Growth, spring phenology and stem quality of four broadleaved species assessed in provenance trials in the Netherlands - Implications for seed sourcing
iForest - Biogeosciences and Forestry, Volume 18, Issue 5, Pages 242-251 (2025)
doi: https://doi.org/10.3832/ifor4930-018
Published: Sep 22, 2025 - Copyright © 2025 SISEF
Research Articles
Abstract
Local seed sourcing from autochthonous tree populations is widely practiced for biodiversity conservation, as they are presumed to be adapted to local environmental conditions. Yet, autochthonous seed sources are not registered in the Netherlands for timber production, as these stands often exhibit poor stem quality due to their coppice history. Here, we study whether the performance of registered local seed sources from autochthonous origin (category “Source identified”) differs from local seed sources selected or improved for forestry purposes. We analyzed survival, growth, spring phenology, and stem form in four single-site provenance trials. In total, seeds from 74 provenances of four broadleaved species (Alnus glutinosa, Betula pubescens, Fagus sylvatica, and Quercus robur) were collected and planted in four provenance trials between 2008 and 2010. Provenance effects were consistently significant for budburst, whereas the effects on survival, growth, and stem form varied by species. Betula pubescens showed a 169% height difference between provenances at age 12, with several autochthonous provenances outperforming seed orchard material. In the other species, several autochthonous provenances performed equally well in terms of growth compared to seed sources in the “Tested” category. Differences in budburst timing were particularly evident in Fagus sylvatica and Quercus robur. In these trials, the autochthonous provenances were among the latest in flushing compared to the other local seed sources. Principal component analysis revealed strong correlations between growth and budburst, especially in Fagus sylvatica and Quercus robur, where earlier flushing was linked to greater height. This highlights potential trade-offs in selecting for both growth and late budburst. Overall, the study demonstrated that even within the ecologically uniform Dutch environment, genetic differences are present between local seed sources. We conclude that autochthonous provenances, despite exhibiting inferior phenotypic characteristics in situ due to past intensive silvicultural practice, can possess good growth and form characteristics, which makes them suitable for seed collection for both ecological and production purposes.
Keywords
Provenance Trials, Growth, Stem Form, Spring Leaf Phenology, Alnus glutinosa, Betula pubescens, Fagus sylvatica, Quercus robur
Introduction
The selection of seed sources used for producing forest reproductive material is of major concern for the vitality, productivity, and persistence of forests ([27], [58], [39]). To mitigate climate change and enhance forest resilience, there is a growing demand for seed sources for high-quality forest reproductive material (FRM) that is well-adapted and genetically resilient ([23]). However, seed sources can differ in their performance for multiple traits, including productivity characteristics such as survival and yield, as well as in their adaptability to environmental challenges, as shown by decades of provenance research ([1], [35]). Even within small geographic ranges, provenances may significantly differ in phenotypic traits ([30], [45], [62], [41], [11]). For example, Müller & Finkeldey ([45]) studied the performance of Fagus sylvatica seedlings from six provenances growing along a temperature and precipitation gradient in Northern Germany in a common garden experiment. Despite the small distances between seed origins, they found significant differences in height and mortality. However, precipitation at the original provenance location had no influence on the performance of the provenances. Similarly, Wunderlich et al. ([62]) found clear differences in growth between locally sourced provenances from the federal state of Baden-Württemberg in trials of Quercus robur and Picea abies in Germany. In their study a relationship between the altitude of origin and height growth was found mainly in Picea abies. Buras et al. ([11]) demonstrated soil-type-specific growth patterns in locally sourced provenances of Quercus robur in the Netherlands, highlighting the importance of soil conditions (e.g., clay versus sand) in evaluating growth performance. This indicates that not only broad geographic trends, but also small-scale variations in microclimate, soil conditions, or site history can drive local adaptation. These findings highlight the importance of carefully selecting provenance in forestry practices, even when sourcing seeds locally, to ensure the optimal growth and adaptability of forest stands. Local seed sources can be autochthonous, meaning that they are populations that have spontaneously colonized the area after the last glaciation or have been artificially propagated from material collected from the same population or within autochthonous populations in close proximity. These trees have grown and reproduced in a particular place for thousands of years ([15]). These populations are presumed to be characterized primarily by their adaptation to local environmental conditions and their unique genetic makeup ([34]). In the Netherlands, not all local seed sources are of autochthonous origin, and therefore, a distinction is made between autochthonous and other local seed sources of non-autochthonous or unknown origin. Forest reproductive material in the Netherlands is regulated under the Seeds and Planting Materials Act ([46]), which enforces Council Directive 1999/105/EC on the marketing of forest reproductive material across the EU. In the national register of approved basic material ([2]), the category “Source identified” is reserved exclusively for local material with autochthonous origin. These autochtho-nous planting materials are primarily used for ecological purposes, including nature restoration or landscaping (e.g., hedge rows) to support biodiversity conservation. While research on the impact of the origin of seed sources on interactions with associated biodiversity remains limited ([7]), there is some evidence that suggests that local seed sources play a key role in supporting pollinators and pathogen resistance ([32], [16], [8]). For instance, non-local provenances of Crataegus monogyna produced buds up to five weeks earlier than local ones, potentially disrupting the plant-Lepidoptera-bird food chain. Local provenances also exhibited the mildest mildew symptoms ([32]). Given the increasing demand for FRM in response to the new Dutch Forest strategy ([40]) - in which large-scale forest restoration plantings are foreseen -, selecting autochthonous planting material that simultaneously supports biodiversity conservation, landscape restoration, and high-quality wood production would be advantageous in the context of the Netherlands’ multifunctional forest management approach.
Autochthonous populations in the Netherlands are relatively scarce ([44]). This scarcity is primarily due to historical deforestation and intensive forest exploitation, large-scale land consolidation during the Middle Ages ([43]). By the beginning of the 19th century, the area of forest had been drastically reduced to only 4% of the total land area ([20]). This was driven by various factors, including the need for wood for construction, shipbuilding, fuel, metal smelting, oak bark for tanneries, as well as land conversion for agriculture and other uses ([9]). From the 1950s onwards, large-scale land consolidation and widespread use of foreign planting material in reforestation efforts further fragmented and declined autochthonous populations ([43]). Evidence for autochthonous status relies on a combination of historical information (e.g., ancient woodland indicators from old topographic maps), ecological site characteristics, and tree characteristics that indicate a high naturalness. For Quercus petraea and Quercus robur, chloroplast DNA haplotype analysis has also been employed to infer autochthony, as it allows a retrospective analysis of the impact of human-mediated seed transfer within a region ([10]). Although there is an interest in planting autochthonous provenances for production purposes, to date none of these seed stands have met the phenotypic criteria required for classification in the higher “Selected” category. As most autochthonous populations are found in ancient woodlands, defined in the Netherlands as forests that appear on the earliest topographical maps (c. 1850) and have existed continuously for several centuries, they show signs of old historical use, in particular coppicing. Coppice woodland was by far the most important forest type in the Netherlands until the beginning of the last century. With the decline of active coppice management, most coppice woodlands have either been converted to high forest or left unmanaged. In cases where conversion occurred, one shoot per stool was typically retained to form the basis of a new high forest ([9], [28]). However, the resulting timber quality was generally poor. The stems of coppice stools may grow straight up, but under the influence of drifting sand, grazing (e.g., by sheep) or sea wind, re-sprouted shoots often exhibit malformed growth ([42]). Today, the structural characteristics of these ancient woodlands still reflect their origins as former coppice systems, despite having undergone several decades of natural, unmanaged development. Due to the low stem quality of these stands, they are not suitable for registration in the category “Selected” nor for the selection of plus trees within these stands for seed orchard establishment. Because these poor phenotypic characteristics resulting from historical forest management practices, mainly coppicing, provide little information about the underlying genetic potential, this can only be assessed by research in provenance trials. But so far, few autochthonous provenances have been included in provenance trials in the Netherlands.
In this study, we evaluated the performance of registered Dutch local seed sources from four widespread European tree species, namely, Fagus sylvatica, Quercus robur, Alnus glutinosa, and Betula pubescens, in provenance trials. Fagus sylvatica and Quercus robur are very common and economically valuable forest tree species that have been extensively studied in earlier provenance trials ([30], [59], [51]). Alnus glutinosa and Betula pubescens are economically less important species in western Europe and have received little attention in provenance studies ([57], [33], [6], [49]). Both are pioneer species that are valued mainly for their ecological functions and abilities to grow under moist conditions ([5], [21]).
We aimed to compare local autochthonous seed sources of category “Source identified” (SI), known to have naturally regenerated and without phenotypic selection, with local planted seed sources in the category “Selected” (S) and in the improved categories “Tested” (T) and “Qualified” (Q) for productivity, stem quality, and budburst. All seed sources were from the Netherlands, which is considered a region of provenance. We were particularly interested in (i) assessing differences between local seed sources in spring leaf phenology, (ii) evaluating the genetic potential of autochthonous seed sources that exhibit poor stem form due to historic management, specifically former coppice, and (iii) identifying provenances that perform well for multiple traits. Ultimately, we provide recommendations for each species on which autochthonous provenances to use in Dutch forestry, taking into account both productivity and ecological objectives.
Material and methods
Plant material and experimental design
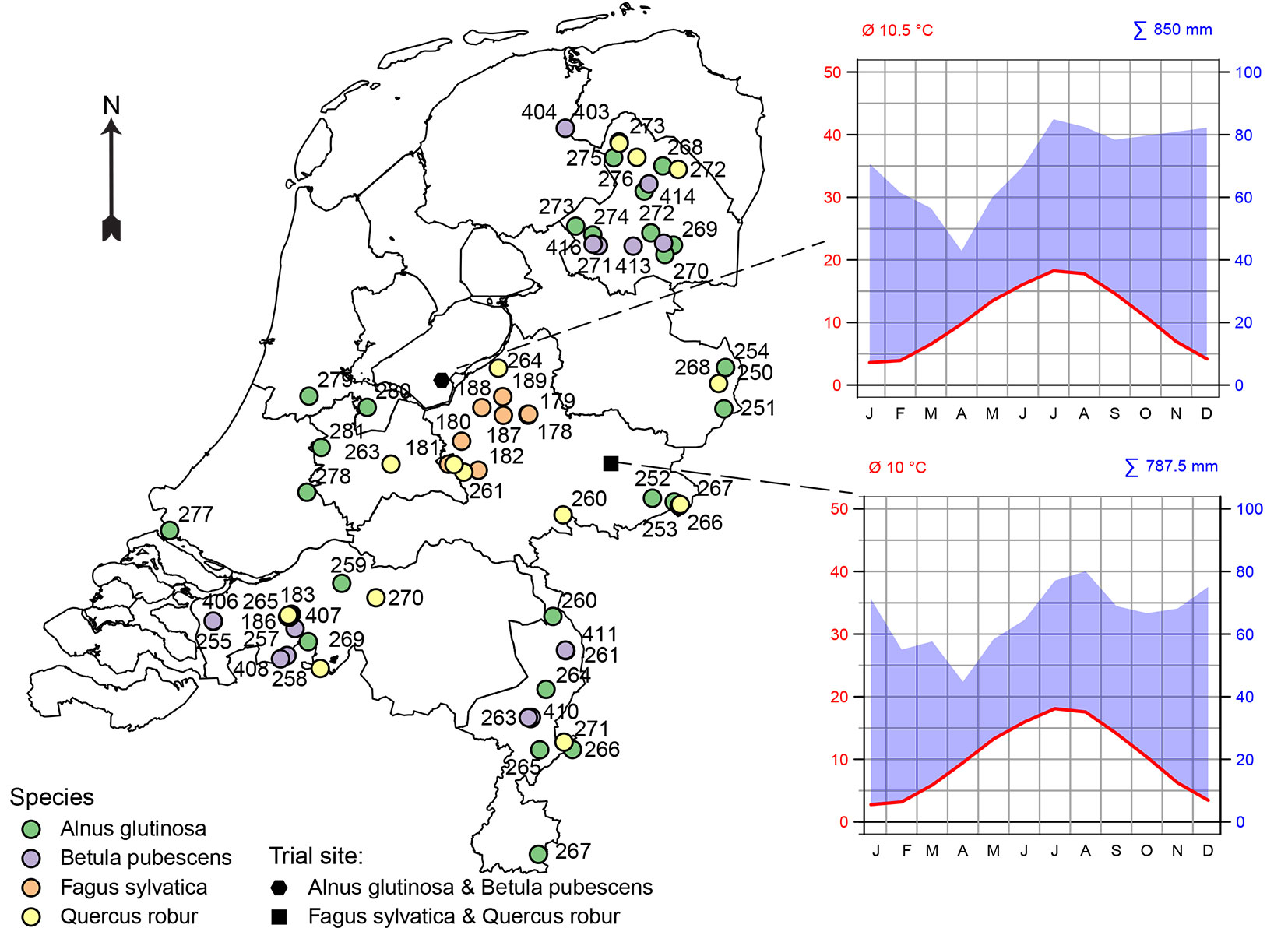
In total 74 provenances were selected for testing: 13 for Fagus sylvatica, 13 for Betula pubescens, 16 for Quercus robur, and 32 for Alnus glutinosa (Fig. 1, Tab. S1 in Supplementary material). The total number of seed sources tested varied by species, depending on the number of seed stands registered and the availability of seed harvests. All provenances were (formerly) registered seed sources of the Dutch register of approved basic material. These include seed stands of autochthonous origin in the category “Source Identified” and planted seed stands of unknown or non-autochthonous origin in the category “Selected” and “Tested” ([38]). The majority of the “Selected” and “Tested” seed stands of Quercus robur and Fagus sylvatica are roadside and estate plantations. For Betula pubescens also a seedling seed orchard (category “Qualified”) was included in the trial. The Netherlands does not distinguish different regions of provenance, so all seed sources in the trials were derived from the same region of provenance (NL). More detailed information about the origin of the provenances and their type of basic material is presented in Tab. S1 (Supplementary material).
Fig. 1 - Provenances included in the provenance trials and locations of the trials in the Netherlands, along with their Walter-Lieth climate diagrams (1990-2019).
The experiment was originally established as a randomised complete block design (RCBD), with three complete replicated blocks. Each block contains all provenances, randomly assigned to plots. Each plot initially consisted of 25 (5 × 5) trees of the same provenance. Each block had exactly as many plots as the number of provenances (32 in Alnus glutinosa, 14 in Betula pubescence, 13 in Fagus sylvatica and 16 in Quercus robur). However, due to limitations in plant material availability, for Quercus robur, Betula pubescens and Fagus sylvatica one or two provenances were represented in only two blocks, resulting in a slightly unbalanced and incomplete block design. The trials of Alnus glutinosa and Betula pubescens were located in the forest area Horsterwold, in the middle of the Netherlands (52° 20′ 34″ N, 05° 29′ 28″ E) on a clayey soil (Fig. 1). Here, two-year-old seedlings were planted at a spacing of 1.5 × 1.25 m in spring 2008. The trials of Quercus robur and Fagus sylvatica were established in the eastern part of the Netherlands (52° 04′ 20″ N, 06° 22′ 41″ E and 52° 04′ 03″ N, 06° 22′ 31″ E, respectively) on a sandy soil (Fig. 1). Here, two-year-old Quercus robur seedlings and Fagus sylvatica three-year-old seedlings were planted at a planting spacing of 1.5 × 1.5 m in spring 2008 and 2010, respectively. No thinning was performed in the trials before the measurements.
Both sites are characterized by a maritime climate with mean temperatures of approximately 10 °C and annual precipitation of 788 mm and 850 mm, respectively (Fig. 1). For each site, climate data from a nearby climate station (De Bilt, Twente, respectively) were downloaded from KNMI. The corresponding Walter-Lieth climate diagrams are depicted in Fig. 1.
Assessments
In all trials, survival, height, diameter at breast height (DBH), budburst, stem straightness, and forking were assessed (Tab. 1). Survival was assessed 11-14 years after planting. Tree height and DBH were measured for every surviving tree 10-12 years after planting (Tab. 1). Budburst was recorded 4 to 8 years after planting for each tree separately in 3 consecutive years following a 5-step scoring protocol: (1) dormant winter bud; (2) buds expanding; (3) budburst, first green is visible; (4) leaves are flushing; and (5) leaves are fully expanded. The assessments were conducted only once a year in the spring. The date of assessment was chosen so that a wide variation in budburst scores occurred among the trees in the trial. In the third year (2016) observations for beech were conducted too late in spring, resulting in a lack of observable variation. Consequently, these data were excluded from further analyses. Stem straightness and forking were assessed 11-14 years after planting. For straightness, a 5-step scale was used: (5) absolutely straight stem; (4) fairly straight (only one direction slightly crooked); (3) slight to moderate bends in different directions; (2) moderate to strong bends; and (1) no straight stem. Also forking was scored on a scale ranging from 5 to 1: (5) no fork; (4) branch with a similar angle; (3) fork(s) only in the upper half of the tree height; (2) fork(s) only in the lower half on the tree height; and (1) forks developed both in upper and in lower half of the tree height.
Tab. 1 - Summary of measured traits per species and age at measurement.
| Trial site |
Species | Trait | Age after planting (years) |
Year of assessment |
|---|---|---|---|---|
| Horsterwold | Alnus glutinosa | Survival | 14 | 2022 |
| Height | 11 | 2019 | ||
| Diameter | 11 | 2019 | ||
| Budburst | 5 | 2013 | ||
| Budburst | 6 | 2014 | ||
| Budburst | 8 | 2016 | ||
| Straightness | 14 | 2022 | ||
| Forks | 14 | 2022 | ||
| Betula pubescens | Survival | 14 | 2022 | |
| Height | 12 | 2020 | ||
| Diameter | 12 | 2020 | ||
| Budburst | 6 | 2014 | ||
| Budburst | 7 | 2015 | ||
| Budburst | 8 | 2016 | ||
| Straightness | 14 | 2022 | ||
| Forks | 14 | 2022 | ||
| Zelle | Quercus robur | Survival | 13 | 2021 |
| Height | 12 | 2020 | ||
| Diameter | 12 | 2020 | ||
| Budburst | 6 | 2014 | ||
| Budburst | 7 | 2015 | ||
| Budburst | 8 | 2016 | ||
| Straightness | 13 | 2021 | ||
| Forks | 13 | 2021 | ||
| Fagus sylvatica | Survival | 11 | 2021 | |
| Height | 10 | 2020 | ||
| Diameter | 10 | 2020 | ||
| Budburst | 4 | 2014 | ||
| Budburst | 5 | 2015 | ||
| Budburst | 6 | 2016 | ||
| Straightness | 11 | 2021 | ||
| Forks | 11 | 2021 |
Statistical analysis
All data analyses were performed with the statistical software R v. 4.2.0 ([48]). A significance level of α=0.05 was used for statistical testing. Each species had one trial located at a single site, and therefore the analyses were conducted separately for each species.
Field experiments are often subject to systematic spatial variation, e.g., due to soil gradients, microtopography, or water availability, which are typically not captured by blocking alone. Ignoring these spatial trends may lead to inflated error variance and biased provenance estimates. Instead of a linear model such as ANOVA with block as a factor, R package “SpATS” ([52]) was used to model spatial variation and adjust trait values for spatial noise. The model in SpATS was ran using the PSANOVA algorithm. This is a semi-parametric linear mixed model, which includes provenance as a fixed effect, and smooth spatial surfaces as random effects over the row and column coordinates of the plots. SpATS models both fixed provenance effects and spatial trends simultaneously, and can be summarised as (eqn. 1):
where the vector y contains the trait values averaged per plot (3 plot means per provenance), β is a vector of fixed terms including the intercept and provenance effects, and X is the respective design matrix. Xs βs represents linear fixed effects for row and column coordinates of each plot. Zs s is the random penalized component modelling the smooth spatial surface. Finally, Zu u is a random component that accounts for discontinuous spatial variation. More details of the model are provided in the original publication ([52]). Best Linear Unbiased Estimates (BLUEs) of the traits were obtained for each provenance, corrected for spatial trends. The SpATS package does not perform an overall test for provenance, therefore the R package ASReml-R ([12]) was used with a similar model as that of SpATS. With ASReml-R, a Wald test of Provenance was conducted. Pairwise comparisons based on t-tests were performed with the function LSD.test in the R package “Agricolae”, using the False Discovery Rate adjustment for multiple testing. Finally, provenances were classified into groups based on pairwise comparisons. For each trait, the input consisted of mean values per plot, computed from individual tree measurements. For height (m) and diameter (cm), directly measured continuous values were averaged per plot. For survival, we used the proportion of surviving trees per plot. For ordinal traits such as budburst, forks, and straightness, individual scores (1-5) were averaged per plot, resulting in continuous values suitable for linear models. These plot averages were used as input for SpATS, along with the corresponding plot coordinates. Pearson’s correlation coefficient (r) was applied to provenance mean values to evaluate the consistency of budburst scores for each provenance across different years. A principal component analysis (PCA) was performed, using the R function prcomp to describe the tested provenances and associations between the traits. PCA is a dimensionality reduction method that converts many correlated traits into a smaller number of uncorrelated variables called principal components, allowing a summarized view of traits and provenances. As input for PCA, adjusted means from SpATS were used. In traits measured over multiple years, a single year was selected. For example, for budburst, the year that displayed the largest variation between provenances was chosen for the PCA. Biplots were produced to visualise the relationships between traits and the relative performance of the provenances. Finally, scatterplots were produced to give an indication of which provenances perform best for three traits representing budburst, growth (height or diameter) and form or survival (x-axis versus y-axis and symbols divided into tertiles). As survival, growth, form and late-flushing are all important characteristics for selecting seed sources, three traits with a significant provenance test out of these were selected.
Results
Test for provenance effect
The Wald test for provenance showed that provenance effects were significant for all traits (α=0.05), although not for all species-trait combinations (Tab. 2). The estimated means for all provenances and traits are given in Tab. S2-S5 (Supplementary material). Below, the results are described in more detail per trait and species.
Tab. 2 - Results of the Wald test of provenance effect for the six traits per species. (Trait): each trait-age combination; (Wald Statistics): Wald test for provenance effect; (DF): degrees of freedom; (P-value): Probability value; (*): P < 0.05; (**): P<0.01; (***): P<0.001; (ns): non-significant.
| Species | Trait | Wald Statistics |
DF | P-value |
|---|---|---|---|---|
| Quercus robur | Survival13 | 48.5 | 15 | *** |
| Budburst6 | 194.3 | 15 | *** | |
| Budburst7 | 189.4 | 15 | *** | |
| Budburst8 | 189.4 | 15 | *** | |
| Height12 | 21.6 | 15 | ns | |
| Diameter12 | 40.4 | 15 | *** | |
| Straightness13 | 115.2 | 15 | *** | |
| Forks13 | 15.6 | 15 | ns | |
| Fagus sylvatica | Survival11 | 20.3 | 12 | ns |
| Budburst4 | 327.1 | 12 | *** | |
| Budburst5 | 339.4 | 12 | *** | |
| Height10 | 34.3 | 12 | *** | |
| Diameter10 | 33.3 | 12 | *** | |
| Straightness11 | 14.5 | 12 | ns | |
| Forks11 | 47.8 | 12 | *** | |
| Alnus glutinosa | Survival14 | 47.9 | 31 | ** |
| Budburst5 | 60.6 | 31 | ** | |
| Budburst6 | 89.1 | 31 | *** | |
| Budburst8 | 68.5 | 31 | *** | |
| Diameter11 | 39.2 | 31 | ns | |
| Height11 | 61.9 | 31 | *** | |
| Straightness14 | 85.9 | 31 | *** | |
| Forks14 | 93.5 | 31 | *** | |
| Betula pubescens | Survival14 | 32.8 | 13 | *** |
| Budburst6 | 64.9 | 13 | *** | |
| Budburst7 | 108.1 | 13 | *** | |
| Budburst8 | 70.7 | 13 | *** | |
| Height12 | 174.2 | 13 | *** | |
| Diameter12 | 259.9 | 13 | *** | |
| Straightness14 | 20.1 | 13 | ns | |
| Forks14 | 15.8 | 13 | ns |
Survival
The overall survival rates per species ranged between 0.48 and 0.90 (Tab. S2-S5). Higher survival rates were recorded in the Fagus sylvatica trial, with an adjusted trial mean of 90% and a range of 77-96% at the age of 11 years after establishment. The Alnus glutinosa and Quercus robur trials showed mean survival rates of 73% at 14 years and 57% at 13 years, respectively. The Betula pubescens trial exhibited the lowest overall survival rate, with a mean of 48% at 14 years, and none of the provenances exceeding 70% survival. The Quercus robur, Alnus glutinosa and Betula pubescens trials showed a significant provenance effect for survival rates. Though, pairwise comparisons based on the LSD measure between provenances after correction for multiple testing did not show significant differences in survival rates among provenances. For the Fagus sylvatica trial there was no significant provenance effect (Tab. 2).
Growth (height and diameter)
Growth differed between species, with Alnus glutinosa displaying the highest growth, achieving a mean height of 10.3 m at age 11 (Tab. S2-S5). This was followed by Betula pubescens with a height of 7.4 m at age 12, while Fagus sylvatica and Quercus robur exhibited the lowest heights of 6.0 m at age 10 and 6.4 m at age 12, respectively. Significant provenance effects for height and diameter were found, except for diameter in the Alnus glutinosa trial and for height in the Quercus robur trial (Tab. 2). Pairwise differences were most pronounced in Betula pubescens, also between autochthonous provenances. Provenance 410 (category SI), attained the highest growth rates with a height of 10.8 m and a diameter of 11.3 cm. The seed orchard trees, included in the trial as two different seed harvest years (403 and 404), ranked among the lowest-performing provenances in growth. In the Alnus glutinosa trial, height ranged from 9.5 m (provenance 268) to 10.9 m (provenance 277), with pairwise comparisons indicating non-significant differences between provenances. Autochthonous seed sources generally exhibited growth comparable to provenances categorized as “Selected” and “Tested”. In the Fagus sylvatica trial, the three autochthonous seed sources (187, 188, and 189 - category SI) showed the lowest growth performance in both height and diameter, although differences between provenances were not significant after adjustment for multiple testing. Among the Quercus robur provenances, the “Selected” and “Tested” categories generally showed the highest diameter growth.
Budburst
Budburst scores showed strong correlation between years, particular in Quercus robur and Fagus sylvatica, indicating high temporal consistency among provenances. For example, for Quercus robur, the correlation between 2014 and 2015, and 2015 and 2016 was r = 0.96 and for 2014-2016 r = 0.97. Less stronger correlations were observed for Alnus glutinosa and Betula pubescens (Tab. 3). Budburst had a clear provenance effect for all species (Tab. 2), though for Alnus glutinosa and Betula pubescens provenances, there was minimal variation in budburst. Although statistically significant differences among specific provenances were detected in some years, the mean budburst rates for the provenances fell within a comparable range (Betula pubescens: 3.0-3.9 in 2016; Alnus glutinosa: 3.5-4.6 in 2015). In contrast, the differences in budburst were more pronounced in the Quercus robur and Fagus sylvatica trials. Here the later-flushing provenances were typically from autochthonous origin (SI category - Tab. S4-S5 in Supplementary material). In the Fagus sylvatica trial, the provenances with later budburst times included the autochthonous provenances 187, 188, and 189 (SI category), followed by the three provenances 177, 178, 179, two of which have the qualification “Tested”. The earliest flushing was observed among the other “Selected” provenances. In the Quercus robur trial, the majority of autochthonous provenances exhibited significantly later budburst than the three “Tested” provenances (260, 261, 262 - Tab. S5 in Supplementary material).
Tab. 3 - Pearson’s correlation coefficient (r) between years for the budburst stage using provenance mean values.
| Species | Budburst stage | ||
|---|---|---|---|
| year 1 × year 2 | year 2 × year 3 | year 1 × year 3 | |
| Alnus glutinosa | 0.67 | 0.5 | 0.59 |
| Betula pubescens | 0.52 | 0.85 | 0.57 |
| Fagus sylvatica | 1 | - | - |
| Quercus robur | 0.96 | 0.96 | 0.97 |
Stem straightness and forking
In Betula pubescens, no significant provenance effect for straightness (p > 0.05) and forking (p > 0.05) was detected (Tab. S3). Forking scores ranged from 3.7 to 4.4, and stem straightness scores ranged from 2.6 to 3.1, indicating that most provenances performed well in terms of form. In the Alnus glutinosa trial there was a significant provenance effect (P < 0.001) for both form traits (Tab. 2). The overall trial mean for forking was 4.4, with small differences among provenances (Tab. S2). For stem straightness, scores ranged from 2.9 to 3.7, with most provenances demonstrating reasonably good straightness, comparable to the provenance 250 (Denekamp-01, category “Tested”). Only, four autochthonous provenances (255, 259, 271, 278) exhibited significantly poorer straightness than Denekamp-01 (Tab. S2 in Supplementary material). In general, the forking scores in the Quercus robur and Fagus sylvatica trials were lower than those observed in the Alnus glutinosa and Betula pubescens trials. In the Quercus robur trial, with scores for forking ranging from 2.7 to 3.8, no significant provenance effect was detected (Tab. S5). Concerning straightness, differences were more pronounced, with the “Tested” and “Selected” provenances ranking among the highest. The straightness scores for the autochthonous provenances varied, ranging from 3.1 (provenance 270) to 2.0 (provenance 275), the latter being the least straight. In the Fagus sylvatica trial, a provenance effect was observed for forking but not for straightness (Tab. 2). The mean forking score across all provenances was 2.7 (Tab. S4). Also, the overall mean for straightness across provenances was relatively low (2.7) and no significant differences between provenances were found (Tab. S4 in Supplementary material).
PCA of all traits
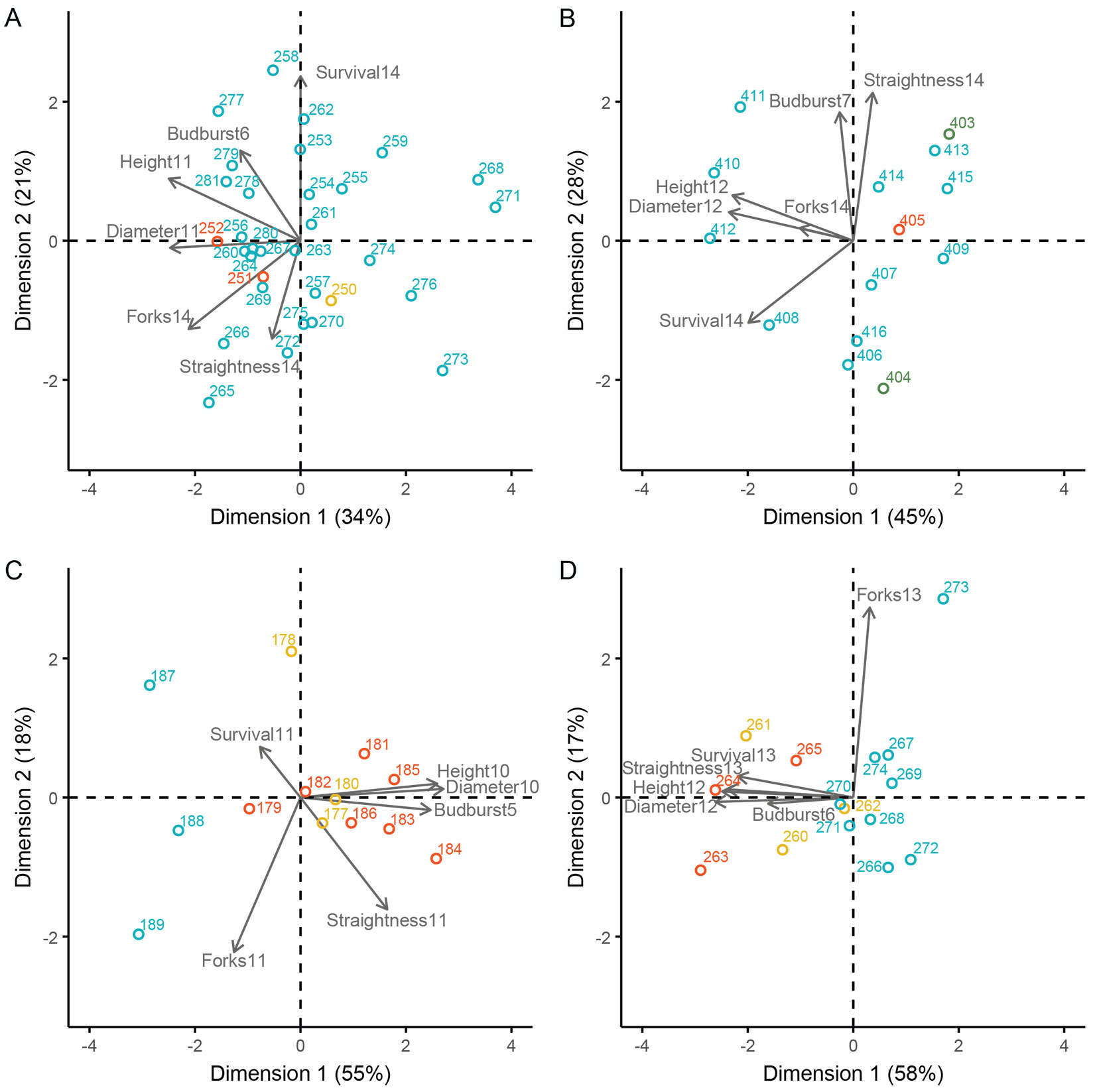
The results of the PCA indicated that growth traits (height and diameter) along the first principal component (PC1) had the most significant influence on determining variation among provenances for all species (Fig. 2). In the case of Alnus glutinosa, the first two principal components explained 55% of the total variation, with PC1 accounting for 33.7% and PC2 for 21.3% (Fig. 2A). The analysis demonstrated that provenances exhibiting good growth and early budburst were distinguished along the x-axis from those with lower growth performance and later flushing. Along PC2, negatively correlated vectors for straightness and survival were found (Fig. 2A). In Betula pubescens, the first two principal components accounted for 72.2% of the variation (PC1 = 44.5%, PC2 = 27.7% - Fig. 2B). The second component was primarily explained by straightness and budburst, with a high straightness being associated with early budburst. For Fagus sylvatica, PC1 accounted for 54.9% of the total variation, indicating that provenances with high growth also exhibited early budburst (Fig. 2C). The second component accounted for 17.9% of the variation, where straightness and forking were most important. In Quercus robur, the first two components explained 74.3% of the variation (PC1 = 57.7%, PC2 = 16.6% - Fig. 2D). The second component was mainly associated with forking, which did not correlate with any of the other traits (Fig. 2D).
Fig. 2 - Plots of provenance scores for principal components 1 and 2 (PC1 vs. PC2) for the four species. The color of the points represent the category of FRM of the provenance (SI = blue, S = red, T = yellow, Q = green). The vectors correspond to the direction and contribution in the plot of the six traits. For provenance IDs and trait abbreviations see Tab. 2and Tab. S1 in Supplementary material. (A) Alnus glutinosa, (B) Betula pubescens, (C) Fagus sylvatica, (D) Quercus robur.
Selecting provenances
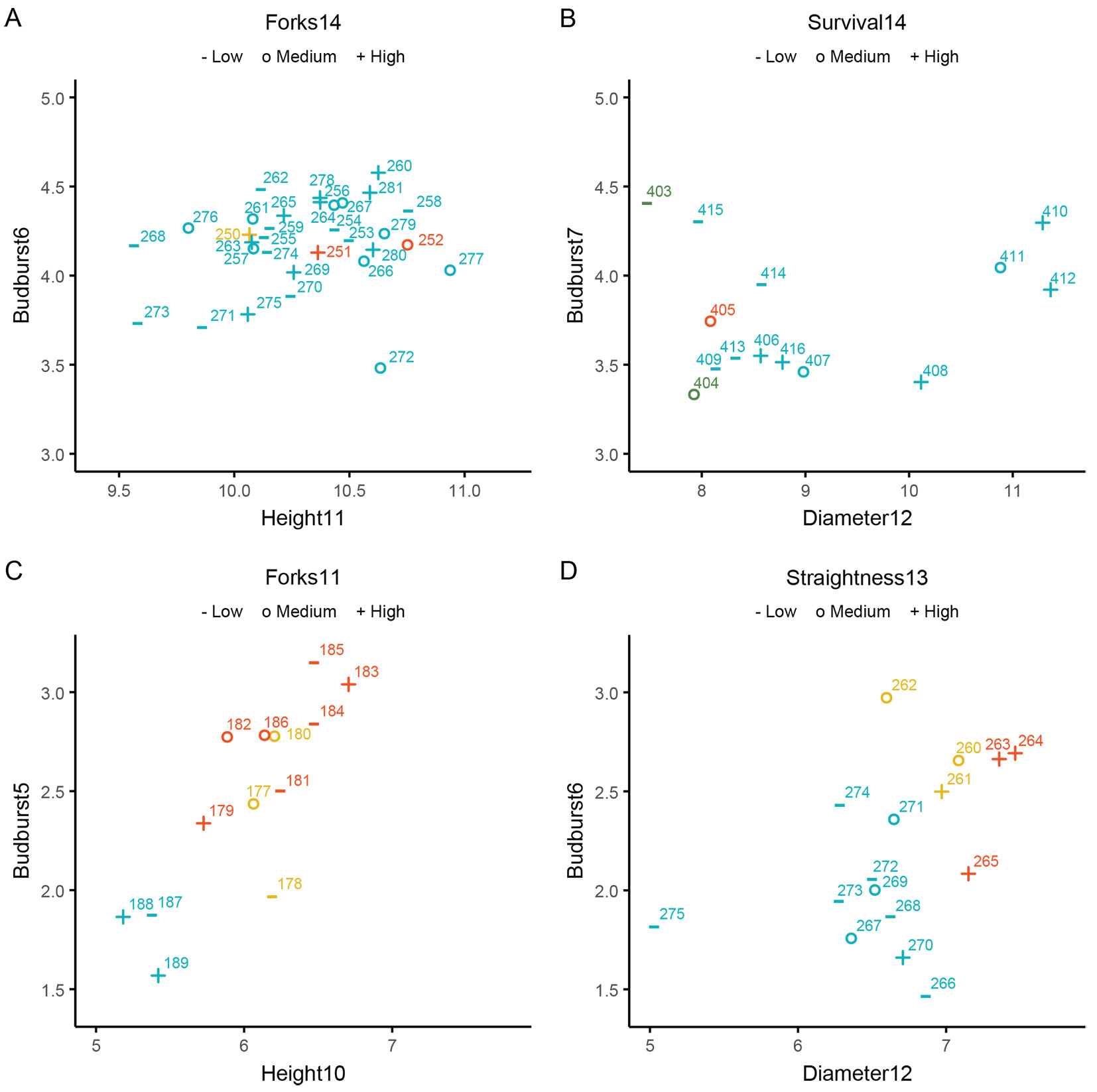
For all species, height and diameter were highly correlated. Additionally, the form traits straightness and forks were correlated with each other, except in the Quercus robur trial. For all species, one of the growth traits was consistently included as one of the traits (x-axis), along with budburst (y-axis). The third trait varied across species. Fig. 3C shows that for Fagus sylvatica, provenances demonstrating superior height growth often have earlier budburst. The autochthonous provenances (cat. SI) are in the lower-left quadrant of the plot, indicating they are later flushing but also have less growth. Forking does not correspond with this relationship, as provenances with high fork class scores (represented by red dots) show varied growth and budburst patterns. In Quercus robur, unlike in Fagus sylvatica, provenances with higher growth (as indicated by diameter) do not have earlier budburst. Autochthonous provenances, most of which flush later, do not cluster in the lower-left quadrant of the plot, but instead exhibit different diameter growth values. Provenances in the category “Selected” and “Tested” are in the upper right quadrant of the plot, indicating that they have in general higher growth and are earlier flushing. Moreover, the coding (plus sign) for straightness suggests they also perform best for straightness. Provenance 275 exhibits remarkably low rates for the combination of diameter growth and straightness compared to the other provenances, while the two autochthonous provenances 265 and 270 perform well for all three traits (Fig. 3D). In the case of Alnus glutinosa, provenances are scattered throughout the plot. It also shows that many taller provenances had also earlier budburst. The “Tested” provenance 250 (Denekamp-01) is situated in the centre for growth and budburst, but with higher forking performance. The scatterplot also shows that there are several autochthonous provenances that perform similar or better than Denekamp-01 for all three traits (for example 263, 269, 275, 280). For Betula pubescens, the plot illustrates that provenances with larger diameters also tend to have higher survival rates, but differ in budburst. The seed orchard seed lots (403, 404) and the “Selected” provenance 405 are among the worst performers.
Fig. 3 - Scatter diagrams of the four species. (A) Alnus glutinosa, (B) Betula pubescens, (C) Fagus sylvatica, (D) Quercus robur. Numbers represent provenance IDs. The color of the symbols represent the category of FRM of the provenance (SI = blue, S = red, T = yellow, Q = green). For provenance ID codes and trait abbreviations see Tab. 2and Tab. S1 in Supplementary material. Higher values for budburst indicate earlier budburst.
Discussion
Variation in survival and growth
Significant provenance effects for survival were observed in Quercus robur, Alnus glutinosa and Betula pubescens, but not in Fagus sylvatica. Also, survival rates varied among species, with Fagus sylvatica exhibiting the highest survival (trial mean 90%) and Betula pubescens the lowest (48%). This suggests that Fagus sylvatica may be better suited to the trial site conditions. Knowing that birches are sensitive to transplanting ([4]), the lower survival in Betula pubescens is probably partly due to a transplanting shock. Betula pubescens trees have a preference for cool, moist soils but face challenges in hot, dry conditions due to their shallow root systems ([47], [5]). Due to their shallow root system, also site-specific management issues such as competition with vegetation can play a role. In contrast, the open conditions of a provenance trial may not reflect the natural forest environment where this species typically thrives.
Growth differences (height or diameter) were found in all species, but the most pronounced provenance effect was observed in Betula pubescens, where the tallest provenance was nearly twice as tall as the shortest, a height difference of 5.2 meters at age 12. Interestingly, four of the eleven autochthonous provenances (cat. SI) in this species outperformed seed orchard and “Selected” materials. In the other species pairwise comparisons showed that autochthonous provenances (cat. SI) exhibit in many cases comparable growth as provenances in the higher categories (S and T). These findings suggest potential for selection within “Source identified” material to use for forests with wood production function.
Differences in spring leaf phenology
Significant provenance effects for budburst were evident in all four species, with the clearest differentiation in timing of budburst and consistency over years observed in Fagus sylvatica and Quercus robur. Autochthonous Fagus sylvatica provenances and most autochthonous Quercus robur provenances showed the latest budburst among the studied provenances. This is noteworthy since the “Tested” Fagus sylvatica provenances (177, 178 and 180) and in particular the “Tested” oak provenance Ede-01 (260), which is an accepted standard, were in earlier trials explicitly selected for their relatively late budburst ([36], [37], [38]). However, in these trials seed sources were recommended as “Tested” based on comparisons with seed sources from 20th century plantation forests and roadside plantations, and occasionally foreign material ([37]), but not with autochthonous seed sources. Late budburst is generally expected in Dutch autochthonous populations due to local adaptation, as delayed flushing reduces the risk of frost damage ([26], [60]). In the Netherlands late frost events occur occasionally, which makes late budburst an important selection criterium for these species. On a broader range-wide scale, longitudinal trends in Fagus sylvatica budburst have been observed, with Northern and Western European provenances generally flushing later than those from Southeast Europe ([60], [29], [25], [50]). In these European-wide trials Dutch autochthonous Fagus sylvatica provenances (e.g., Aarnink, Elspeet) have consistently been recorded as among the latest to flush ([25], [50], [22]). Similarly, Dutch Quercus robur provenances also exhibit late flushing in comparisons of provenances at a regional scale across Northwestern Europe, as confirmed in provenance trials in Ireland ([24]), Denmark ([31]) and Southern England ([61]). For Quercus robur, Wilkinson et al. ([61]) found that the southern provenances were always earlier than those from more northern latitudes. For Alnus glutinosa and Betula pubescens differences between provenances were less pronounced. Early successional species, such as Alnus glutinosa and Betula pubescens, generally have earlier budburst than the mid- to late-successional species Quercus robur and late-successional Fagus sylvatica ([13]). Despite its early budburst, Alnus glutinosa is reported to be relatively tolerant to spring frost ([56], [14]). Therefore, the selective pressure of spring frost on populations in this species may be weaker than in more frost-sensitive species, leading to less differentiation in budburst timing among provenances. Moreover, budburst was not considered in the only provenance trial of Alnus glutinosa previously conducted in the Netherlands ([57]). Apparently, budburst was not seen as an important trait to select for, while good growth and stem form were. Neither is there much literature on provenance differences for budburst on European or regional scale. Baliuckas & Pliura ([3]) assessed budburst of Alnus glutinosa in field trials located in different eco-geographic regions of Lithuania, finding a southeast-northwest clinal variation pattern. Though, the most comprehensive common garden experiment performed in Pennsylvania (USA) with 48 Alnus glutinosa provenances from the European range, showed that populations were rather uniform in budburst timing and no clear pattern of climate differences was detected ([19]). Studies for Betula pubescens are even scarcer. Billington & Pelham ([6]) examined seven natural Scottish Betula pubescens populations in a single progeny test and found significant differences among populations and among families within populations.
Provenance differences at a small geographic scale
Despite the small geographic scale of our sampling, using provenances sourced exclusively from the Netherlands, we detected modest, yet significant provenance effects across most species-trait combinations, highlighting genetic differentiation even within a relatively climatologically and ecologically uniform lowland area as the Netherlands. While local adaptation was not assessed, the observed genetic differentiation in budburst and growth, both key adaptive traits, at such a small geographic scale is interesting. Adaptive genetic differentiation in European forest species is driven by environmental gradients such as temperature, precipitation, soil composition, and altitude. The Netherlands lacks substantial environmental gradients, as climatological or altitudinal variation is low, comparable to the lowland regions of Belgium (Flanders) or Denmark. Indeed, De Kort et al. ([17]) analyzed Alnus glutinosa populations in conventional provenance trials combined with population genomics but found very limited evidence of adaptation at the scale of Belgian provenance regions. On the other hand, Jensen ([30]) demonstrated that even within the relatively small region of Jutland, significant genetic variation in flushing time exists among native oak provenances, strongly influenced by proximity to the coast. In another study in Denmark, Lobo et al. ([41]) found genetic differentiation at a fine scale in phenology in six woody species, including Betula pubescens. They demonstrated that local populations can be genetically differentiated, even when separated by distances of only 10 to 35 km, in areas with low altitude variation and spring temperatures that vary by only 1 °C to 2.5 °C. As the observed variation could only be partly explained by the climate at the site of origin, they suggested it is likely a product of both natural selection and non-adaptive forces. Such a similar explanation might be given for the results of the Dutch provenances, where even without strong selective pressures, natural processes such as restricted gene flow, historical events, and random genetic drift might have contributed to the observed differentiation at such small geographic scale.
Historical management effects on stem form
Due to past management practices, such as coppicing, most autochthonous Dutch seed sources have been considered inferior in stem quality. As these stands cannot be visually selected for their phenotypic superiority, their Forest Reproductive Material is classified as “Source identified” and not as “Selected”. However, our findings indicate that under trial conditions, several of these autochthonous provenances have the potential to produce high-quality stems comparable to provenances used as standards, suggesting their suitability for production purposes. It is important to recognize that these trials are still in their early stages, and assessing form characteristics at age 11-14 may be premature. For fast-growing pioneer species such as Alnus glutinosa, as well as Betula pubescens, which are short-lived species, this age might be sufficient. However, for slower-growing and long-living species like Quercus robur and Fagus sylvatica, which develop their final stem quality later, these form assessments may be relatively early to give reliable conclusions.
Practical implications for seed sourcing
Provenances that exhibit a combination of desirable traits, including good growth, late flushing, good stem form and high survival rates, are the most suitable for Dutch forestry with a production-oriented objective. The autochthonous provenances included in this study are among the first to be evaluated for these traits. Our results suggest a correlation between growth and timing of budburst, particularly in Fagus sylvatica. Here, the best-growing provenances also exhibited earlier budburst, potentially complicating the selection of provenances that optimize both traits. Several studies on Fagus sylvatica have shown that, in certain parts of its range, late budburst - an adaptive strategy to avoid late frosts - has been linked to reduced height growth ([25], [50], [18]). Based on our findings, we propose recommendations for seed sourcing, in particular to register certain autochthonous (category SI) provenances for production purposes. Given the anticipated increase in demand for planting stock in the Netherlands, we recommend prioritizing the highest-performing SI provenances for seed harvesting when wood production is a key objective. Conversely, lesser performing autochthonous SI provenances should be avoided or only harvested in cases where biodiversity conservation or landscape restoration is the priority in the plantings. Additionally, increased harvesting in autochthonous stands requires careful consideration of genetic diversity, as some of these forests are small remnants. To ensure a genetically diverse and resilient seed supply, for small stands, mixing of SI seed sources with similar characteristics could be considered.
Conclusions
Our study demonstrates that even at a small geographic scale, local seed sources exhibit genetic differences in spring leaf phenology, growth, and form traits. Budburst, a key adaptive trait in frost-prone regions like the Netherlands, varied significantly, particularly in Fagus sylvatica and Quercus robur with autochthonous sources generally flushing among the latest. The results also demonstrate that several autochthonous seed sources showed good stem form, despite past coppicing, indicating their potential for use in production forestry. This was evident in all species. We identified provenances in each species that combine favorable traits such as good growth, acceptable form, high survival, and late budburst. These findings support the registration and use of certain autochthonous local seed sources for both timber production and ecological purposes. The results cannot be directly extrapolated to inform provenance selection for other planting sites. Studies indicate that site effects often have a greater impact than provenance effect ([54], [53], [55]). However, the test sites reflect typical growing conditions for the four species in the Netherlands, which is a single provenance region, characterized by minimal climatological or ecological variation. Thus, our recommendations for seed sourcing can be broadly applied across the country. Nevertheless, soil characteristics remain a critical factor as shown by Buras et al. ([11]), who found soil-type-specific growth patterns in Quercus robur provenances. Future research should prioritize multi-site trials that incorporate diverse soil types to further refine provenance recommendations.
Acknowledgements
We are grateful to the State Forest Service and Estate Zelle for their support in establishing and maintaining the provenance trials. We thank Toon Helmink, Otto Vaessen, and Judith van Tol for their help with fieldwork. This research was funded by the Board for Plant Varieties, as part of the provenance testing project (CGO) for the “Dutch List of recommended varieties and provenances of trees”.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Hinke Wiersma
Inge Verbeek
Paul Copini 0000-0002-5547-2609
Centre for Genetic Resources, The Netherlands, Wageningen University and Research, P.O. Box 47, 6700 AA, Wageningen (The Netherlands)
Biometris, The Netherlands, Wageningen University and Research, P.O. Box 47, 6700 AA, Wageningen (The Netherlands)
Corresponding author
Paper Info
Citation
Buiteveld J, Wiersma H, Paulo M-J, Verbeek I, Copini P (2025). Growth, spring phenology and stem quality of four broadleaved species assessed in provenance trials in the Netherlands - Implications for seed sourcing. iForest 18: 242-251. - doi: 10.3832/ifor4930-018
Academic Editor
Marco Borghetti
Paper history
Received: Jun 23, 2025
Accepted: Aug 28, 2025
First online: Sep 22, 2025
Publication Date: Oct 31, 2025
Publication Time: 0.83 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2025
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 2790
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 822
Abstract Page Views: 937
PDF Downloads: 944
Citation/Reference Downloads: 0
XML Downloads: 87
Web Metrics
Days since publication: 153
Overall contacts: 2790
Avg. contacts per week: 127.65
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Juvenile growth response of European beech (Fagus sylvatica L.) to sudden change of climatic environment in SE European trials
vol. 2, pp. 213-220 (online: 22 December 2009)
Research Articles
Predicting phenology of European beech in forest habitats
vol. 11, pp. 41-47 (online: 09 January 2018)
Research Articles
Identification and molecular characterization of LTR and LINE retrotransposable elements in Fagus sylvatica L.
vol. 2, pp. 119-126 (online: 10 June 2009)
Research Articles
Bud flush phenology and nursery carryover effect of paper birch provenances
vol. 8, pp. 809-817 (online: 19 May 2015)
Research Articles
Fine-scale spatial genetic structure in a multi-oak-species (Quercus spp.) forest
vol. 8, pp. 324-332 (online: 05 September 2014)
Research Articles
A comparative study of growth and leaf trait variation in twenty Cornus wilsoniana W. families in southeastern China
vol. 10, pp. 759-765 (online: 02 September 2017)
Editorials
Workshop COST E52 “Evaluation of beech genetic resources for sustainable forestry”
vol. 2, pp. 104 (online: 10 June 2009)
Research Articles
Genetic control of intra-annual height growth in 6-year-old Norway spruce progenies in Latvia
vol. 12, pp. 214-219 (online: 25 April 2019)
Technical Reports
Categorization of field trials with GM plants in the Netherlands: applicable to field trials with GM forest trees?
vol. 8, pp. 222-225 (online: 31 August 2014)
Research Articles
Age trends in genetic parameters for growth and quality traits in Abies alba
vol. 9, pp. 954-959 (online: 07 July 2016)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword