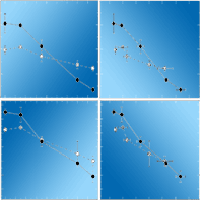
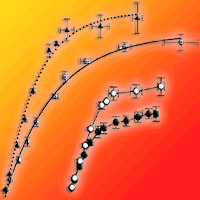
The main goal of this study was to obtain detailed information on photosynthetic responses of evergreen broad-leaved tree species to ozone (O3). For this, two-year-old seedlings of Castanopsis sieboldii, Quercus glauca, and Q. myrsinaefolia were grown for one growing season, from 15 May to 27 October 2014 under three levels of gas treatments, charcoal-filtered air and 1.0 time and 1.5 times ambient O3 concentrations. We analysed the intercellular CO2 concentration-response curve of the net photosynthetic rate, i.e., the A/Ci curve, in July and October, and growth measurement was carried out at the end of the experiment in October. We observed a difference in O3 susceptibility among the species. Negative effects of O3 were observed on the growth and photosynthetic traits of C. sieboldii, while no significant effects on these traits were noted in the two Quercus species. The decrease in light-saturated net photosynthetic rate (Asat) of C. sieboldii under elevated O3 was accompanied with a significant decrease in the maximum rate of carboxylation (Vcmax). Decreases of leaf nitrogen content and nitrogen use efficiency to Rubisco are considered as factors contributing to lower Vcmax in C. sieboldii seedlings under elevated O3. In addition to the decrease in Vcmax, O3 exposure induced marginal increase of stomatal limitation of photosynthesis. These results indicate that both biochemical and diffusion processes in photosynthesis are responsible for the decrease in Asat of C. sieboldii under elevated O3.
Keywords
, , , ,
Citation
Watanabe M, Kinose Y, Izuta T (2018). Photosynthesis of three evergreen broad-leaved tree species, Castanopsis sieboldii, Quercus glauca, and Q. myrsinaefolia, under elevated ozone. iForest 11: 360-366. - doi: 10.3832/ifor2493-011
Academic Editor
Silvano Fares
Paper history
Received: May 17, 2017
Accepted: Feb 25, 2018
First online: May 04, 2018
Publication Date: Jun 30, 2018
Publication Time: 2.27 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 48812
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41146
Abstract Page Views: 3368
PDF Downloads: 3325
Citation/Reference Downloads: 10
XML Downloads: 963
Web Metrics
Days since publication: 2838
Overall contacts: 48812
Avg. contacts per week: 120.40
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 4
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Bernacchi CJ, Singsaas EL, Pimentel C, Portis Jr AR, Long SP (2001)Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell and Environment 24: 253-259.
CrossRef |
Gscholar
(2)
Büker P, Feng Z, Uddling J, Briolat A, Alonso R, Braun S, Elvira S, Gerosa G, Karlsson PE, Le Thiec D, Marzuoli R, Mills G, Oksanen E, Wieser G, Wilkinson M, Emberson LD (2015)New flux based dose-response relationships for ozone for European forest tree species. Environmental Pollution 206: 163-174.
CrossRef |
Gscholar
(3)
Evans JR (1989)Photosynthesis and nitrogen relationships in leaves of C
3 plants. Oecologia 78: 9-19.
CrossRef |
Gscholar
(4)
Farquhar GD, Sharkey TD (1982)Stomatal conductance and photosynthesis. Annual Review of Plant Physiology 33: 317-345.
CrossRef |
Gscholar
(5)
Farquhar GD, Von Caemmerer S, Berry JA (1980)A biochemical model of photosynthetic CO
2 assimilation in leaves of C
3 species. Planta 149: 78-90.
CrossRef |
Gscholar
(6)
Hikosaka K, Terashima I (1995)A model of the acclimation of photosynthesis in the leaves of C
3 plants to sun and shade with respect to nitrogen use. Plant, Cell and Environment 18: 605-618.
CrossRef |
Gscholar
(7)
Hoshika Y, Watanabe M, Inada N, Koike T (2012)Growth and leaf gas exchange in three birch species exposed to elevated ozone and CO
2 in summer. Water, Air and Soil Pollution 223: 5017-5025.
CrossRef |
Gscholar
(8)
Hoshika Y, Watanabe M, Inada N, Koike T (2013a)Model-based analysis of avoidance of ozone stress by stomatal closure in Siebold’s beech (
Fagus crenata). Annals of Botany 112: 1149-1158.
CrossRef |
Gscholar
(9)
Hoshika Y, Watanabe M, Inada N, Mao Q, Koike T (2013b)Photosynthetic response of early and late leaves of white birch (
Betula platyphylla var.
japonica) grown under free-air ozone exposure. Environmental Pollution 182: 242-247.
CrossRef |
Gscholar
(10)
IUSS Working Group WRB (2015)World reference base for soil resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. World soil resources reports no. 106, FAO, Rome, Italy, pp. 192.
Online |
Gscholar
(11)
Jordan DB, Ogren WL (1984)The CO2/O2 specificity of ribulose 1.5-bisphosphate carboxylase/oxygenase. Dependence on ribulose bisphosphate concentration, pH and temperature. Planta 161: 308-313.
CrossRef |
Gscholar
(12)
Karlsson PE, Braun S, Broadmeadow M, Elvira S, Emberson LD, Gimeno BS, Le Thiec D, Novak K, Oksanen E, Schaub M, Uddling J, Wilkinson M (2007)Risk assessments for forest trees: the performance of the ozone flux versus the AOT concepts. Environmental Pollution 146: 608-616.
CrossRef |
Gscholar
(13)
Kinose Y, Azuchi F, Uehara Y, Kanomata T, Kobayashi A, Yamaguchi M, Izuta T (2014)Modeling of stomatal conductance to estimate stomatal ozone uptake by
Fagus crenata, Quercus serrata, Quercus mongolica var.
crispula and
Betula platyphylla. Environmental Pollution 194: 235-245.
CrossRef |
Gscholar
(14)
Kinose Y, Fukamachi Y, Okabe S, Hiroshima H, Watanabe M, Izuta T (2017)Photosynthetic responses to ozone of upper and lower canopy leaves of
Fagus crenata Blume seedlings grown under different soil nutrient conditions. Environmental Pollution 223: 213-222.
CrossRef |
Gscholar
(15)
Kitao M, Löw M, Heerdt C, Grams TEE, Häberle K-H, Matyssek R (2009)Effects of chronic elevated ozone exposure on gas exchange responses of adult beech trees (
Fagus sylvatica) as related to the within-canopy light gradient. Environmental Pollution 157: 537-544.
CrossRef |
Gscholar
(16)
Kitaoka S, Koike T (2004)Invasion of broad-leaf tree species into a larch plantation: seasonal light environment, photosynthesis and nitrogen allocation. Physiologia Plantarum 121: 604-611.
CrossRef |
Gscholar
(17)
Kohno Y, Matsumura H, Ishii T, Izuta T (2005)Establishing critical levels of air pollutants for protecting East Asian vegetation - A challenge. In: “Plant responses to air pollution and global change” (Omasa K, Nouchi I, De Kok LJ eds). Springer-Verlag, Tokyo, Japan, pp. 243-250.
CrossRef |
Gscholar
(18)
Lambers H, Chapin III FS, Pons TL (2008)Plant physiological ecology (2nd edn). Springer, New York, USA, pp. 605.
Gscholar
(19)
Li P, Calatayud V, Gao F, Uddling J, Feng Z (2016)Differences in ozone sensitivity among woody species are related to leaf morphology and antioxidant levels. Tree Physiology 36: 1105-1116.
CrossRef |
Gscholar
(20)
Long SP, Bernacchi CJ (2003)Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany 54: 2393-2401.
CrossRef |
Gscholar
(21)
Loreto F, Fares S (2013)Biogenic volatile organic compounds and their impacts on biosphere-atmosphere interactions. In: “Climate change, air pollution and global challenges: understanding and perspectives from forest research” (Matyssek R, Clarke N, Cudlin P, Mikkelsen TN, Tuovinen J-P, Wieser G, Paoletti E eds). Elsevier, Oxford, UK, pp. 57-75.
CrossRef |
Gscholar
(22)
McAinsh MR, Evans NH, Montgomery LT, North KA (2002)Calcium signalling in stomatal responses to pollutants. New Phytologist 153: 441-447.
CrossRef |
Gscholar
(23)
Mills G, Harmens H, Hayes F, Pleijel H, Büker P, Gonzáles-Fernández I, Alonso R, Bender J, Bergmann E, Bermejo V, Braun S, Danielsson H, Gerosa G, Grünhage L, Karlsson PE, Marzuoli R, Schaub M, Simpson D (2017)III. Mapping critical levels for vegetation. In: “Manual on methodologies and criteria for modelling and mapping critical loads and levels and air pollution effects, risks and trends” (ICP Modelling and Mapping ed). UNECE Convention on Long-range Transboundary Air Pollution, Geneva, Switzerland, pp. 1-66.
Online |
Gscholar
(24)
Naja M, Akimoto H (2004)Contribution of regional pollution and long-range transport to the Asia-Pacific region: analysis of long-term ozonesonde data over Japan. Journal of Geophysical Research 109: D21306.
CrossRef |
Gscholar
(25)
Nakanishi S, Ohba T, Takeda Y, Hattori T (1983)Illustration of vegetation in Japan. vol. I - Forest vegetation. Hoikusha Publishing Co. Ltd., Osaka, Japan, pp. 216.
Gscholar
(26)
Niinemets U, Tenhunen JD (1997)A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species
Acer saccharum. Plant, Cell and Environment 20: 845-866.
CrossRef |
Gscholar
(27)
Niinemets U, Tenhunen JD, Canta NR, Chavis MM, Faria T, Pereira JS, Reynolds JF (1999)Interactive effects of nitrogen and phosphorus on the acclimation potential of foliage photosynthetic properties of cork oak,
Quercus suber, to elevated atmospheric CO
2 concentrations. Global Change Biology 5: 455-470.
CrossRef |
Gscholar
(28)
Ohara T, Akimoto H, Kurokawa J, Horii N, Yamaji K, Yan X, Hatasaka T (2007)An Asian emission inventory of anthropogenic emission sources for the period 1980-2020. Atmospheric Chemistry and Physics 7: 4419-4444.
CrossRef |
Gscholar
(29)
Overmyer K, Kollist H, Tuominen H, Bets C, Langebartels C, Wingsle G, Kangasjärvi S, Brader G, Mullineauz P, Kangasjärvi J (2008)Complex phenotypic profiles leading to ozone sensitivity in
Arabidopsis thaliana mutants. Plant, Cell and Environment 31: 1237-1249.
CrossRef |
Gscholar
(30)
R Development Core Team (2017)R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, pp. 3520.
Online |
Gscholar
(31)
Takashima T, Hikosaka K, Hirose T (2004)Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous
Quercus species. Plant, Cell and Environment 27: 1047-1054.
CrossRef |
Gscholar
(32)
Tani A, Kawawata Y (2008)Isoprene emission from the major native
Quercus spp. in Japan. Atmospheric environment 42: 4540-4550.
CrossRef |
Gscholar
(33)
Tissue DT, Lewis JD (2010)Photosynthetic responses of cottonwood seedlings grown in glacial through future atmospheric [CO
2] vary with phosphorus supply. Tree Physiology 30: 1361-1372.
CrossRef |
Gscholar
(34)
Torsethaugen G, Pell EJ, Assmann SM (1999)Ozone inhibits guard cell K
+ channels implicated in stomatal opening. Proceedings of the National Academy of Sciences USA 96: 12577-12582.
CrossRef |
Gscholar
(35)
Vahisalu T, Puzõrjova I, Brosché M, Valk E, Lepiku M, Moldau H, Pechter P, Wang Y-S, Lindgren O, Salojärvi J, Loog M, Kangasjärvi J, Kollist H (2010)Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. The Plant Journal 62: 442-453.
CrossRef |
Gscholar
(36)
Von Caemmerer S (2000)Biochemical models of leaf photosynthesis. CSIRO Publishing, Australia, pp. 152.
Online |
Gscholar
(37)
Warren CR, Adams MA (2004)Evergreen trees do not maximize instantaneous photosynthesis. Trends in Plant Science 9: 270-274.
CrossRef |
Gscholar
(38)
Watanabe M, Yamaguchi M, Tabe C, Iwasaki M, Yamashita R, Funada R, Fukami M, Matsumura H, Kohno Y, Izuta T (2007)Influences of nitrogen load on the growth and photosynthetic responses of
Quercus serrata seedlings to O
3. Trees 21: 421-432.
CrossRef |
Gscholar
(39)
Watanabe M, Yamaguchi M, Matsumura H, Kohno Y, Izuta T (2008)Effects of ozone on growth and photosynthesis of
Castanopsis sieboldii seedlings grown under different nitrogen loads. Journal of Agricultural Meteorology 64: 143-155.
CrossRef |
Gscholar
(40)
Watanabe M, Ryu K, Kita K, Takagi K, Koike T (2012)Effects of nitrogen load on the growth and photosynthesis of hybrid larch F1 (
Larix gmelinii var.
japonica ×
L. kaempferi) grown on serpentine soil. Environmental and Experimental Botany 83: 73-81.
CrossRef |
Gscholar
(41)
Watanabe M, Hoshika Y, Inada N, Wang X, Mao Q, Koike T (2013)Photosynthetic traits of Siebold’s beech and oak saplings grown under free air ozone exposure. Environmental Pollution 174: 50-56.
CrossRef |
Gscholar
(42)
Watanabe M, Hoshika Y, Inada N, Koike T (2014)Canopy carbon budget of Siebold’s beech (
Fagus crenata) sapling under free air ozone exposure. Environmental Pollution 184: 682-689.
CrossRef |
Gscholar
(43)
Wittig VE, Ainsworth EA, Long SP (2007)To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last 3 decades of experiments. Plant, Cell and Environment 30: 1150-1162.
CrossRef |
Gscholar
(44)
Wittig VE, Ainsworth EA, Naidu SL, Karnosky DF, Long SP (2009)Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Global Change Biology 15: 396-424.
CrossRef |
Gscholar
(45)
Yamaguchi M, Watanabe M, Iwasaki M, Tabe C, Matsumura H, Kohno Y, Izuta T (2007)Growth and photosynthetic responses of
Fagus crenata seedlings to O
3 under different nitrogen loads. Trees 21: 707-718.
CrossRef |
Gscholar
(46)
Yamaguchi M, Watanabe M, Matsumura H, Kohno Y, Izuta T (2011)Experimental studies on the effects of ozone on growth and photosynthetic activity of Japanese forest tree species. Asian Journal of Atmospheric Environment 5: 65-87.
CrossRef |
Gscholar
(47)
Yamaji K, Ohara T, Uno I, Kurokawa J, Pochanart P, Akimoto H (2008)Future prediction of surface ozone over east Asia using models-3 community multiscale air quality modeling system and regional emission inventory in Asia. Journal of Geophysical Research 113: D08306.
Gscholar
(48)
Zhang W, Feng Z, Wang X, Niu J (2012)Responses of native broadleaved woody species to elevated ozone in subtropical China. Environmental Pollution 163: 149-157.
CrossRef |
Gscholar