Bud flush phenology and nursery carryover effect of paper birch provenances
iForest - Biogeosciences and Forestry, Volume 8, Issue 6, Pages 809-817 (2015)
doi: https://doi.org/10.3832/ifor1367-008
Published: May 19, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
Paper birch (Betula papyrifera Marsh) is an ecologically valuable species with a broad geographic distribution across the North America. Its diversity, versatility and enduring nature make it an ideal candidate for a selective breeding program in this region. However, an understanding of the genecology of this species is fundamental to deploy it successfully. Ten paper birch provenances were collected from British Columbia (BC, Canada) and northern Idaho (USA) along elevational transects to determine whether observed bud flush phenology was due to genetics and /or environmental variation or their interaction. Seedlings were grown at three different nurseries: University of Idaho (46°44’N), Landing (50°17’N) and Little Forestry (54°00’N) and planted in a randomized single tree interlocking block design in three common gardens at Sandpoint, ID (48°13’N), Skimikin, BC (50°45’N) and Red Rock, BC (53°45’N). Results indicate that variation in the timing of bud flush is a complex interaction among local genetic characteristics and environmental conditions of the growing site. Birch bud flush followed a general geographic trend where provenances at the southern common garden (Sandpoint) required less time (Day of Year, DoY) and fewer growing degree days (GDD) compared to central (Skimikin) and northern (Red Rock) common gardens. Although there were significant differences in the timing of bud flush among provenances along an elevational gradient, none of the regions showed the expected linear elevational cline, trends were inconsistent. Further, birch bud flush was significantly influenced by nursery displacement effects in the initial year of establishment but disappeared within three years. These results provide an opportunity to characterize bud flush phenology of paper birch and would be useful for improving operational paper birch seed transfer programs in BC.
Keywords
Betula papyrifera, Common Garden, Elevational Cline, Growing Degree Day (GDD), Day Of Year (DoY), Nursery Carry Over Effect, Provenance Trial, Seed-transfer
Introduction
Paper birch (Betula papyrifera Marsh) is one of the most widely distributed birch species in North America. Recently, researchers have shown that the presence of birch in mixedwood stands plays a significant role in nutrient cycling, and in the disease and insect pest control for conifers species ([5], [1], [49], [17]). Moreover, the presence of birch in young conifer plantations improves soil nutrient status and reduces the risk of root disease ([6], [48], [44]). All these beneficial characteristics help to promote birch as a new commercial reforestation species in British Columbia (BC - [5], [12], [18], [19]). However, knowledge concerning geographic variation or patterns of genetic variation within and among provenances is scarce ([5], [6], [16], [9]). Therefore, further study in relation to variability in birch’s phenotypic traits is needed prior to establishing seed zones and seed transfer guidelines.
Transfer of a provenance to a new environment may predispose it to both positive and negative impacts on growth depending on transfer direction ([22], [53]). Moreover, when tree species are transferred to a new environment they must regulate the timing of bud flush and flowering during the spring, as well as the timing of growth cessation and dormancy development during late summer and fall, in order to reduce the risk of frost damage ([21]). In this regard, bud flush phenology is critical because it determines the beginning of the growing season and the probability of damage due to late spring frosts ([4]). However, the annual rhythm of bud development in many forest trees species (such as birch, Douglas fir - Pseudotsuga menziesii Mirb.) are controlled by chilling temperature, duration of chilling (for birch temperatures ≤ 5°C for a few weeks) and accumulation of spring heat sums ([24], [40]). In addition, these chilling characteristics do not just determine susceptibility to frost damage. Chilling also affects bud and leaf development ([40]) and can vary from species to species ([35]) or differ within species by latitudinal origin ([14]). After meeting the chilling requirement, positive temperatures lead to bud development and the process may accelerate when temperature starts to increase ([25]). However, extended photoperiod may overcome the lack of chilling if trees are exposed to long days before meeting their chilling requirement irrespective of provenance origin ([8]). Although air temperature appears to be the most significant environmental factor influencing the initiation of bud flush in Betula spp., photoperiod and soil temperature may also play important roles ([10], [31], [16]).
Apart from the above mechanisms regulating spring bud flush, observations from common garden studies at different geographic locations suggest variability in spring bud phenology within species. Studies in northern Europe suggest northern provenances of B. pubescens Ehrh., B. nana L., B. pendula ([51], [36]) and Acer platanoides L. ([56]) flush before southern provenances in a continental climate. Furthermore, continental provenances of B. pendula and B. pubescens flush earlier than those of coastal provenances ([37]), while inland provenances of Ulmus glabra (Huds.) flushed earlier than those of coastal provenances ([38]). Conversely, other studies have demonstrated that southern provenances of Picea sitchensis (Bong.) Carr. and B. papyrifera flush slightly before northern provenances, and coastal populations of Pseudotsuga menziesii var. menziesii and B. papyrifera flush before continental provenances at a continental site ([39], [11], [16]. Therefore, it is important to determine the potential transfer responses before initiating any transfer guidelines.
A significant change in abiotic factors can occur in mountainous regions over a short distance ([54]). Therefore, elevational transects provide an ideal methodology for studying the variation of plant functional traits in response to environmental factors. Based on the study by Myking ([37]), low elevation provenances of Betula spp. flush before high elevation provenances. A similar trend was also observed in provenance trials with other species, Fagus sylvatica L. in Polish and Finnish provenance trials ([7], [56]). Conversely, Sharik & Barnes ([46]) observed no elevational trend for flushing in a Betula spp. provenance trial in the USA. Therefore, an investigation of phenology responses along elevational transects is prerequisite before developing any seed transfer guidelines.
Nursery practices may lead to alteration of a population’s phenotypic expression. This may ultimately influence the genetic acclimation (genotype by environment interaction - phenotype - in a single generation) of forest trees to plantation sites ([3]). Consequently, any nursery with an environment greatly different from the site of seed origin may alter seedling growth and development after planting ([3], [15]). Moreover, when northern provenances grown at southern nurseries are planted back in the north, their flushing phenology may be out of phase with that of the local populations because bud flush phenology depends not only on the present (growing) environment, but also on the environment in which the buds were formed ([56], [20]). Therefore, study of nursery carryover effects is another factor needed to consider before developing any seed transfer guidelines.
From a previous investigation on geographic variability of paper birch provenances across BC, Hawkins & Dhar ([16]) suggested some regional and population differentiation for certain traits. Despite promising results from this initial research, the populations of that study were not collected along elevational transects. Also, there was no investigation of nursery effects on birch bud flush. Therefore, a study was designed to investigate genecology (geographic variation in bud flush) of paper birch provenances at three different common garden locations in British Columbia (Canada) and Northern Idaho (USA) as well as to determine if there was any nursery carryover effect on birch bud flush. Campbell & Sorensen ([3]) and Hawkins ([15]) suggested that a nursery environment/climate different from the stocks’ site of origin could impact its field performance. In addition, our study also determined whether provenance responses were related to their site of origin (clinal variation).
Materials and methods
Provenances
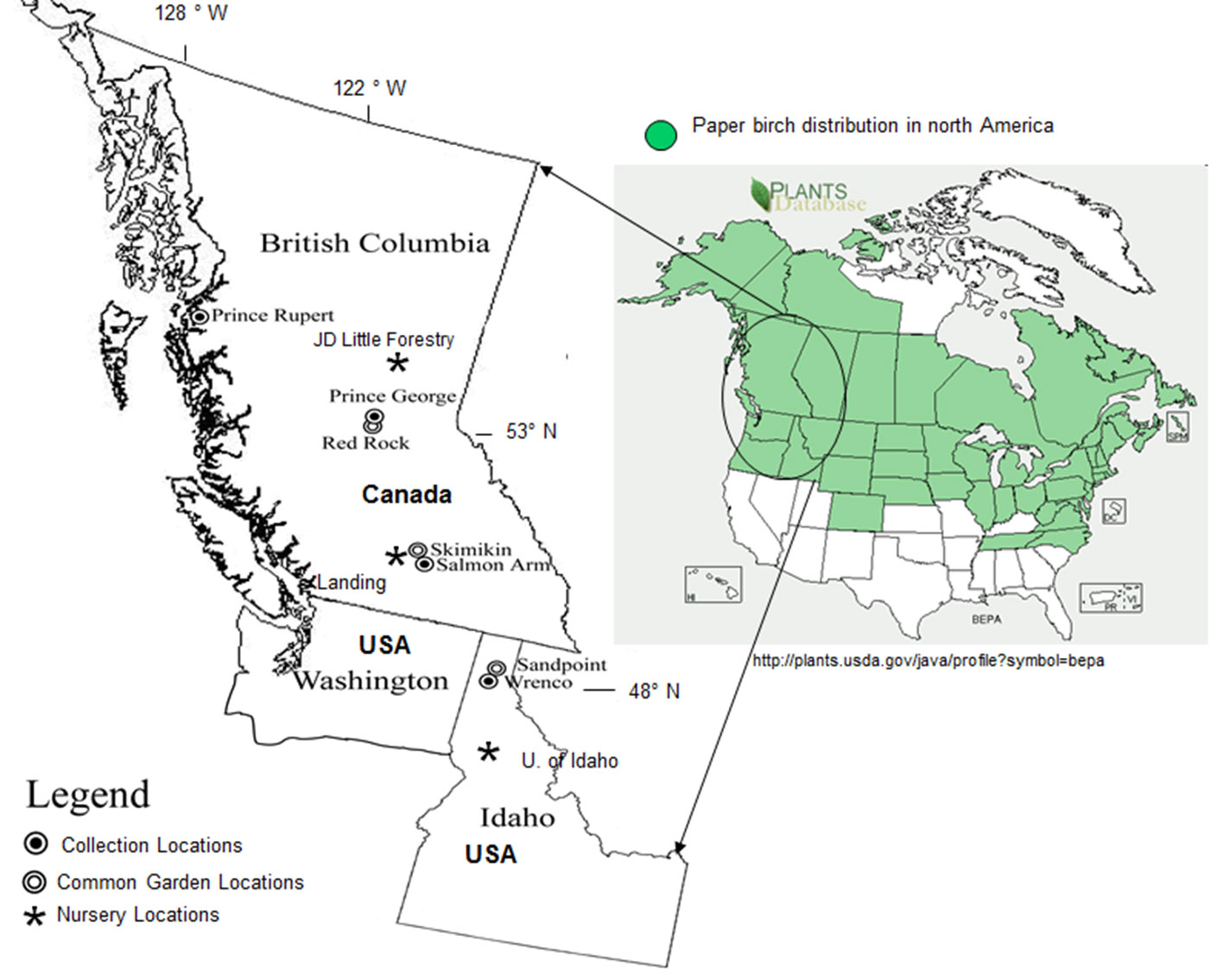
In 1998, paper birch seed was collected from four different geographic regions: Salmon Arm, Prince George, Prince Rupert (British Columbia) and Idaho (Idaho, USA) (Fig. 1, Tab. 1). For Prince Rupert, Prince George and Salmon Arm regions, 5-8 provenance samples (depending on elevation range) were collected along elevational transects. Stands within each region were chosen within ± 10 to 20 m of a pre-determined elevation, beginning at the bottom of an elevational transect and continuing every 100 m thereafter. Following initial analysis, 3 provenances from each of 3 BC regions were selected (lowest, mid and highest elevation) for further experimentation. One population from northern Idaho was also sampled. At all sites, five non-clonal (half-sib or no genetic relationship as birch generally does not reproduce vegetatively in BC) trees within each stand (provenance) were chosen for seed collection. These trees were of good health, form and had produced seed. Seed was collected and bulked to represent a provenance for each elevation.
Fig. 1 - Paper birch provenance collection sites, common garden and nursery locations, and paper birch geographic distribution in North America.
Tab. 1 - Geographic origin and climatic variables of different paper birch provenances, nurseries and common gardens. Mean annual temperature (MAT), mean temperature of the coldest month (MTCM), mean annual precipitation (MAP), extreme temperature difference (TD), and days of frost free period (FFP). Climate BC Model (⇒ http://climatemodels.forestry.ubc.ca/climatebc) based on 30-year mean climatic data, 1981-2009. (#): Salmon Arm and Prince George sources are continental while Prince Rupert is a maritime climate. (*): All nursery locales and common garden sites have a continental climate.
| Region# | Code | Elevation (m a.s.l.) | Latitude (N) |
Longitude (W) | MAT (°C ) |
MTCM (°C) |
MAP (mm) |
TD (°C) |
FFP (Day) |
|---|---|---|---|---|---|---|---|---|---|
| Idaho | 13 | 870 | 48° 13′ | 116° 42′ | 6.8 | -2.6 | 958 | 20.8 | 134 |
| Salmon Arm | 31 | 460 | 50° 42′ | 119° 25′ | 7.0 | -3.7 | 783 | 21.9 | 146 |
| Salmon Arm | 33 | 760 | 50° 42′ | 119° 25′ | 5.8 | -4.5 | 783 | 21.4 | 132 |
| Salmon Arm | 37 | 1200 | 50° 42′ | 119° 25′ | 4.0 | -5.7 | 783 | 20.7 | 111 |
| Prince George | 51 | 700 | 53° 55′ | 122° 28′ | 4.3 | -6.1 | 618 | 21.1 | 104 |
| Prince George | 54 | 1000 | 53° 55′ | 122° 28′ | 3.2 | -6.8 | 618 | 20.6 | 88 |
| Prince George | 56 | 1200 | 53° 55′ | 122° 28′ | 2.4 | -7.3 | 618 | 20.3 | 78 |
| Prince Rupert | 61 | 210 | 55° 47′ | 128° 45′ | 6.8 | -3.1 | 1472 | 19.2 | 168 |
| Prince Rupert | 63 | 400 | 55° 47′ | 128° 45′ | 6.0 | -3.8 | 1472 | 19.3 | 156 |
| Prince Rupert | 66 | 750 | 55° 47′ | 128° 45′ | 4.5 | -5.2 | 1472 | 19.4 | 132 |
| Nursery location* | |||||||||
| University of Idaho (UI) | 735 | 46° 44′ | 116° 58′ | 9.1 | 0.2 | 650 | 19.9 | 160 | |
| Landing (LN) | 400 | 50° 17′ | 119° 16′ | 7.8 | -3.3 | 635 | 22.5 | 149 | |
| Little Forestry Center (NW) | 650 | 54° 00′ | 122° 28′ | 3.9 | -8.6 | 679 | 24.3 | 106 | |
| Common garden location | |||||||||
| Sandpoint (SP) | 640 | 48° 13′ | 116° 40′ | 7.4 | -2.6 | 763 | 21.0 | 145 | |
| Skimikin (SK) | 550 | 50° 47′ | 119° 24′ | 7.1 | -3.7 | 512 | 22.4 | 141 | |
| Red Rock (RR) | 725 | 53° 45′ | 122° 43′ | 4.1 | -6.7 | 766 | 21.5 | 102 | |
Nurseries and common gardens trial
All 10 provenances of paper birch seedlings were grown at three different nurseries (NW: Canfor J.D. Little Forestry Centre, Prince George, BC; LN: Landing Nursery, Vernon, BC; UI: the University of Idaho, Moscow, ID - Tab. 1) to determine the importance of nursery practices and environment on the growth and acclimation of the seedlings after planting ([5]). Seeds were hand sown at each nursery in early May 1999 in PSB 515A styroblocks (Beaver Plastics, Edmonton, AB - 284 seedlings m2), lifted in November 1999 and placed in cold storage until the spring of 2000 when they were planted. In spring 2000, seedlings from all three nurseries were planted at 3 common gardens: late April in Sandpoint (Idaho), early May in Skimikin (Salmon Arm) and mid-May in Red Rock (Prince George - Tab. 1). In this study we did not measure any nursery practice as each nursery had unique cultural regimes to optimize seedling growth with respect to local climatic conditions. As this was a preliminary study, it can be viewed as a benchmark for any follow-up studies.
All seedlings were identified according to provenance, geographic region and nursery for tracking their response in the common gardens. In total, 16 seedlings per provenance-nursery were planted at 2 × 2 m spacing in a randomized single tree interlocking block design (16 trees per provenance planted as 4 trees in each of 4 replications for each of the 3 nurseries: 16 × 3 = 48 seedlings per provenance), allowing for comparisons among geographic region (4), provenance within geographic regions (10), nursery (3) and among common gardens (3). The experiment at different common garden locations was conducted under natural light (photoperiod), temperature and precipitation conditions. The average duration of photoperiod and temperature during the early growing season (April-June) varied by 20-90 minutes and up to 3 °C among the three common garden locations. Precipitation was similar at Sandpoint and Red Rock, while Skimikin was much drier (Tab. 1).
Assessment of bud flush
All ten provenances planted at Red Rock, Sandpoint and Skimikin were surveyed for spring bud flush in 2001 and 2003 (Tab. 1). The bud flush survey involved recording bud burst for 10 buds on a single branch, preferably the terminal. If the terminal did not have 10 buds, then buds on the next highest branch were examined and so on until a total of 10 buds were surveyed. For each survey, the total number of buds burst (for each tree) was recorded until all 10 buds flushed. The date of individual bud flush was identified when the first green ragged edges visually appear between the bud scales, almost like the opening of a “clam shell” ([16]). All phenological assessments were done at least twice a week, and all trees were surveyed at all three common garden locations. In this study, 80% bud flush was chosen as the point of analysis, meaning the duration required to have 8 of 10 buds flush.
Climate data
All temperature data were acquired from the nearest weather reporting station (on site for Skimikin and Sandpoint, and within 10 km for Red Rock) to each common garden location. Red Rock is situated of the same glacial plain as the reporting station, therefore distance was not a concern for weather data. Temperature data were used to calculate growing degree days (GDD) or heat sum for each common garden location in each year. Budflush_GDD were calculated based on mean daily temperature starting on January 1st of each year using the formula (eqn. 1):
(heat accumulated when T > Tthres, and if T < Tthres then heat sum = 0) where Tmax is the maximum temperature and Tmin the minimum temperature recorded over each 24-h period, Tthres is the threshold temperature. The threshold temperature for budflush_GDD calculation was 0 °C ([20], [41], [45], [16]). Day length was calculated on the 21st day of each month for each population and common garden using the US Naval Observatory tables (⇒ http://www.weatherimages.org/latlonsun.html). The different climatic variables were estimated for each provenance by using Climate BC version 4.72, ([55] - ⇒ http://climatemodels.forestry.ubc.ca/climatebc/).
Data analysis
All data were analyzed based on 80% bud flush using SYSTAT® (version 12, SYSTAT Software Inc) general linear model (GLM). During GLM, Type III sums of squares (Yates’s weighted squares of means) was used as our data were unbalanced (number of provenance per region was not equal). In the literature there is ongoing debate about the use of sums of squares for unbalanced data. Some suggest using Type II sums of squares (Yates’s weighted squares of means - [32], [28]), while other researchers suggest using Type III sums of squares ([29], [47], [23]). We used Type III sums of squares for the GLM.
Initially, both provenance and region were considered as main effects for statistical analysis. When region was considered as a main effect, all provenances within a region were pooled to form an analytical unit and the analysis was run with four regions (Prince George, Prince Rupert, Salmon Arm and Idaho). However, GLM for both provenance and geographic region showed similar results for significance and F values. Therefore, a nested GLM was conducted, where different provenances are nested within region (independent variable). This was considered a main effect and modeled in the GLM procedure for 80% bud flush and growing degree days (budflush_GDD - dependent variable) to describe the geographic variation for paper birch provenances. The final model used in the analysis was as follows (eqn. 2):
where B80% is the bud flush proportion (80%, the number of days required to reach 8 of 10 buds flushed), m is the grand mean, G is the common garden location, N is the nursery, P(R) is the provenance (P) nested within the geographic region (R), and ε is the error. A separate model was run for each year (2001 and 2003) due to different sample sizes. In this study, seed source was the only random factor and all the others were fixed.
Subsequently pairwise comparisons were conducted using Tukey’s multiple comparison tests to determine specific differences among provenances. The relationship between bud flush performance of provenances and their source climate for a given individual test site was used to improve and validate bud flush response functions. Therefore, simple regression analyses were carried out to look at the response of provenances planted at different common gardens as function of the climate differences between provenance source locations and the respective common gardens. Variables were duration of frost free period (FFP), mean annual temperature (MAT), degree days (DD) and mean annual precipitation (MAP). A similar regression analysis was also done with response variables (Day of Year, DoY and budflush_GDD) to predict the effect of seed transfer distance. Seed transfer distance was calculated based on the latitudinal difference of the seed origin and common garden location (latitude of seed origin - latitude of common garden location). To simplify the analysis of relationships between climate variables of provenance source location and bud flush, we employed a principle component analysis (PCA). The PCA was conducted using the programming language R version 3.0.1 ([43]). For all response variables, general normality tests were carried out with SYSTAT® version 12.0 and transformation was made when required.
Results
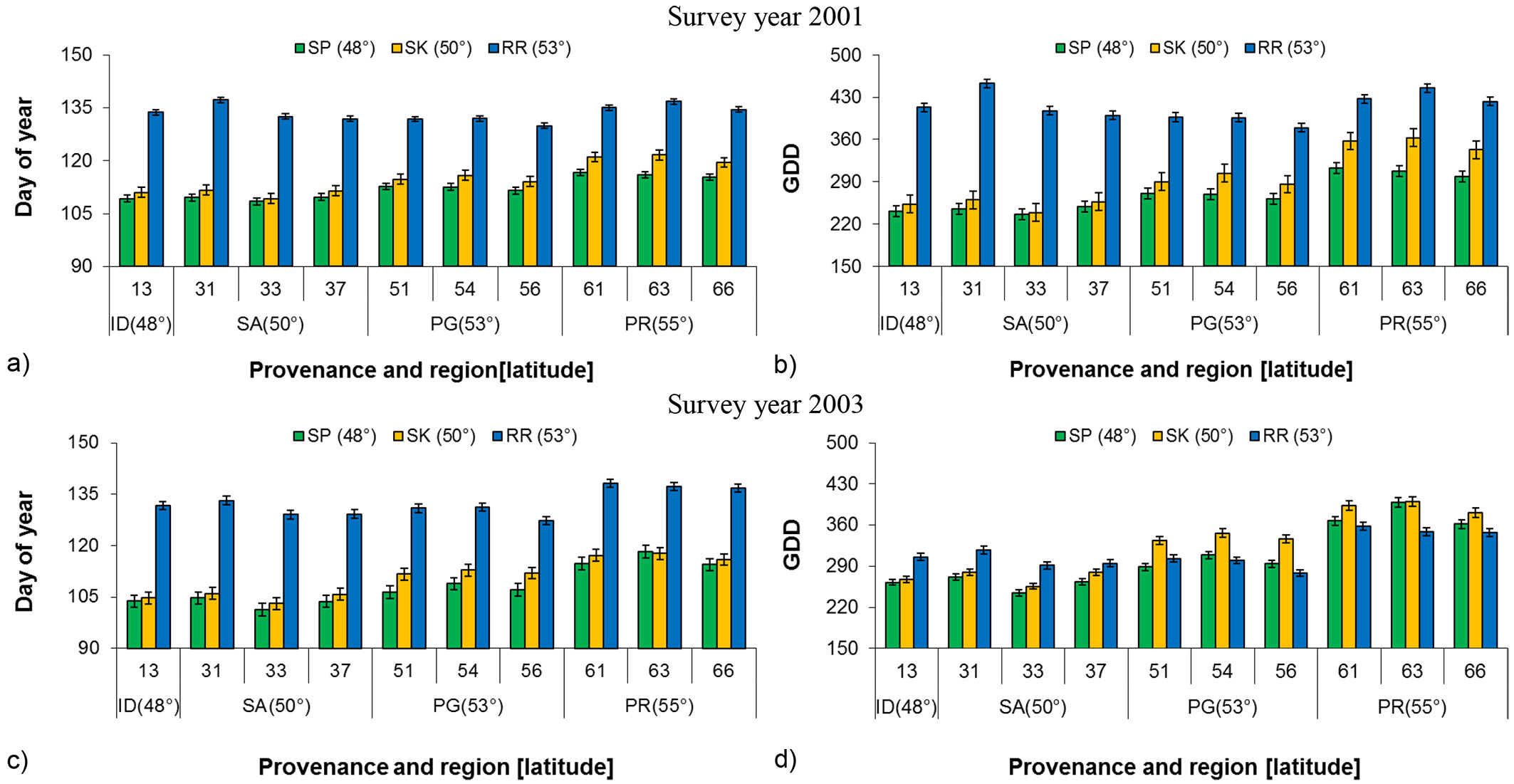
Based on GLM analysis, of 80% bud flush among the common gardens was significantly different for budflush_DoY and budflush_GDD or heat sum requirement in both years (Tab. 2). Generally budflush_DoY and budflush_GDD increased with increased common garden latitude (Fig. 2). Bud flush at the Sandpoint (southern) common garden was on an average 22 days in 2001 and 24 days in 2003 earlier than at the Red Rock (northern) common garden (Fig. 2a, Fig. 2c). When individual provenances were considered, provenance 33 from the Salmon Arm region required the least number of days for bud flush in both years at all three common garden locations, which was followed by the Idaho provenance (13). Considering heat requirement (budflush_GDD based on 0 °C), different provenances responded differently at different common garden locations (Fig. 2b, Fig. 2d). However in all instances, Prince Rupert provenances (61, 63 and 66) required the greatest number of days (budflush_DoY) and amount of heat (budflush_GDD) to flush. Tukey’s test for multiple comparisons (α = 0.05) showed a significant difference among geographic regions in all years, except for between Salmon Arm and Idaho in 2001 (P = 0.084) and 2003 (P = 0.792). This might be due to the close proximity and similar environmental conditions of the two geographic regions. When provenances were nested within geographic region, both budflush_DoY and budflush_GDD showed significant total population variation in 2003 and close to significant in 2001, indicating the existence of genetic differences among provenances and common garden locations’ environmental impact (Tab. 2).
Tab. 2 - Results of the GLM analysis for days (budflush_DoY) to 80 % spring bud flush and growing degree days (budflush_GDD) in 2001 and 2003. Significant results (α = 0.05) are reported in italic.
| Variable | Factor | Sum-of-Squares | df | F-Ratio | P |
|---|---|---|---|---|---|
| 2001: Budflush_DoY (n = 755) |
Garden | 62 795.43 | 2 | 1 061.34 | <0.001 |
| Nursery | 283.28 | 2 | 4.79 | 0.009 | |
| Provenance (Region) | 322.89 | 6 | 1.93 | 0.073 | |
| Garden × Nursery | 32.43 | 4 | 0.27 | 0.895 | |
| Garden × Provenance (Region) | 509.55 | 12 | 1.44 | 0.144 | |
| Nursery × Provenance (Region) | 288.43 | 12 | 0.81 | 0.638 | |
| Error | 21 181.59 | 716 | - | - | |
| 2001: Budflush_GDD (n = 755) |
Garden | 2 678 785.94 | 2 | 456.04 | <0.001 |
| Nursery | 26 367.71 | 2 | 4.49 | 0.012 | |
| Provenance (Region) | 29 742.09 | 6 | 1.69 | 0.060 | |
| Garden × Nursery | 3 542.93 | 4 | 0.30 | 0.877 | |
| Garden × Provenance (Region) | 52 862.91 | 12 | 1.50 | 0.119 | |
| Nursery × Provenance (Region) | 25 647.90 | 12 | 0.73 | 0.725 | |
| Error | 2 102 921.44 | 716 | - | - | |
| 2003: Budflush_DoY (n = 758) |
Garden | 80 615.06 | 2 | 787.95 | <0.001 |
| Nursery | 50.17 | 2 | 0.49 | 0.613 | |
| Provenance (Region) | 1 437.13 | 6 | 4.68 | <0.001 | |
| Garden × Nursery | 46.90 | 4 | 0.23 | 0.922 | |
| Garden × Provenance (Region) | 863.72 | 12 | 1.41 | 0.157 | |
| Nursery × Provenance (Region) | 484.55 | 12 | 0.79 | 0.662 | |
| Error | 36 780.63 | 719 | - | - | |
| 2003: Budflush_GDD (n = 758) |
Garden | 62 164.03 | 2 | 8.04 | <0.001 |
| Nursery | 4 193.38 | 2 | 0.54 | 0.582 | |
| Provenance (Region) | 125 855.48 | 6 | 5.43 | <0.001 | |
| Garden × Nursery | 2 414.51 | 4 | 0.16 | 0.960 | |
| Garden × Provenance (Region) | 63 335.04 | 12 | 1.37 | 0.177 | |
| Nursery × Provenance (Region) | 28 667.61 | 12 | 0.62 | 0.828 | |
| Error | 2 779 393.37 | 719 | - | - |
Fig. 2 - Least square mean (LSM) of budflush_DoY and budflush_GDD for 80% bud flush (± SE) by provenance, region and latitude at each common garden in 2001 and 2003. (GDD): Growing degree days; (SP): Sandpoint; (SK): Skimikin; (RR): Red Rock; (SA): Salmon Arm; (ID): Idaho; (PG): Prince George; (PR): Prince Rupert.
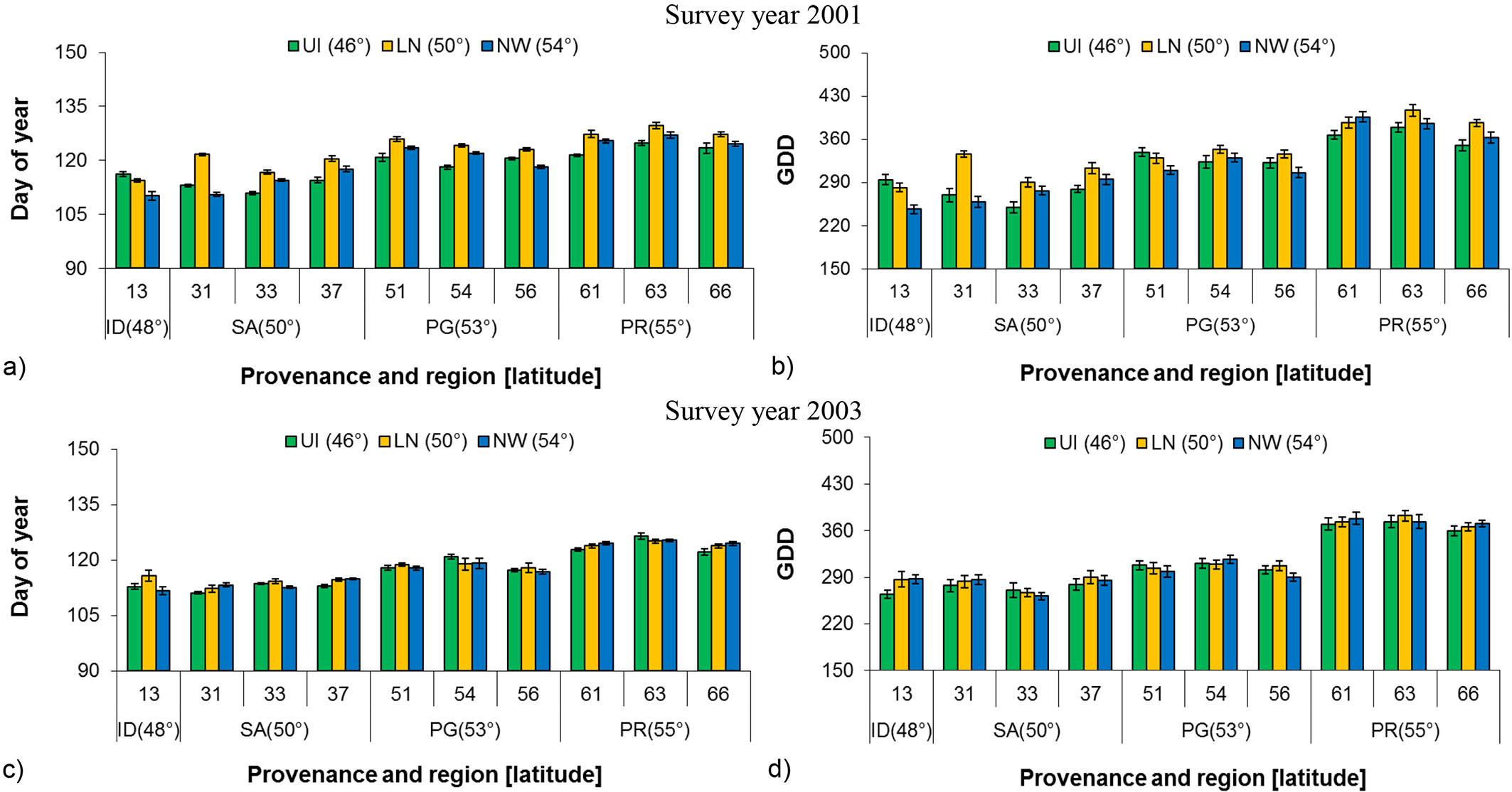
A significant difference among nurseries for birch bud flush was observed for both budflush_DoY and budflush_GDD at the beginning of seedling establishment (2001), but the relationship was not significant in 2003 (Tab. 2). Provenances grown at Landing Nursery required the greatest number of days (budflush_DoY) and budflush_GDD as compared to the other two nurseries in 2001, while in 2003 the difference was considerably less, thus also indicating the absence of a nursery carryover effect (Fig. 3a, Fig. 3b, Fig. 3c, Fig. 3d).
Fig. 3 - Least square mean (LSM) of Budflush_DoY and budflush_GDD in 2001 and 2003 to 80 % bud flush (± SE) by provenance, region and latitude at each nursery with common garden pooled. (GDD): Growing degree days; (SP): Sandpoint; (SK): Skimikin; (RR): Red Rock; (SA): Salmon Arm; (ID): Idaho; (PG): Prince George; (PR): Prince Rupert.
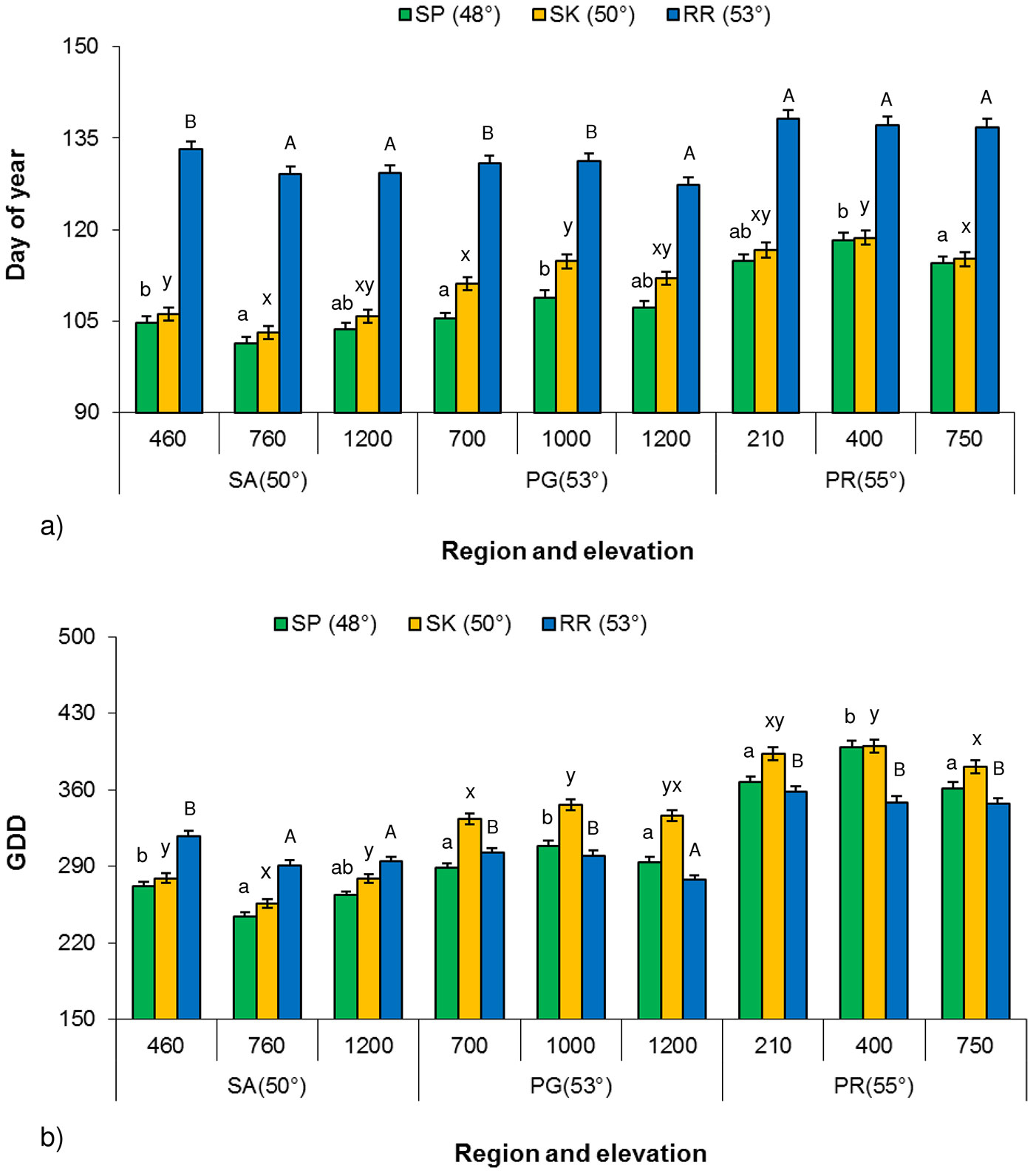
The influence of elevation on paper birch bud flush showed an inconsistent trend at all three common garden locations. There were significant differences (P < 0.001) in the timing of bud flush (budflush_DoY) and budflush_GDD among provenances along an elevational transect for each of the Prince George, Prince Rupert and Salmon Arm regions at all three common garden locations, except Prince Rupert provenances at Red Rock (Fig. 4a, Fig. 4b). The influence of elevation showed an inconsistent trend and different regions required different budflush_DoY and budflush_GDD to flush. For example, the middle elevation provenance (760 m a.s.l.) from Salmon Arm region required fewer budflush_DoY and less budflush_GDD for flush at all three common garden locations than provenances from higher (1200 m a.s.l.) or lower (460 m a.s.l.) elevation. Prince George provenances showed the opposite trend such as the middle elevation provenance (1000 m a.s.l.) requiring more budflush_DoY (Fig. 4a) and budflush_GDD to flush (Fig. 4b).
Fig. 4 - Least square mean (LSM) of Budflush_DoY and budflush_GDD for 80% bud flush (± SE) by elevation at each common garden location along elevational transects for each climatic region in 2003. (SP): Sandpoint; (SK): Skimikin; (RR): Red Rock; birch bud flush values followed by the same letter (a, b, c or A, B, C, or x, y, z) within each region along elevational transect are not significantly different (α = 0.05, Tukey’s test for multiple comparison with region along elevational transect, meter a.s.l.).
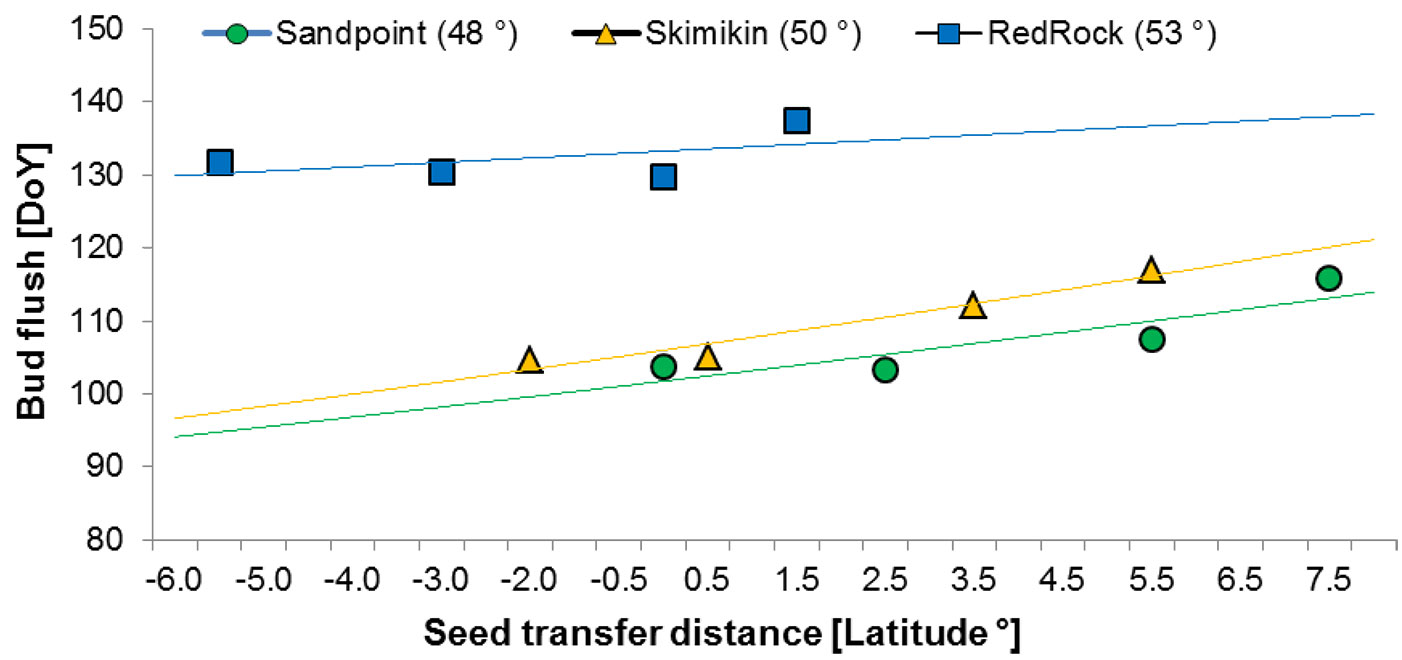
A linear relationship (P < 0.001) was observed between the timing of bud flush (budflush_DoY) and latitudinal seed transfer distance (Fig. 5). The most rapid bud flush was observed with decreasing seed transfer distance towards the south at the southern common gardens, whereas at the northern common garden the opposite trend was observed (Fig. 5). However without any apparent detrimental effect on phenology (e.g., buds fail to flush,, multiple dominant buds), provenances may be transferred up to 5° of latitude toward the north and 7° of latitude toward the south.
Fig. 5 - Day of the Year to 80% bud break by provenance in relation to latitudinal seed transfer distance for Red Rock (upper line), Skimikin (middle line) and Sandpoint (lower line) common gardens. (DoY): Day of year; (Seed transfer distance): latitude of seed origin - latitude of garden location.
When regression analysis was carried out for budflush_DoY, budflush_GDD and seed transfer distance for bud flush against provenance source location climatic variables (MAT, DD, FFP and MAP), there was no correlation or a very poor relationship (low R2) was observed. None of the source variables explained more than 25% of the observed variation (see Tab. SM1 - Appendix 1). Therefore, further principal components analysis (PCA) was conducted to determine the impact of source location climate variables on bud flush (Tab. SM1 and Fig. SM3 - Appendix 1). The PCA identified three main variation components, which in total explained 99.1% of the data set variability. Component I contributed 66.7% to the variance (eigenvalue = 2.7), component II contributed 32.2% (eigenvalue = 1.29) and component III contributed 0.2% eigenvalue = 1.22). FPP, MAT and DD showed strong correlations with the component I, while MAP with the component II.
Discussion
Paper birch planted at Sandpoint (Idaho, USA) flushed slightly earlier than those planted at Skimikin (BC), and much earlier than those planted at Red Rock (BC - Fig. 2). This indicated that birch bud flush follows a general geographic trend and progresses from south to north ([26], [16]). A similar phenological trend was also observed in a genetic study on paper birch carried out by Morgenstern ([33]). Apart from the geographic (common garden) location, it is evident that the observed bud flush is a consequence of local temperature or photoperiod or their interaction ([30]). When we consider the average temperature in January, February, March and April at the common garden location, Sandpoint (-3.5, -0.9, +3.7, +7.4 °C, respectively) was warmer than Skimikin (-4.6, -1.2, +3.6, +6.7 °C) and Red Rock (-9.2, -5.9, -0.4, +4.6 °C). This implies that temperature during the winter and spring played an important role in regulating the timing of birch bud flush, which is consistent with other studies ([20], [50], [31], [42], [16]). According to Heide ([20]), warmer weather in January, February and March considerably reduced the thermal requirement for Betula spp. bud flush if the fall chilling requirement had been met. This suggests that if a southward transfer is associated with warmer temperature then bud flush will be faster, while for a northward transfer, associated with cooler temperatures, bud flush should take longer.
Besides air temperature, photoperiod may also play an important regulatory role on birch bud flush because air temperature may vary significantly from year to year, while photoperiod does not ([21]). Therefore, many temperate and boreal plant species rely on photoperiod to constrain their development to a “safe period” and the consequence of this photoperiodic constraint appears to increase with increasing latitude of seed origin ([34]). Although this was not a controlled photoperiod experiment, previous controlled photoperiod and translocation experiments in this region suggest that photoperiod had a significant impact on paper birch bud flush ([16]). Therefore, we may assume that photoperiod itself or an interaction between local temperature and photoperiod could have an impact on birch flush. Li et al. ([30]) observed that long photoperiod promoted bud flush at warmer temperatures. In addition, local soil temperature may be another contributory factor which could influence spring bud phenology of paper birch. The Red Rock common garden soil remains below zero in the early growing season compared to the Skimikin and Sandpoint common gardens. Birch with warm roots (14 oC throughout the experimental period) flushed significantly earlier than those with cold roots (mean 0 °C) in greenhouse and controlled growth chamber experiments ([16]).
The nursery carryover effect on paper birch bud flush appears to be overcome within three years of initial seedling establishment. This implies that nursery effect may not be a significant residual factor in the timing of paper birch bud flush. In another study, the same group of provenances also showed a nursery carryover effect for height growth up to four years after planting ([9]). A similar result was also reported by Carlson et al. ([6]) in other paper birch provenance trials in British Columbia. The reasons for a nursery carryover effect were not identified, but it may be due to temperature effects during seedling dormancy induction in the nursery. Southern nurseries are much warmer than northern ones during the fall period. The nursery carryover effect was present in the stock planted in all common garden location as bud flush phenology depends not only on the present (growing) environment, but also on the environment in which the buds were formed ([56], [20]). Based on the study by Westergaard & Eriksen ([56]), low temperatures during bud formation of Acer platanoides L. resulted in a more shallow dormancy level, while Heide ([20]) indicated that high autumn temperatures delay spring bud flush in Betula species. Bowden-Green & Rooke ([2]) and Lang ([27]) reported that a photoperiod at the nursery location shorter than the seed source photoperiod may have a direct impact on seedling size variation, as photoperiod can markedly alter the vegetative development of woody plants, particularly the timing of growth cessation.
In this study, a clear population differentiation was expected as the elevational differences of the highest and lowest collected provenances were large and might limit gene flow ([54]). Nonetheless, the results indicate an inconsistent trend and birch bud flush did not proceed from the lowest elevation to the highest as previously suggested by Myking ([37]) and Sulkinoja & Valanne ([51]). This was unexpected, but not unprecedented, as Sharik & Barnes ([46]) found an absence of elevational differentiation among populations of two Betula species from the Appalachian Mountains grown in a Michigan common garden. They concluded that factors other than temperature do regulate bud flush, likely the photoperiod or a photoperiod-temperature interaction ([33]). Further investigation with a greater number of provenances is required to validate whether this elevational trend on birch bud flush is stable.
When provenances from continental or inland regions (Prince George) were compared with those of a maritime or coastal region (Prince Rupert), the order of bud flush followed a longitudinal or inverse continentality trend, i.e., continental populations flushed earlier than maritime populations. A similar result was reported by Wielgolaski & Inouye ([57]) in a Pinus sylvestris L. provenance trial where continental provenances flushed before coastal provenances at a continental site. This might be due to a maritime climatic effect. According to Veen ([52]), provenances from maritime origin respond more slowly than continental provenances when brought to a continental site, because maritime climates experience gradually increasing temperatures in spring with long periods of late frosts; whereas continental climates experience rapidly increasing temperatures with short periods of late frosts. As a result, coastal provenances are exposed to forcing temperatures much earlier (late winter/early spring) than continental provenances, thereby they are “prevented” from growing by a longer chilling requirement, while provenances from a continental climatic region require a minimum amount of heat because there is less chance of late spring frosts ([37]). Seed transfer from a continental to a coastal climate may result in premature bud flush and frost damage as frost resistance during bud break in paper birch is low ([13]).
The coefficient of determination for the linear regressions to 80% bud flush are very low in all combinations, which indicate that factors other than latitude must be involved in explaining the variation among the provenances. Differences in climatic adaptation among the birch population might exist as origin of some of the provenances locations had different climatic conditions compared to that of the common gardens ([53]). The best correlation with climate variables was found at Skimikin garden, which is almost in the middle of the provenances source location. Looking at latitudinal seed transfer distance, our results suggest that latitudinal transfer northward up to 5° and southward up to 7° may be possible without any detrimental effect on bud flush, as this only leads to a later bud flush than that of local provenances. Similar observations were also reported by Hawkins & Dhar ([16]) in another birch provenance trial in BC. For the same provenances trial, Dhar et al. ([9]) reported that a transfer of 5° latitude in both directions had no negative impact on B. papyrifera height growth. Given the small number of provenances in the study, this information should be used with caution.
Conclusion
The overall results of this short term experiment suggests that: (1) there is no single factor that determines the onset of bud flush in paper birch in a common garden; rather it is a complex interaction among local genetic characteristics and environmental conditions at the growing site; (2) none of the regions showed the expected elevational cline for bud flush; rather, inconsistent trends were observed; (3) nursery displacement affects bud flush in the initial year of establishment but disappeared within a short time period; (4) seed transfer may be extended up to 5° northward and 7° southward with apparent minimal negative implications. However. caution may be needed when transferring provenances from inland regions to the coast. Although this information is useful for initiating seed zone development and seed transfer guideline for paper birch provenances in BC, the result should be considered with caution as findings for this study were based on a small number of provenances. Further study with several common garden experiments at different latitudes and elevations is highly recommended to determine the intra- and inter-population variability and plasticity of this species.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Nicole Balliet
Christopher DB Hawkins
Mixedwood Ecology and Management Program, University of Northern British Columbia, 3333 University Way, V2N 4Z9 Prince George, BC (Canada)
University of British Columbia, 3333 University Way, Kelowna, BC (Canada)
Yukon Research Centre, Yukon College, Whitehorse, YT (Canada)
Vicky G Berger
Kalamalka Research Station, BC Ministry of Forests and Range, 3401 Reservoir Road, Vernon, BC (Canada)
Department of Forest Resources, University of Idaho, Moscow, ID (USA)
Corresponding author
Paper Info
Citation
Dhar A, Balliet N, Hawkins CDB, Carlson MR, Berger VG, Mahoney R (2015). Bud flush phenology and nursery carryover effect of paper birch provenances. iForest 8: 809-817. - doi: 10.3832/ifor1367-008
Academic Editor
Andrea Piotti
Paper history
Received: Jun 03, 2014
Accepted: Mar 16, 2015
First online: May 19, 2015
Publication Date: Dec 01, 2015
Publication Time: 2.13 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 52962
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 44164
Abstract Page Views: 3118
PDF Downloads: 4274
Citation/Reference Downloads: 23
XML Downloads: 1383
Web Metrics
Days since publication: 3937
Overall contacts: 52962
Avg. contacts per week: 94.17
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 2
Average cites per year: 0.18
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Growth, spring phenology and stem quality of four broadleaved species assessed in provenance trials in the Netherlands - Implications for seed sourcing
vol. 18, pp. 242-251 (online: 22 September 2025)
Research Articles
Juvenile growth response of European beech (Fagus sylvatica L.) to sudden change of climatic environment in SE European trials
vol. 2, pp. 213-220 (online: 22 December 2009)
Review Papers
Genetic diversity and forest reproductive material - from seed source selection to planting
vol. 9, pp. 801-812 (online: 13 June 2016)
Research Articles
Genetic control of intra-annual height growth in 6-year-old Norway spruce progenies in Latvia
vol. 12, pp. 214-219 (online: 25 April 2019)
Review Papers
Fifteen years of forest tree biosafety research in Germany
vol. 5, pp. 126-130 (online: 13 June 2012)
Research Articles
Delineation of seed collection zones based on environmental and genetic characteristics for Quercus suber L. in Sardinia, Italy
vol. 11, pp. 651-659 (online: 04 October 2018)
Research Articles
Age trends in genetic parameters for growth and quality traits in Abies alba
vol. 9, pp. 954-959 (online: 07 July 2016)
Research Articles
Patterns of genetic variation in bud flushing of Abies alba populations
vol. 11, pp. 284-290 (online: 13 April 2018)
Research Articles
Comparison of genetic parameters between optimal and marginal populations of oriental sweet gum on adaptive traits
vol. 11, pp. 510-516 (online: 18 July 2018)
Research Articles
Seedling emergence capacity and morphological traits are under strong genetic control in the resin tree Pinus oocarpa
vol. 17, pp. 245-251 (online: 16 August 2024)
iForest Database Search
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords