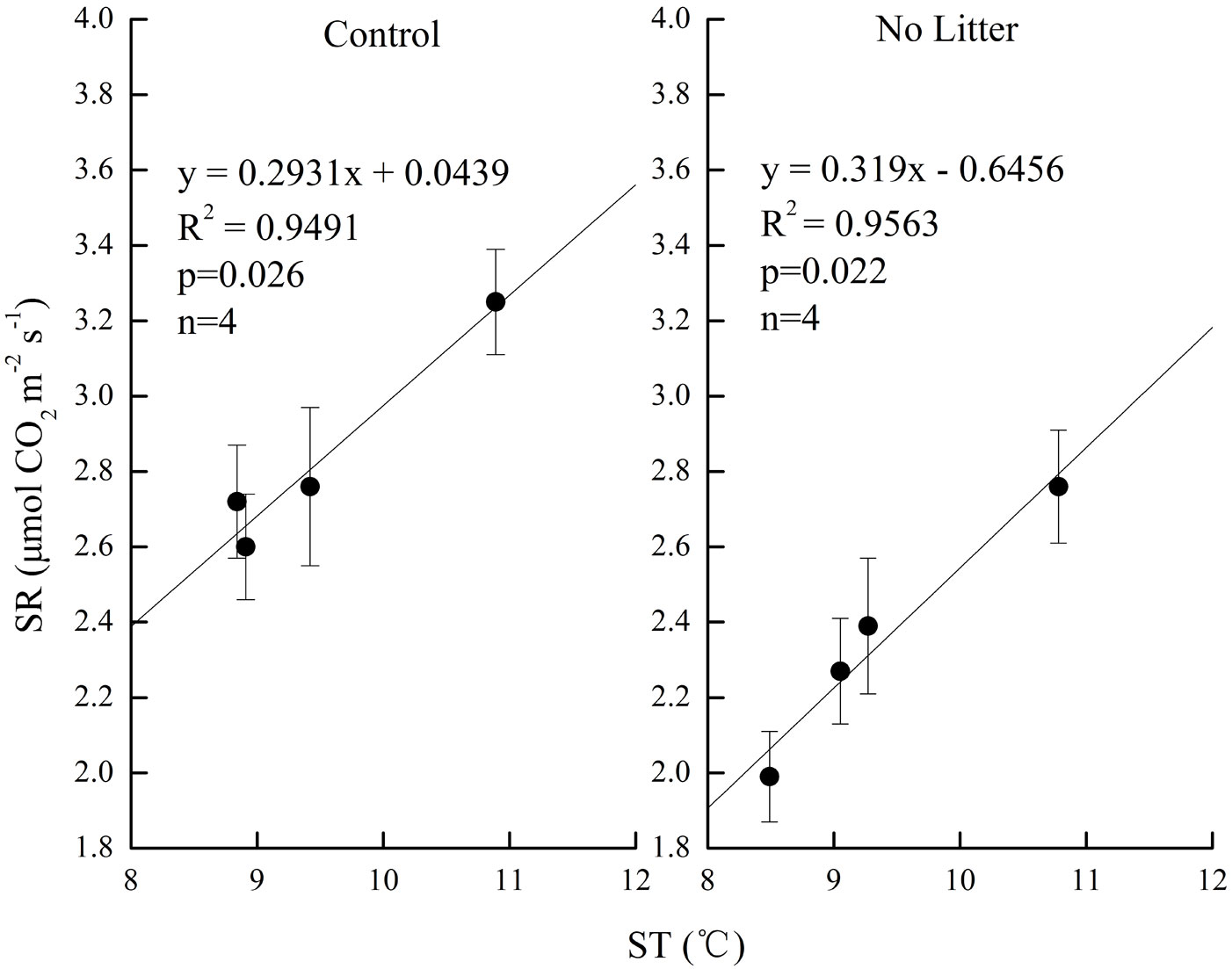
Soil respiration decreased with elevation in our study. Correlation analysis was carried out to reveal the relationship of soil respiration with soil temperature, as well as the relationship of soil respiration with soil water content along the altitudinal gradient in the birch secondary forest. Results showed that soil respiration had significant relationships with soil temperature in the Control treatment (R=0.974, p<0.05, n=4) and No Litter treatment (R=0.978, p<0.05, n=4), while it was not significantly correlated with soil water content (p>0.05, n=4). Regression analysis was further used to fit the dependence of soil respiration on soil temperature. In both treatments, a significant linear regression relationship was found between soil respiration and soil temperature along the altitudinal gradient. Soil temperature accounted for 94.9% and 95.6% of the variation in soil respiration in Control and No Litter treatments, respectively (Fig. 2).
Fig. 2 - Results of regression analysis between soil respiration (SR, in μmol CO2 m-2 s-1) and soil temperature (ST, in °C) along the altitudinal gradient, in the two treatments (Control and No Litter). Values are mean ± standard error (n=16 at altitudes of 2910 m, 3135 m, 3300 m and n=17 at altitude of 3492 m a.s.l.).
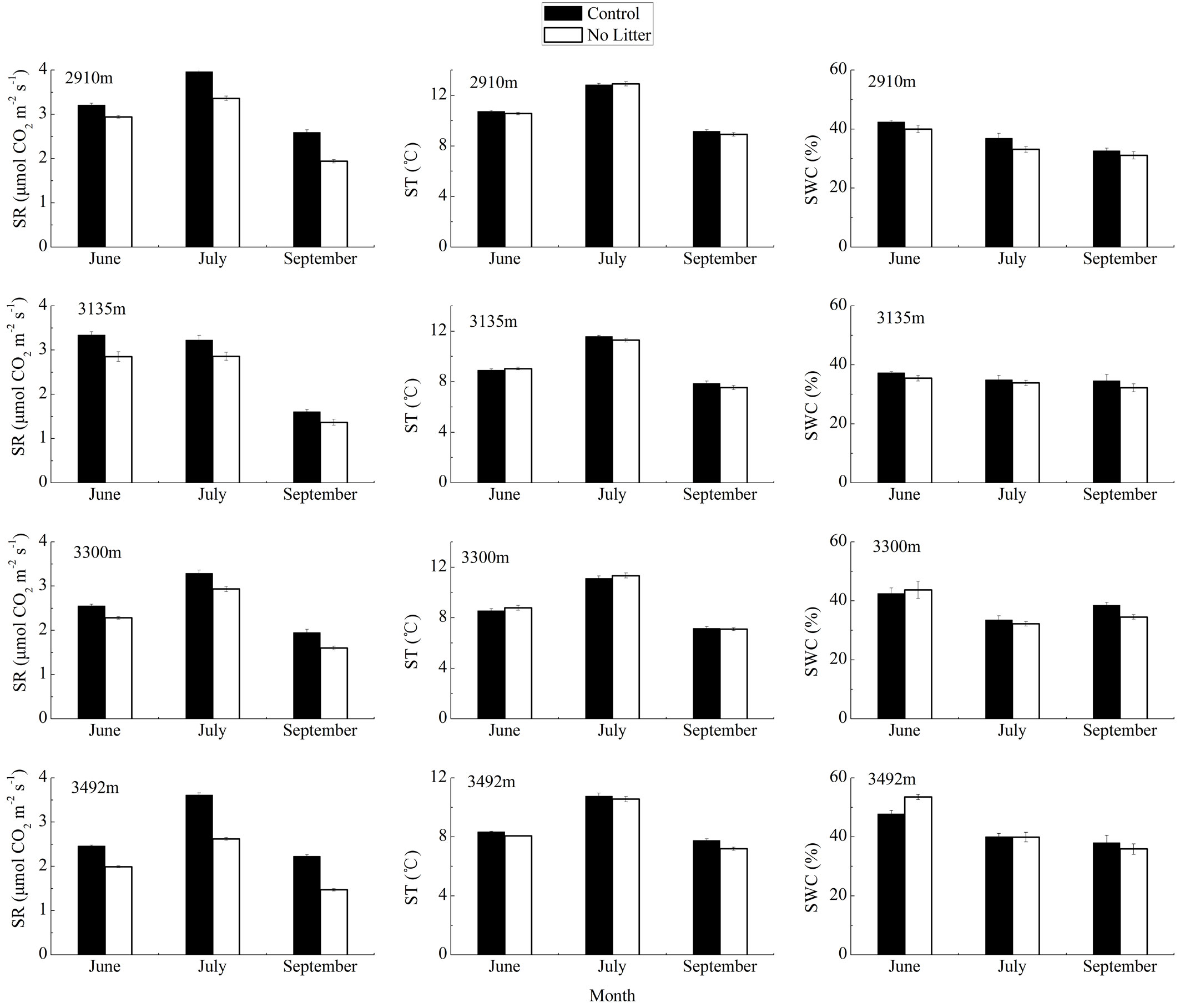
Temporal variation in soil respiration
At all elevations, soil respiration rate in both treatments showed the highest values in July 2011 and the lowest in September 2011. At each altitude, temporal variations in soil respiration rates were dependent on soil temperatures changes, but different from the changes in soil water content which reached its maximum in June 2010 (Fig. 3).
Fig. 3 - The temporal variation of soil respiration (SR, in μmol CO2 m-2 s-1), soil temperature (ST, in °C) and soil water content (SWC, in %) at each elevation, in the two treatments (Control and No Litter). Values are mean ± standard error (n=6 in June 2010; n=5 in July 2011; n=5 at all altitudes in September 2011, with the exception of 3492 m, n=6).
Correlation analysis was carried out at each elevation to reveal the relationship between soil respiration, soil temperature and water content using repeated measurements across all dates. Results showed that soil respiration was positively correlated with soil temperature (p<0.05) at all altitudes, but not significantly correlated with soil moisture in both treatments (p>0.05).
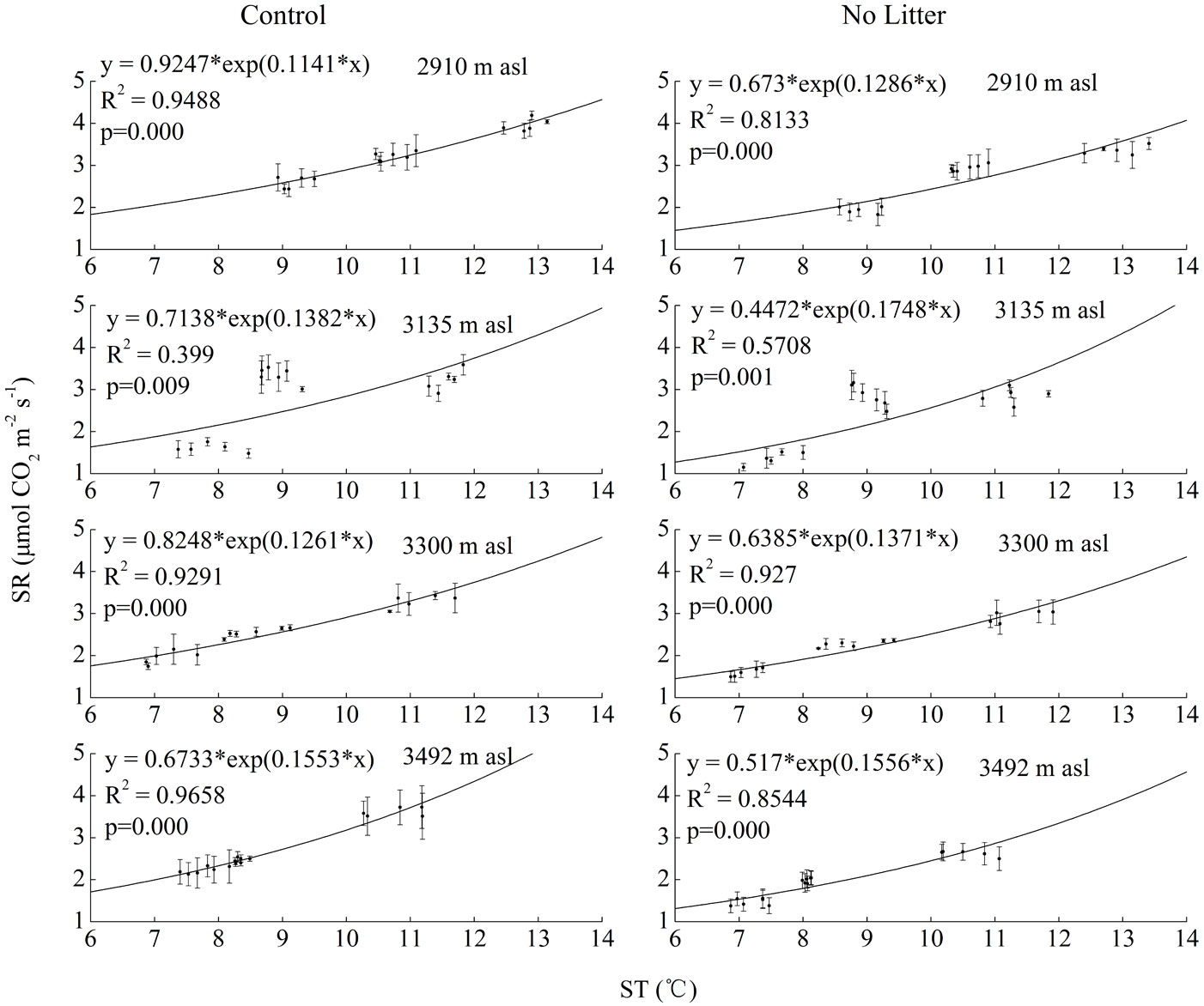
At each altitude, a significant exponential regression relationship between soil respiration and soil temperature both in Control and No Litter treatments was found (p<0.05). At 2910 m, 3135 m, 3300 m, 3492 m asl, soil temperature accounted for 94.9%, 39.9%, 92.9% and 96.6% of variation in soil respiration in the Control treatment, and 81.3%, 58.0%, 92.7% and 85.4% in the No Litter treatment (Fig. 4).
Fig. 4 - Results of regression analysis between soil respiration (SR, in μmol CO2 m-2 s-1) and soil temperature (ST, in °C) at each elevation, in the two treatments (Control and No Litter). Values are mean ± standard error (n=3).
Discussion
Dependence of soil respiration on temperature along the altitudinal gradient
In the present study, soil respiration decreased with elevation. Soil temperature accounted for most of the variation in soil respiration along the altitudinal gradient in both treatments. Our results showed that temperature changes associated with the altitudinal gradient could have a large impact on soil respiration. Many components of climate and local environment (including temperature and soil characteristics) vary along elevation gradients ([16]), leading to the variation in soil respiration. In recent years, several studies have used altitudinal gradients as an approach for testing the effect of environmental variables on soil respiration ([21], [40], [25], [31]). However, the results obtained up to date were conflicting, with no clear indications of increasing or decreasing trends in soil respiration along the gradient. Shi et al. ([25]) found that soil respiration rate decreased with elevation, whereas Wu et al. ([32]) concluded that soil CO2 emission increased with elevation. Zimmermann et al. ([40]) reported that soil respiration did not vary significantly across the full transect spanning almost 3000 m in elevation. Diverse results were probably related to the heterogeneity of sampling locations where the main factors regulating soil respiration were different.
Lower explanation rate at 3135 m a.s.l.
A significant exponential relationship between soil respiration and soil temperature at each elevation was found in this study. However, such correlation was weaker at 3135 m a.s.l. than at the other three altitudes, suggesting that other site-specific parameters other than soil temperature might be responsible for the observed variation in soil respiration. Soil properties also potentially affect soil respiration ([2]). Carbon (C) is known as the basic element used by soil microorganisms during the decomposition process ([26]). Despite that correlation between soil respiration and soil C content was not pronounced or was weakly significant in several field experiments ([4]), many measurements suggest that soil respiration had positive correlation with soil C content ([7], [27], [1], [23]). For example, Gough & Seiler ([7]) reported a weak positive relationship between soil CO2 efflux and percent soil C in loblolly pine plantations located in the South Carolina, USA. La Scala et al. ([23]) also reported that CO2 emissions in soil increases with carbon content of a tropical bare soil. Nitrogen (N) is one of the key factors for plant growth in terrestrial ecosystems ([11], [33]). Enrichment in N would stimulate soil respiration by increasing plant growth and the amount and decomposition of litter ([6]). However, previous studies have shown controversy results on impacts of N addition on soil respiration ([12], [9]). Specifically, Janssens et al. ([12]) reported soil carbon efflux declines following nitrogen addition either through fertilization or atmospheric nitrogen deposition. Haynes & Gower ([9]) also found a negative effect of N fertilization on soil respiration in red pine plantations in northern Wisconsin, USA. Although we did not attain sufficient data to statistically explain the relationship between soil respiration, soil C and N content in the present study, we analyzed soil properties of the experimental sites in June 2010, finding that total carbon (TC), soil organic matter (SOC), and total nitrogen (TN) were higher at the altitude of 3135 m than at the other three altitudes (Tab. 1). Therefore, we speculated that the variation in SR at 3135 m a.s.l. was more likely to be driven by a combination of soil properties such as TC, SOC and TN along with soil temperature, rather than being a consequence of soil temperature alone.
The effect of soil water content on soil respiration
Too high or too low soil moisture can limit soil respiration. Water saturation limits aeration and low soil moisture leads to desiccation, reduced substrate access or diffusion, which restricts microbial metabolism ([20]). The optimum soil water content is usually somewhere near the field capacity, when macropore spaces are mostly air-filled, thus facilitating O2 diffusion. When micropore spaces are mostly water-filled, the diffusion of soluble substrates is facilitated ([38]). In agreement with other studies carried out in the Miyaluo Forest District ([3], [39]), soil water content in the present study had no obvious influence on soil respiration either along the altitudinal gradient or at each elevation. Sufficient rain as well as rich water resources might be responsible for little influence of soil moisture on soil respiration in this area.
Litter contribution
Litter decomposition is an important component of soil respiration. Raich & Nadelhoffer ([17]) estimated that the aboveground litter inputs in many forest ecosystems contribute approximately 33% of the annual C loss through soil CO2 efflux, suggesting that the aboveground litter input exerts an important influence on soil C dynamics ([14]). Rey et al. ([20]) found that the contribution of aboveground litter decomposition ranged from 29% in the autumn, when rainfall was high and leaves started to fall, to 15% in the summer, when low soil water contents limited litter decomposition, in a coppice oak forest in Central Italy. Sulzman et al. ([28]) estimated that aboveground litter decomposition contributed 19% to soil respiration in an old growth coniferous forest located in the central Cascade Mountains, Oregon (USA). Similar to their estimates, the contribution of litter to soil respiration in our study averaged 17% ranging between 13% and 27%. Soil respiration was significantly reduced in the No Litter treatment compared to the Control treatment at all altitudes, suggesting that litter removal had a great effect on soil CO2 emission in the birch secondary forest. In Control and No Litter treatments, there was a significant linear regression relationship between soil respiration and soil temperature along the altitudinal gradient. At each altitude, there was a significant exponential regression relationship between them in both treatments. Therefore, litter removal did not change the soil respiration dependence from soil temperature.
Conclusions
We investigated the variation of soil respiration and its relationship with environmental factors along an altitudinal gradient in the birch secondary forest, for which limited data are available so far. Our results demonstrated that:
- soil respiration rate decreased with elevation;
- the soil temperature might be the dominant factor affecting soil respiration;
- the litter layer contribution to soil respiration averaged 17% in the birch secondary forest.
Our results suggest that the predicted increase in the atmospheric temperature might increase soil respiration in the birch secondary forest, at least in the summer season. However, the impact of an increase in temperature on the overall soil carbon budget of the forest should properly consider the whole annual C cycle, and the possible temperature-mediated effects on the nutrient cycle.
Acknowledgements
We would like to thank Bojie Fu, Yihe Lv and three anonymous reviewers for their valuable comments on earlier versions of the manuscript. We also thank Fei He and Dedong Zhou, Xuerong Li and Jinwei Liu for their help in the field. In addition, many thanks are owed to Debra Forthman and Changhong Su for their useful suggestions about language presentation. This work was supported financially by the Strategic Priority Research Program of Chinese Academy of Sciences (No. XDA05060100) and the National Science Foundation of China (No. 41071039 and No. 31000210).
References
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Guohua Liu
Zongshan Li
Li Gong
Meng Wang
State Key Laboratory of Urban and Regional Ecology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085 (China)
Li Gong
Meng Wang
Graduate University of Chinese Academy of Sciences, Beijing 100039 (China)
Institute of Geographical Sciences, Henan Academy of Sciences, Zhengzhou 450052 (China)
Institute of Botany, Chinese Academy of Sciences, Beijing 100093 (China)
Corresponding author
Paper Info
Citation
Luo S, Liu G, Li Z, Hu C, Gong L, Wang M, Hu H (2014). Soil respiration along an altitudinal gradient in a subalpine secondary forest in China. iForest 8: 526-532. - doi: 10.3832/ifor0895-007
Academic Editor
Giorgio Matteucci
Paper history
Received: Nov 16, 2012
Accepted: Sep 04, 2014
First online: Dec 01, 2014
Publication Date: Aug 02, 2015
Publication Time: 2.93 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2014
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 55908
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 46759
Abstract Page Views: 3538
PDF Downloads: 4108
Citation/Reference Downloads: 23
XML Downloads: 1480
Web Metrics
Days since publication: 4094
Overall contacts: 55908
Avg. contacts per week: 95.59
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 6
Average cites per year: 0.55
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
The manipulation of aboveground litter input affects soil CO2 efflux in a subtropical liquidambar forest in China
vol. 12, pp. 181-186 (online: 10 April 2019)
Research Articles
Spatial heterogeneity of soil respiration in a seasonal rainforest with complex terrain
vol. 6, pp. 65-72 (online: 07 February 2013)
Research Articles
Seasonal dynamics of soil respiration and nitrification in three subtropical plantations in southern China
vol. 9, pp. 813-821 (online: 29 May 2016)
Research Articles
Short-time effect of harvesting methods on soil respiration dynamics in a beech forest in southern Mediterranean Italy
vol. 10, pp. 645-651 (online: 20 June 2017)
Research Articles
Soil fauna communities and microbial activities response to litter and soil properties under degraded and restored forests of Hyrcania
vol. 14, pp. 490-498 (online: 11 November 2021)
Research Articles
Soil respiration and carbon balance in a Moso bamboo (Phyllostachys heterocycla (Carr.) Mitford cv. Pubescens) forest in subtropical China
vol. 8, pp. 606-614 (online: 02 February 2015)
Research Articles
Thinning effects on soil and microbial respiration in a coppice-originated Carpinus betulus L. stand in Turkey
vol. 9, pp. 783-790 (online: 29 May 2016)
Research Articles
Comparison of soil CO2 emissions between short-rotation coppice poplar stands and arable lands
vol. 11, pp. 199-205 (online: 01 March 2018)
Research Articles
Effect of different dolomitic limestone dosages on soil respiration in a mid-altitudinal Norway spruce stand
vol. 12, pp. 357-365 (online: 05 July 2019)
Research Articles
Wood-soil interactions in soil bioengineering slope stabilization works
vol. 2, pp. 187-191 (online: 15 October 2009)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword