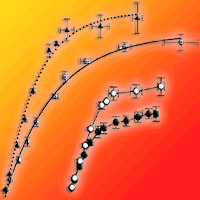
Three-year-old seedlings of two Tunisian provenances of cork oak (Quercus suber L.) differing in climatic conditions at their geographical origin were subjected to increasing light intensities. Gaâfour was the provenance from the driest site and Feija from the wettest site. Low-light adapted seedlings from both provenances were exposed to two light treatments: full sunlight (HL) and low light (LL, 15% sunlight) for 40 days. The CO2-response curve of leaf net photosynthesis (An-Ci curve) established under saturated photon flux density was used to compare photosynthetic parameters between leaves subjected to continuous low light (LL leaves) and leaves transferred from low to high light (HL leaves). Transfer from low to high light significantly increased net photosynthesis (An) and dark respiration (Rd) in Gaâfour provenance but not in Feija. After transfer to high irradiance, specific leaf area (SLA) did not change in either provenance. This suggested that the increase in photosynthetic capacity on a leaf area basis in HL leaves of Gaâfour provenance was not due to increased leaf thickness. Only the seedlings from the Gaâfour provenance were able to acclimate to high light by increasing Vcmax and Jmax.
Keywords
, , , ,
Citation
Rzigui T, Cherif J, Zorrig W, Khaldi A, Nasr Z (2017). Adjustment of photosynthetic carbon assimilation to higher growth irradiance in three-year-old seedlings of two Tunisian provenances of Cork Oak (Quercus suber L.). iForest 10: 618-624. - doi: 10.3832/ifor2105-010
Academic Editor
Silvano Fares
Paper history
Received: May 07, 2016
Accepted: Mar 06, 2017
First online: May 17, 2017
Publication Date: Jun 30, 2017
Publication Time: 2.40 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2017
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 51526
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 43118
Abstract Page Views: 3043
PDF Downloads: 3968
Citation/Reference Downloads: 62
XML Downloads: 1335
Web Metrics
Days since publication: 3209
Overall contacts: 51526
Avg. contacts per week: 112.40
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2017): 8
Average cites per year: 0.89
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Aranda I, Castro L, Pardos M, Gil L, Pardos JA (2005)Effects of the interaction between drought and shade on water relations, gas exchange and morphological traits in cork oak (
Quercus suber L.) seedlings. Forest Ecology and Management 210: 117-129.
CrossRef |
Gscholar
(2)
Balaguer L, Martinez-Ferri E, Pérez-Corona ME, Baquedano FJ, Castillo FJ, Manrique E (2001)Population divergence in the plasticity of the response of
Quercus coccifera to the light environment. Functional Ecology 15: 124-135.
CrossRef |
Gscholar
(3)
Bernacchi CJ, Singsass EL, Pimentel C, Portis AR, Long SP (2001)Improved temperature response function for models of Rubisco-limited photosynthesis. Plant, Cell and Environment 24: 253-259.
CrossRef |
Gscholar
(4)
Boardman NK (1977)Comparative photosynthesis of sun and shaded plants. Annual Review of Plant Physiology 28: 355-77.
CrossRef |
Gscholar
(5)
Brooks JR, Sprugel DG, Hinckley TM (1996)The effects of light acclimation during and after foliage expansion on photosynthesis of
Abies amabilis foliage within the canopy. Oecologia 107: 21-32.
CrossRef |
Gscholar
(6)
Calzavara AK, Bianchini E, Mazzanatti T, Oliveira HC, Stolf-moreira R, Pimenta JA (2015)Morphology and ecophysiology of tree seedlings in semideciduous forest during high-light acclimation in nursery. Photosynthetica 53: 597-608.
CrossRef |
Gscholar
(7)
Colin MO, Clive GJ (2001)Plants and resource mosaics: a functional model for predicting patterns of within-plant resource heterogeneity to consumers based on vascular architecture and local environmental variability. Oikos 94: 493-504.
CrossRef |
Gscholar
(8)
Evans JR, Poorter H (2001)Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant, Cell and Environment 24: 755-767.
CrossRef |
Gscholar
(9)
Evans JR, Seemann JR (1989)The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences, and control. In: “Photosynthesis” (Briggs WR ed). Liss, New York, USA, pp. 183-205.
Gscholar
(10)
Farquhar GD, Sharkey TD (1982)Stomatal conductance and photosynthesis. Annual Review of Plant Physiology 33: 317-345.
CrossRef |
Gscholar
(11)
Farquhar GD, Von Caemmerer S, Berry JA (1980)A biochemical model of photosynthetic CO
2 assimilation in leaves of C3 species. Planta 149: 78-90.
CrossRef |
Gscholar
(12)
Fernàndez MJ, Fleck I (2016)Photosynthetic limitation of several representative subalpine species in the Catalan Pyrenees in summer. Plant Biology 18 (4): 638-648.
CrossRef |
Gscholar
(13)
Ghouil H, Montpied P, Epron D, Ksontini M, Hanchi B, Dreyer E (2003)Thermal optima of photosynthetic functions and thermostability of photochemistry in crok oak seedlings. Tree Physiology 23: 1031-1039.
CrossRef |
Gscholar
(14)
Grassi G, Bagnaresi U (2001)Foliar morphological and physiological plasticity in
Picea abies and
Abies alba saplings along a natural light gradient. Tree Physiology 21: 959-967.
CrossRef |
Gscholar
(15)
Gratani L (2014)Plant phenotypic plasticity in response to environmental factors. Advanced in Botany 2014: 1-17.
CrossRef |
Gscholar
(16)
Hanba YT, Kogami H, Terashima I (2002)The effect of growth irradiance on leaf anatomy and photosynthesis in
Acer species differing in light demand. Plant, Cell and Environment 25: 1021-1030.
CrossRef |
Gscholar
(17)
Harley PC, Sharkey TD (1991)An improved model of C3 photosynthesis at high CO
2: reversed O
2 sensitivity explained by lack of glycerate re-entry into the chloroplast. Photosynthesis Research 27: 169-178.
Online |
Gscholar
(18)
Katahata SI, Naramoto M, Kakubari Y, Mukai Y (2007)Photosynthetic capacity and nitrogen partitioning in foliage of the evergreen shrub
Daphniphyllum humile along a natural light gradient. Tree Physiology 27: 199-208.
CrossRef |
Gscholar
(19)
Long SP, Bernacchi CJ (2003)Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany 54: 2393-2401.
CrossRef |
Gscholar
(20)
Makino A, Osmond B (1991)Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiology 96: 355-362.
CrossRef |
Gscholar
(21)
Matesanz S, Valladares F (2014)Ecological and evolutionary responses of Mediterranean plants to global change. Environmental and Experimental Botany 103: 53-67.
CrossRef |
Gscholar
(22)
Niinements U, Cescatti A, Rodeghiero M, Tosens T (2006)Complex adjustments of photosynthetic potentials and internal diffusion conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species
Quercus ilex. Plant, Cell and Environment 29: 1159-1178.
CrossRef |
Gscholar
(23)
Oguchi R, Hikosaka K, Hirose T (2003)Does the photosynthetic light-acclimation need change in leaf anatomy? Plant, Cell and Environment 26: 505-512.
CrossRef |
Gscholar
(24)
Oguchi R, Hikosaka K, Hirose T (2005)Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant, Cell and Environment 28: 916-927.
CrossRef |
Gscholar
(25)
Pandey S, Kushwaha R (2005)Leaf anatomy and photosynthetic acclimation in Valeriana jatamansi L. grown under high and low irradiance. Photosynthetica 43: 85-90.
CrossRef |
Gscholar
(26)
Patrick LD, Ogle K, Tissue DT (2009)A hierarchical Bayesian approach for estimation of photosynthetic parameters of C3 plants. Plant, Cell and Environment 32: 1695-1709.
CrossRef |
Gscholar
(27)
Pearcy RW, Sims AD (1994)Photosynthetic acclimation to changing light environments: scaling from the leaf to the whole plant. In: “Exploitation of Experimental Heterogeneity by Plants: Ecophysiological Process Above- and Below-ground” (Caldwell MM, Pearcy RW eds). Academic Press, San Diego, CA, USA, pp. 145-174.
CrossRef |
Gscholar
(28)
Price GD, Von Caemmerer S, Evans JR, Siebke K, Anderson JM, Badger MR (1998)Photosynthesis is strongly reduced by antisense suppression of chloroplastic cytochrome bf complex in transgenic tobacco. Australian Journal of Plant Physiology 25 (4): 445.
CrossRef |
Gscholar
(29)
Ramírez-Valiente JA, Valladares F, Sánchez-Gómez D, Delgado A, Aranda I (2014)Population variation and natural selection on leaf traits in cork oak throughout its distribution range. Acta Oecologica 58: 49-56.
CrossRef |
Gscholar
(30)
Ramirez-Valiente JA, Sanchez-Gómez D, Aranda I, Valladarres F (2010)phenotypic plasticity and local adaptation in leaf ecophysiological traits of 13 contrasting cork oak populations under different water availabilities. Tree Physiology 30: 618-627.
CrossRef |
Gscholar
(31)
Sharkey TD (1985)Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. The Botanical Review 51: 53-105.
CrossRef |
Gscholar
(32)
Sims DA, Pearcy WP (1992)Response of leaf anatomy and photosynthetic capacity in
Alocasia macrorrhiza (Araceae) to a transfer from low to high light. American Journal of Botany 79: 449-455.
CrossRef |
Gscholar
(33)
Sims DA, Seemann JR, Luo Y (1998)The significance of differences in mechanisms of photosynthetic acclimation to light, nitrogen and CO
2 for return on investment in leaves. Functional Ecology 12 (2): 185-194.
CrossRef |
Gscholar
(34)
Staudt M, Ennajah E, Mouillot F, Joffre R (2008)Do volatile organic compound emissions of Tunisian cork oak populations originating from contrasting climatic conditions differ in their responses to summer drought? Canadian Journal of Forest Research 38: 2965-2975.
CrossRef |
Gscholar
(35)
Sun Y, Zhu J, Sun OJ, Yan Q (2016)Photosynthetic and growth responses of
Pinus koraiensis seedlings to canopy openness: implications for the restoration of mixed-broadleaved Korean pine forests. Environmental and Experimental Botany 129: 118-126.
CrossRef |
Gscholar
(36)
Terashima I, Evans JR (1988)Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant and Cell Physiology 29: 143-155.
CrossRef |
Gscholar
(37)
Terashima I, Miyazawa SI, Hanba YT (2001)Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO
2 diffusion in the leaf. Journal of Plant Research 114 (1): 93-105.
CrossRef |
Gscholar
(38)
Vaz M, Pereira JS, Gazarini LC, David TS, David JS, Rodrigues A, Marocco J, Chaves MM (2010)Drought-induced photosynthetic inhibition and autumn recovery in two Mediterranean oak species (
Quercus ilex and
Quercus suber). Tree Physiology 30: 946-956.
CrossRef |
Gscholar
(39)
Watling JR, Press MC (2000)Infection with the parasitic angiosperm
Striga hermonthica influences the response of the C3 cereal
Oryza sativa to elevated CO
2. Global Change Biology 6: 919-930.
CrossRef |
Gscholar