Soil of the parent plant and AMF mix improve Cerrado’s seedlings growth in forest nurseries
iForest - Biogeosciences and Forestry, Volume 15, Issue 3, Pages 197-205 (2022)
doi: https://doi.org/10.3832/ifor3833-015
Published: May 28, 2022 - Copyright © 2022 SISEF
Research Articles
Abstract
The soil microbiota plays an extremely important role in the growth and survival of plants. The presence of some microorganisms can positively and significantly impact the growth of tree species, which can improve the performance of seedlings after planting for commercial purposes and/or for ecosystem restoration. The present study aimed to evaluate the initial growth of seedlings of Hancornia speciosa and Brosimum gaudichaudii associated with microorganisms from the soil of the parent tree and/or inoculated with arbuscular mycorrhizal fungi (AMF). Four substrates were tested: T1 (control) = Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS); T2 = Aut-dRYL + Aut-CS + inoculum of AMF (Mix); T3 = Aut-dRYL + Aut-CS + soil of the parent plant (SPP); and T4 = Aut-dRYL + Aut-CS + SPP + Mix. The AMF inoculum comprised a mix of the species Gigaspora decipiens, Rhizophagus clarus, and Scutellospora heterogama. Seedling growth was determined 270-350 days after transplanting by measuring the following parameters: mycorrhizal colonization rate (MC), abundance of spores (AS), height (H), stem diameter (D), H/D ratio, volume of roots (VR), dry matter of shoot (SDM), roots (RDM), total (TDM), shoot / root dry matter ratio (SDM/RDM), height / shoot dry matter ratio (H/SDM), and Dickson quality index (DQI). The results showed that the addition of SPP improved the growth and DQI of the seedlings, while the AMF mix minimally changed both growth and DQI. The use of symbiotic microorganisms in the nursery in Brazil is scarse due to the difficulty of acquiring these microorganisms and the lack of commercialization of specific isolates for species native to the Cerrado biome. The present study evaluated the use of soil from naturally occurring areas as a source of inoculum. The higher growth and biomass production of inoculated plants support the use of SPP as a form of inoculum and/or inoculation with native AMF to produce seedlings of H. speciosa and B. gaudichaudii.
Keywords
Forest Nurseries, Biological Inoculants, Dickson Quality Index
Introduction
The Brazilian savannah, known as “Cerrado” has been suffering distressing deforestation in the past few decades. Recent data show that less than 50% of the Cerrado’s original vegetation is totally or partially conserved ([4]). The main consequences of deforestation involve the extinction of some animal and plant species, the loss of biodiversity, and the impact on the streambed of many rivers that are essential to the local communities ([35]).
To reverse the process of degradation in the Cerrado, a viable alternative consists of reforesting deforested areas using native species from this biome. However, the low efficiency of propagation under natural conditions, inherent of some native species of Cerrado, along with predatory extraction, makes it challenging to naturally regenerate these areas. Thus, the use of recovery techniques, such as planting seedlings of native species grown in the nursery, are important to promote the restoration of the biome.
The development of research on naturally occurring species in the Cerrado biome aims to identify their application in alternative agricultural production systems (APS - [16]). APS help to reforest the cultivated area and generate income for growers through multiple products, such as wood, fruits, leaves, flowers, bark, and resins ([43]). Among the Cerrado fruit species with potential for use in APS, Hancornia speciosa and Brosimum gaudichaudii stand out.
Hancornia speciosa is a tree species of the Apocynaceae family showing difficult propagation under natural conditions because of its recalcitrant seeds which undergo a rapid dehydration of the embryo ([40]). Moreover, the pulp of the fruits causes inhibition of the seeds’ germination, which thus requires seed pre-treatment ([40]).
Brosimum gaudichaudii belongs to the Moraceae family and is very popular because of its medicinal properties. Currently, it is subject to increasing predatory extraction ([49]) that significantly reduced the populations of this species ([14]).
The successful establishment of forest tree seedlings depends on several factors, such as nutrition and the composition of the substrate of cultivation. In some cases, the low survival of seedlings in the nursery and in the field is linked to the absence of symbiotic microorganisms in the cultivation substrate. The soil microbiota plays an important role in the growth and survival of plants, both in nursery conditions and after planting in the field, with arbuscular mycorrhizal fungi (AMF) being among the most representative microorganisms which efficiently promote plant growth ([19], [26]).
Due to the great biodiversity in tropical areas, especially in Brazil, there is a wide variety of soil microorganisms which can be beneficial to plant growth by performing several important processes such as phosphate solubilization, atmospheric N fixation, and production of plant regulators, among others ([34]). Therefore, the use of soil from the natural range of a given species can be used as a source of inoculum during seedling production. This practice can also be driven by the low cost of the inoculum.
The role of AMF is essential for plant communities in natural environments, as these microorganisms improve the growth of host plants, especially under stress conditions ([31]), such as many species of the Cerrado. This biome is characterized by a short and irregular rainy season and low nutrient supply in the soil ([37], [46]). AMF can promote in the host species increased water and nutrients uptake through their hyphae, increased photosynthetic rate and regulation of gas exchange ([5]).
Despite the benefits generated by AMF to plants, there is little information regarding which AMF species are efficiently associated with tree species native to the Cerrado. Therefore, a viable alternative is to inoculate trees with the most common AMF species, such as Gigaspora decipiens, Rhizophagus clarus, and Scutellospora heterogama, which are abundant and have low host specificity. The aforementioned AMF species are frequently found in the Cerrado, which shows a high diversity of AMF ([39], [17], [41]). Previous studies reported satisfactory results in the growth of native Cerrado seedlings using the aforementioned AMF species ([1], [21]). Furthermore, the results of those studies indicated that H. speciosa and B. gaudichaudii can also benefit from the symbiotic association with AMF.
Recent studies estimate that 21.000 km² of forests are destroyed every year in Brazil. In response to deforestation, the Brazilian government has made a commitment, through the National Plan for the Recovery of Native Vegetation (PLANAVEG), to recover at least 12 million hectares of native vegetation throughout the country until 2030 ([6]). As a consequence, there is an increased demand for seedlings of species native to Brazil to serve projects for economic and/or environmental purposes. In the present study, we evaluated potential techniques to improve the growth and quality of seedlings native from Cerrado, which is one of the most affected and degraded biomes in Brazil ([46]).
The greatest challenge in the production of seedlings of native species in Brazil is the lack of information on the management of forest nurseries, especially regarding the nutritional aspects and the selection of substrates. In addition, current clean and low-cost technologies used to improve plant growth, such as the use of symbiotic microorganisms, are seldom applied in the production of these seedlings. To the best of our knowledge, there are no published studies that evaluated the performance of H. speciosa or B. gaudichaudii seedlings (two of the most common Cerrado species) grown on substrates made with soil from the parent trees, which is an important source of native microorganisms with a high capacity to benefit plant growth.
Due to the great importance of these microorganisms in helping plant growth and the need to obtain tree seedlings for reforestation, the present study aimed to evaluate the initial growth of seedlings of H. speciosa and B. gaudichaudii, associated with symbiotic microorganisms from the soil of the parent trees and/or inoculated with AMF.
Material and methods
The present study was carried out at the Laboratory of Mycorrhizal Associations, Institute of Biotechnology Applied to Agriculture (BIOAGRO) at the Federal University of Viçosa (UFV), Minas Gerais, Brazil. Plants of H. speciosa and B. gaudichaudii were grown from January to December 2017 in a greenhouse of the Department of Microbiology at UFV. The city of Viçosa (20° 45′ S, 42° 55′ W; altitude 648 m a.s.l.) has a mean annual rainfall of 1221 mm and a mean annual temperature of 19.4 °C, with an average maximum and minimum temperature of 26.4 and 14.8 °C, respectively. The region’s climate belongs to the Cwb type (rainy summers and cold, dry winters), according to the Köppen classification ([2]).
The seeds and soil of the parent trees of H. speciosa and B. gaudichaudii were collected in the Cerrado biome, near the city of Curvelo, MG (18° 45′ S, 44° 25′ W, altitude 632 m a.s.l.). Seeds were collected in December 2016 from 10 parent trees, spaced at a distance of 100 m each other. The soil (SPP) was collected at a depth of 0-20 cm in three points at a distance of 1 m from the parent tree. The SPP was sieved in the field (4 mm diameter mesh); subsequently, the soil collected from all the parent plants was mixed. In order to maintain the viability of the native microorganisms present in the soil, the SPP was stored in a cold chamber (5 °C).
The seeds of H. speciosa and B. gaudichaudii were also stored at 5 °C and conditioned in hermetically sealed plastic bags containing moist sand to avoid dehydration, according to Vanitha et al. ([53]). Prior to sowing, the seeds were soaked in water at room temperature for a period of 24 hours. Seed germination took place at the Forestry Research Nursery of the Department of Forest Engineering of the UFV.
Description, installation, and experimental procedures
Seedlings were grown in plastic planters with a volume of 2 L. All the materials used (planters, dRYL, and CS - see below) were autoclaved twice at 121 °C (1.0 atm) for one hour. Basic fertilization and transplanting of seedlings were carried out 15 days after autoclaving. The composition of the substrate in each planter varied among treatments (Tab. 1). Three different types of substrate were used: (i) autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL), collected at UFV; (ii) soil of the parent plants (SPP), collected in the area of natural occurrence of the species; and (iii) autoclaved commercial substrate (Aut-CS), which consists of coconut fiber, vermiculite, charcoal and pine bark. The addition of AMF mix (Mix) to the soil was also evaluated. Four substrates (treatments) were tested: T1 (control) = Aut-dRYL + Aut-CS; T2 = Aut-dRYL + Aut-CS + Mix; T3 = Aut-dRYL + Aut-CS + SPP; and T4 = Aut-dRYL + Aut-CS + SPP + Mix.
Tab. 1 - Substrate composition for the growth of H. speciosa and B. gaudichaudii seedlings. (Aut-dRYL): autoclaved dystrophic red-yellow latossol; (Aut-CS): autoclaved commercial substrate; (SPP): soil from parent plant; (Mix): AMF Mix (Gigaspora decipiens, Rhizophagus clarus and Scutellospora heterogama).
| Treatment | Materials | Ratio | |||
|---|---|---|---|---|---|
| Aut-dRYL | Aut-CS | SPP | Mix | ||
| T1 | Yes | Yes | No | No | 1:1 (v:v) |
| T2 | Yes | Yes | No | Yes | 1:1 (v:v) |
| T3 | Yes | Yes | Yes | N | 1:1:1 (v:v:v) |
| T4 | Yes | Yes | Yes | Yes | 1:1:1 (v:v:v) |
The AMF inoculum utilized was composed of Gigaspora decipiens, Rhizophagus clarus, and Scutellospora heterogama. The AMF used in the experiment were retrieved from the International Glomeromycota Culture Collection at the Regional University of Blumenau (FURB), Santa Catarina, Brazil. The isolates were reproduced in soil: sand (1: 1, v: v), being cultivated with brachiaria (Urochloa brizantha Hochst Stapf). The inoculum used was composed of spores and root fragments present in the soil: sand (1: 1, v: v) mixture mentioned above.
Physical-chemical analysis of the soil was carried out for characterization purposes. The analyses were conducted using standard methods ([48]). The results are illustrated in Tab. 2.
Tab. 2 - Physico-chemical characteristics of the soil sample used for the production of H. speciosa and B. gaudichaudii seedlings. (dRYL): dystrophic red-yellow latossol - total sand = 0.320 kg kg-1, silt = 0.110 kg kg-1, clay = 0.570 kg kg-1; (SPP-BG): soil from parent plant (Brosimum gaudichaudii) - total sand = 0.178 kg kg-1, silt = 0.350 kg kg-1, clay = 0.472 kg kg-1; (SPP-HS): soil from parent plant (Hancornia speciosa) - total sand = 0.207 kg kg-1, silt = 0.365 kg kg-1, clay = 0.428 kg kg-1; (pHH20): ratio 1:2.5; (P) (K): Mehlich Extractor 1; (Ca2+) (Mg2+) (Al3+): extractor: KCl 1 mol L-1; (H++Al3+): extractor CaOAc 0.5 mol L-1, pH 7.0; (SB): sum of bases; (CECt): effective cation exchange capacity; (CECT): cation exchange capacity, pH 7.0; (V): bases saturation; (m): aluminum saturation; (OM): organic matter (Corg × 1.724) - Walkley-Black method.
| Variable | Units | Sample | ||
|---|---|---|---|---|
| dRYL | SPP-BG | SPP-HS | ||
| pH | H2O | 4.79 | 4.67 | 4.63 |
| P | mg dm-3 | 0.7 | 2.6 | 1.5 |
| K+ | mg dm-3 | 6 | 81 | 47 |
| Mg2+ | cmolc dm-3 | 0.01 | 0.46 | 0.09 |
| Ca2+ | cmolc dm-3 | 0.11 | 0.69 | 0.16 |
| Al3+ | cmolc dm-3 | 0.92 | 1.14 | 1.24 |
| H++Al3+ | cmolc dm-3 | 3.93 | 5.7 | 3.9 |
| SB | cmolc dm-3 | 0.14 | 1.36 | 0.37 |
| CECt | cmolc dm-3 | 1.06 | 2.5 | 1.61 |
| CECT | cmolc dm-3 | 4.04 | 7.06 | 4.27 |
| V | % | 3.5 | 19.3 | 8.7 |
| m | % | 86.8 | 45.6 | 77 |
| OM | dag kg-1 | 1.66 | 3.05 | 2.08 |
Fifty cubic centimeters of inoculum of each AMF species were used to quantify the number of spores present in the AMF mix (total abundance). The wet sifting technique ([24]) in meshes of 0.42 and 0.044 mm was applied, followed by centrifugation in water and 50% sucrose solution. The quantification of the total spore abundance of each sample was carried out in Petri dishes with channels under a stereomicroscope. The substrate was inoculated with a ratio of 200-250 spores per planter. The same procedure was applied for the quantification of AMF spores in the SPP. The SPP substrate was inoculated using 15 and 19 spores per 50 cm-3 for H. speciosa and B. gaudichaudii, respectively.
The experiment was carried out in a completely randomized design (CRD), with four substrate formulations (Tab. 1) and six replicates, totaling 24 experimental units (seedlings) per species under study. Each experimental unit received basic fertilization, consisting of 50 mg dm-3 of K (KCl) and 100 mg dm-3 of P (NaH2PO4·H2O).
The seedlings were transplanted 40 days after sowing (two seedlings per planter). For the treatments with AMF inoculation, the mix of fungi was applied in holes made in the substrate for the allocation of seedlings, which facilitates the contact between the mix and the roots of the seedlings. In total, 5 ml of each fungal isolate was inoculated per seedling, totaling 30 ml of inoculum per planter.
During the experiment the moisture content of the substrate was maintained close to 60% of the field capacity. Every 15 days after the seedlings were transplanted, fertilization was applied using the nutrient solution (50 mL per planter with no P) described by Furlani & Clark ([23]) and adapted by Oliveira et al. ([38]). The influence of the environment on plant growth was mitigated by randomly changing the position of the planters on the bench. The seedlings were thinned 30 days after transplanting (DAT), and only the most vigorous plant of each planter was left in.
The total height of the aerial part (H) and stem diameter (D) of the seedlings were measured at 270 and 350 DAT for H. speciosa and B. gaudichaudii, respectively. The H of the seedlings was measured from the ground up to the apical bud using a ruler, while D was measured with a digital caliper. Each plant was split into root and shoot. After the roots were washed out, the volume of the root system (VR) was measured using a graduated (ml) beaker filled with water.
The abundance of AMF spores (AS) was determined according to Gerdemann & Nicolson ([24]). Soil samples were collected from all the planters; sub-samples of approximately 1 g of fine roots (≤ 2 mm) were collected from the seedlings and preserved in a solution composed of 5% formaldehyde, 5% glacial acetic acid, and 90% ethyl alcohol (v: v: v). The roots were washed and diaphanized with 10% KOH (v: v) overnight, and then left in a water bath at 90 °C for two hours. Afterwards, 50% H2O2 (v: v) was added to the KOH solution, obtaining a 10: 1 ratio between the KOH: H2O2 solutions (v: v), until the roots became translucent. The KOH and H2O2 were removed and the roots were transferred to a solution of 1% HCl for 5 minutes. Subsequently, the root fragments were colored with trypan blue ([7]) and preserved in a solution of lactoglycerol. The percentage of mycorrhizal colonization was determined with the aid of a stereomicroscope using the checkered Petri dish method ([25]). The plants were dried in an oven with forced air circulation at a temperature of 60 °C for 72 h. After drying, the total dry matter mass (TDM), the shoot dry matter mass (SDM), and the root dry matter mass (RDM) of the plants were determined using an analytical scale with an accuracy of 0.01 g. The Dickson quality index of seedlings (DQI - [15]) was calculated as follows (eqn. 1):
Data collection and analysis
Significant differences between treatments were evaluated by the analysis of variance (one-way ANOVA) and the post-hoc Tukey test (α=0.05), using the statistical software SISVAR ([18]).
Results
Mycorrhizal colonization and abundance of spores
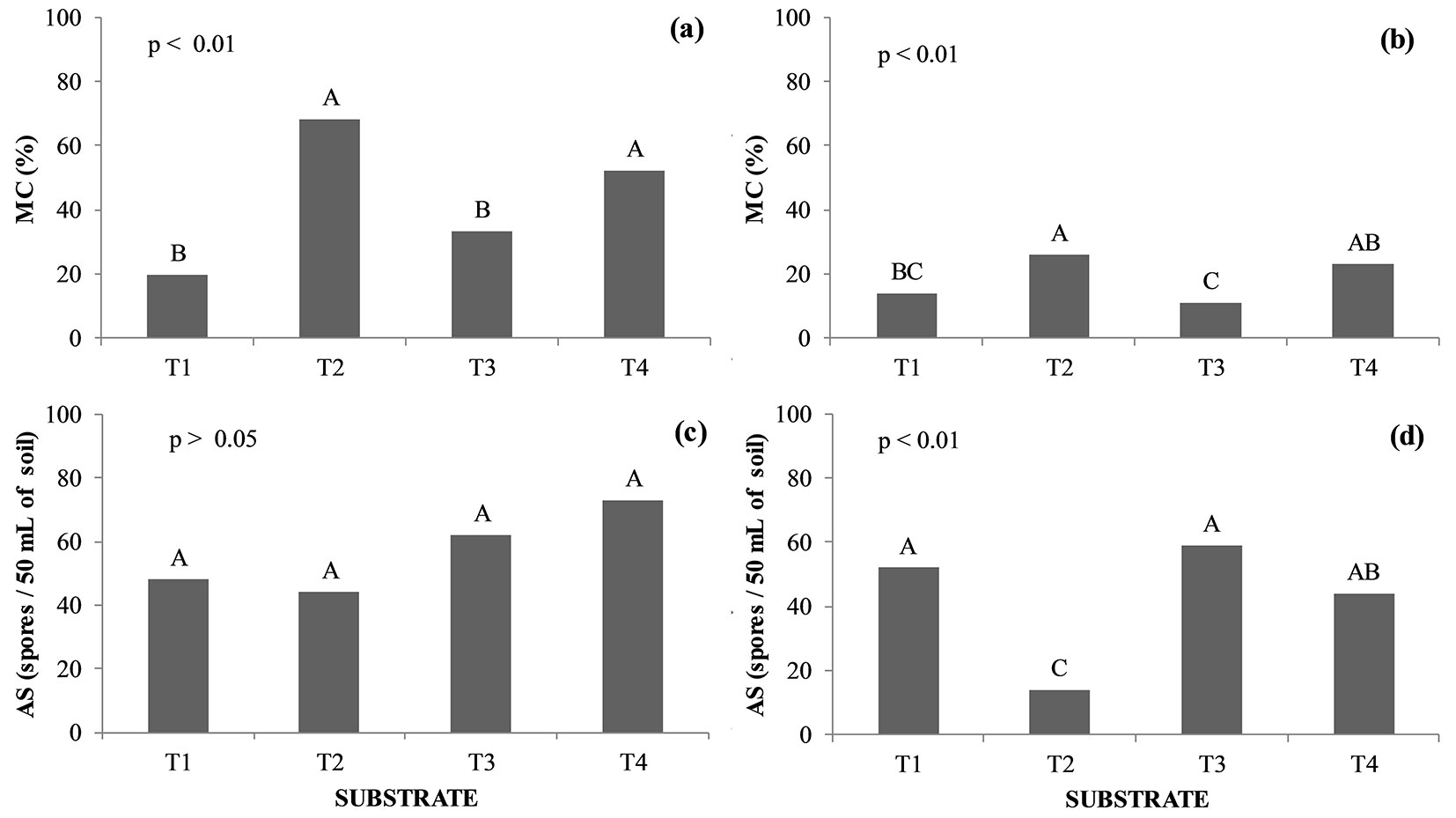
The rate of mycorrhizal colonization (MC) in seedlings of H. speciosa and B. gaudichaudii was influenced by the composition of the substrate (p < 0.01 - Fig. 1a, Fig. 1b, respectively). The abundance of spores (AS) in the substrate were different among treatments for B. gaudichaudii (p < 0.01), but not for H. speciosa (p > 0.05 - Fig. 1c, Fig. 1d). For both species, it was observed that substrates containing AMF mix (T2) presented higher MC values than those observed in T3 (Fig. 1).
Fig. 1 - Mycorrhizal colonization rate (MC) and abundance of spores (AS) of Hancornia speciosa (a, c) and Brosimum gaudichaudii (b, d) seedlings. Treatments marked with the same capital letter do not significantly differ (p>0.05) after Tukey test. (T1): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS); (T2): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + inoculum of AMF (Mix); (T3): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + rhizospheric soil of the parent plant (RSPP); (T4): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + rhizospheric soil of the parent plant (RSPP) + inoculum of AMF (Mix).
When the native AMF from the SPP and the AMF mix were used together (T4) an increase (p < 0.05) in the MC of seedlings was recorded for both H. speciosa and B. gaudichaudii, as compared to T3 (no mix). However, B. gaudichaudii seedlings grown on the substrate T3 showed the lowest MC values in this treatment; the plants had the largest increments in all the morphological characteristics evaluated, except for H/D.
There was no significant difference (p > 0.05) between treatments regarding the abundance of spores (AS) in the substrate of H. speciosa seedlings (Fig. 1c). However, there was a trend towards greater sporulation of the AMF in the substrates that contained rhizospheric soil of the parent plant (SPP - treatments T3 and T4). B. gaudichaudii seedlings grown on T2 substrate presented the lowest (p < 0.05) sporulation of AMF compared to the remaining treatments (T1, T3, and T4 - Fig. 1d). Although the substrate of T1 (control - autoclaved dRYL + autoclaved commercial substrate) had no application of inoculum sources, the seedlings subjected to this treatment presented colonization rates of 20% and 14% for H. speciosa and B. gaudichaudii, respectively.
Growth and plant dry matter mass production
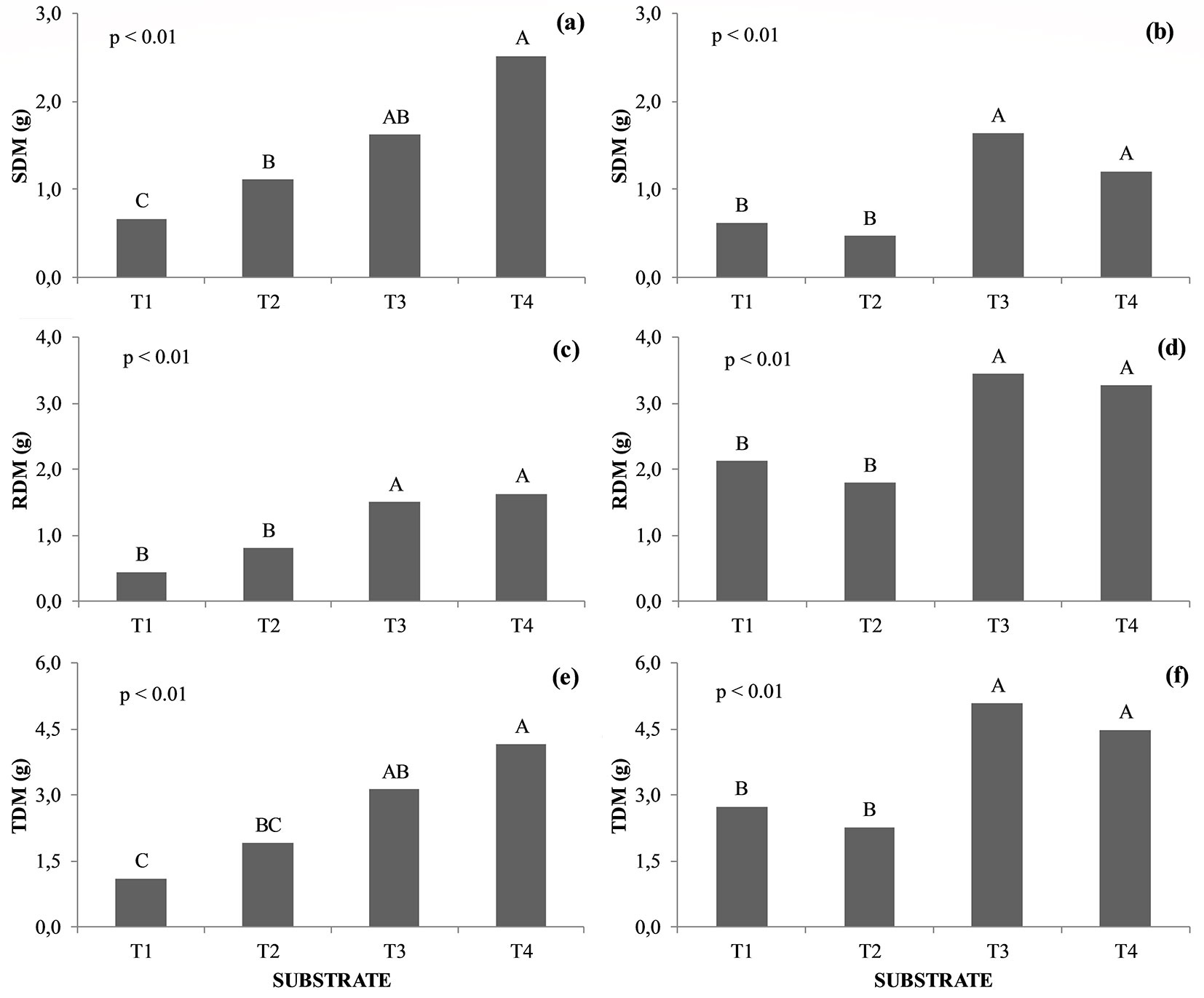
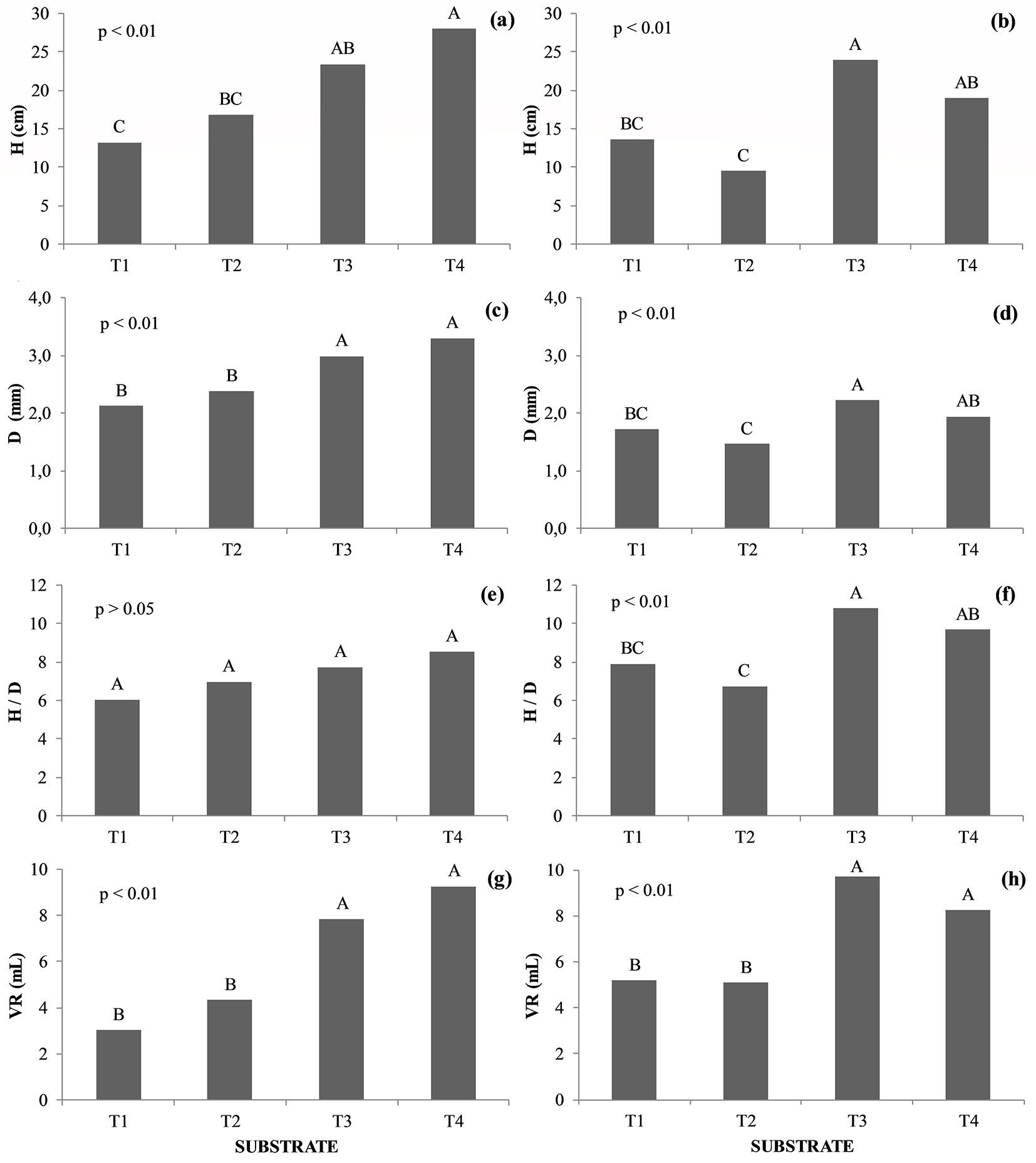
Seedlings of B. gaudichaudii had higher shoot height (H) and stem diameter (D) under treatment T3 (SPP as inoculum source) when compared to T2, in which the source inoculum was the AMF mix (Fig. 2b, Fig. 2d). Seedlings of H. speciosa and B. gaudichaudii showed no statistical difference (p > 0.05) as for H, D, H/D, and VR when cultivated on substrates T1 and T2. The only difference between these treatments was the absence (T1) or presence (T2) of the AMF mix in the soil.
Fig. 2 - Height (H), stem diameter (D), H/D ratio and volume of roots (VR) in Hancornia speciosa (a, c, e, g) and Brosimum gaudichaudii (b, d, f, h) seedlings. Treatments marked with the same capital letter do not significantly differ (p>0.05) after Tukey test. (T1): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS); (T2): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + inoculum of AMF (Mix); (T3): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + rhizospheric soil of the parent plant (RSPP); (T4): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + rhizospheric soil of the parent plant (RSPP) + inoculum of AMF (Mix).
No significant difference was observed in the VR of H. speciosa and B. gaudichaudii plants cultivated on the substrates T3 and T4 (p > 0.05). However, seedlings cultivated on T3 and T4 showed higher VR than T1 and T2 (Fig. 2e, Fig. 2f). The application of the AMF mix in the substrate generated a benefit for H. speciosa seedlings, as the VR of plants grown on T4 substrates was 18% larger than that of plants cultivated in T3. Regarding B. gaudichaudii plants, this effect was the opposite, as the seedlings grown on T3 had 18% higher VR than those subject to T4 treatment.
Seedlings of H. speciosa cultivated on T4 substrate had higher total dry matter mass (TDM) compared to those from T1 and T2 treatments. It was observed that the SDM, RDM, and TDM increments in T4 were 276%, 270%, and 277% higher, respectively, compared to T1. On the other hand, T4 seedlings produced 127%, 104%, and 117% more SDM, RDM, and TDM, respectively, compared to T2 (Fig. 3a, Fig. 3c, and Fig. 3e). Similar results were observed in plants of B. gaudichaudii. In the T3 and T4 substrates, these variables were higher than those observed in the T1 and T2 treatments (Fig. 3b, Fig. 3d, and Fig. 3f).
Fig. 3 - Dry matter of shoot (SDM), roots (RDM), and total (TDM) in Hancornia speciosa (a, c, e) and Brosimum gaudichaudii (b, d, f) seedlings. Treatments marked with the same capital letter do not significantly differ (p>0.05) after Tukey test. (T1): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS); (T2): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + inoculum of AMF (Mix); (T3): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + rhizospheric soil of the parent plant (RSPP); (T4): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + rhizospheric soil of the parent plant (RSPP) + inoculum of AMF (Mix).
Plant growth relationship
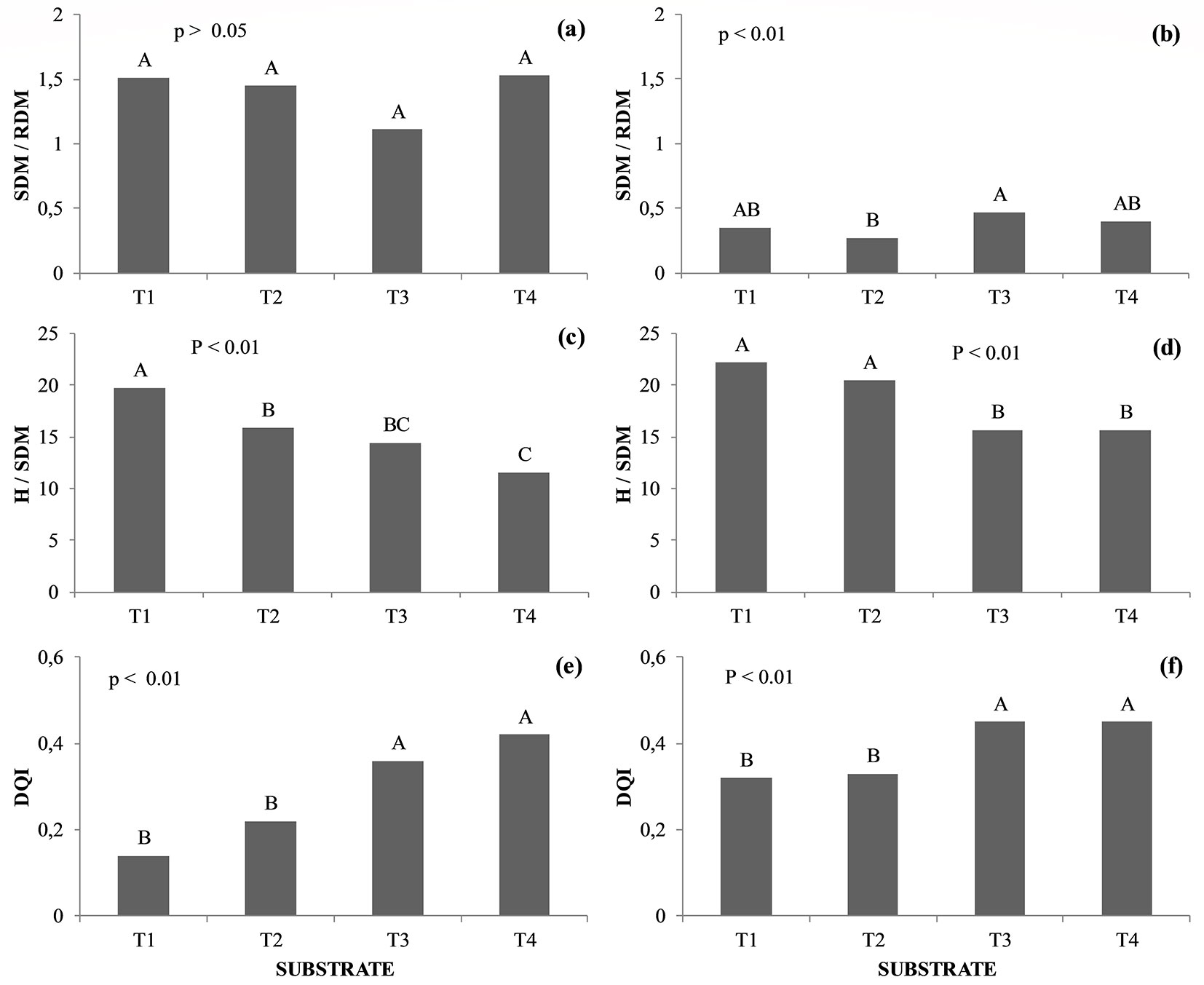
There was no significant difference in the SDM/RDM ratio of H. speciosa seedlings in response to the different treatments (p > 0.05 - Fig. 4a). For B. gaudichaudii seedlings, the highest SDM/RDM values were recorded at T3, which were significantly higher (p < 0.01) than the values at T2 (Fig. 4b). H. speciosa and B. gaudichaudii presented lower H/SDM for treatments which had the presence of SPP in the substrate (Fig. 4c and Fig. 4d, respectively).
Fig. 4 - Shoot / root dry matter ratio (SDM/RDM), height/shoot dry matter ratio (H/SDM), and Dickson quality index (DQI) in Hancornia speciosa (a, c, e) and Brosimum gaudichaudii (b, d, f) seedlings. Treatments marked with the same capital letter do not significantly differ (p>0.05) after Tukey test. (T1): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS); (T2): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + inoculum of AMF (Mix); (T3): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + rhizospheric soil of the parent plant (RSPP); (T4): Autoclaved dystrophic Red-Yellow Latosol (Aut-dRYL) + autoclaved commercial substrate (Aut-CS) + rhizospheric soil of the parent plant (RSPP) + inoculum of AMF (Mix).
The DQI of plants of H. speciosa and B. gaudichaudii grown on substrates T3 and T4 had higher values (p <0.01) when compared to plants from treatments T1 and T2 (Fig. 4e, Fig. 4f). The application of SPP (T3) determined an increase of approximately 156% and 61% in the DQI of H. speciosa seedlings, compared to the T1 (control) and T2 substrates, respectively. A similar trend was observed for B. gaudichaudii seedlings in T3, with an increase of approximately 39% in DQI compared to T1 and T2. The addition of AMF Mix (T4) provided an increase of 17% in the DQI of H. speciosa plants compared to T3, which was not observed for B. gaudichaudii.
Discussion
In the present study, we evaluated the growth of seedlings of two native species of the Cerrado biome (Hancornia speciosa and Brosimum gaudichaudii) aimed to understand whether the inoculation with symbiotic microorganisms from the soil of the parent plants and/or the application of mixed strains of AMF (Gigaspora decipiens, Rhizophagus clarus, and Scutellospora heterogama) isolates would favor plant growth during the nursery phase. The results showed that the inoculation with symbiotic microorganisms significantly promote the growth and dry mass production of H. speciosa and B. gaudichaudii seedlings.
The higher rate of root colonization in B. gaudichaudii e H. speciosa observed in T4 (AMF native from SPP + AMF mix) compared to T3 (AMF native from SPP) substantially differ from the findings of Machineski et al. ([32]), who observed that the mixture of AMF G. clarum and G. margarita generated low values of mychorrizal colonization (MC) of the roots in Ricinus communis. The authors also mention that, in some cases, the mixture of AMF can cause a reduction in MC rates, due to the existence of competition between microorganisms.
An interesting observation is related to the comparison between T3 (AMF native from SPP) and T2 (AMF from Mix), where the MC was higher at T2 in plants of H. speciosa and B. gaudichaudii. This demonstrates that, under the studied conditions, the fungi used as inoculum (G. decipiens, R. clarus, and S. heterogama) were more efficient than native AMF in colonizing host roots. However, there was a trend towards greater growth and dry mass production in seedlings of H. speciosa and B. gaudichaudii grown in T3 compared to T2. This finding indicates that the microorganisms native to the soil, though showing lower rates of root colonization (specially in B. gaudichaudii) did benefit the seedlings, and demonstrates the efficacy of native microorganisms in stimulating plant growth.
The ability of native AMF to change the competitive dynamics of plants in the natural environment is the subject of several studies ([47]). For instance, Lee et al. ([30]) studied the reproduction of Microstegium vimineum plants in growth chambers in response to the use of native AMF inoculum. The authors reported that AMF favored an increase in plant biomass and P uptake. In addition, inoculated plants showed characteristics that stimulate tillering, which is advantageous for the colonization of new territories. These results highlights the mechanisms underlying the greater competitive ability of plants promoted by microorganisms.
SPP may also have been a source of inoculum for other beneficial microorganisms besides AMF, such as plant growth promoting bacteria (PGP), which act as an additional biostimulant for the growth and production of seedlings. Bacterial communities associated with AMF spores can mediate several processes relevant to plant growth, such as disease protection and nutrient uptake. Battini et al. ([3]) demonstrated that PGP associated with Rhizophagus intraradices spores carry out beneficial processes to plants, such as siderophore production, mineral P solubilization and IAA production.
The application of beneficial microorganism as inoculum is a promising biotechnology to promote plant growth in forest nurseries. However, the use of these symbiont microorganisms to improve seedling production is yet poorly explored due to the difficulty of acquisition and the lack of commercialization of specific isolates for native species of the Cerrado biome. Thus, the use of SPP can be an alternative for seedling producers, since this source of inoculum likely contain native microorganisms that are beneficially associated with Cerrado plants. Furthermore, SPP has a low acquisition cost.
The microorganisms present in the SPP can provide greater growth and survival of plants in the field, especially under limiting climatic conditions, such as those in the Cerrado. Remke et al. ([42]) observed positive results on growth and survival of Pinus ponderosa seedlings inoculated with the biota of their parent trees. The authors reported that during the hot and dry period plants inoculated with the biotic community of their parent trees showed higher growth and survival, which were attributed especially to the reduction of water and thermal stress mediated by the domestic microbiota.
The occurrence of root colonization in control plants was an unexpected result. Normally, during the production of seedlings in the greenhouse, low rates of contamination with microorganisms incidentally occur in autoclaved substrates. The presence of mycorrhizal colonization in the control treatment (T1) can be explained by the possible partial sterilization of AMF propagules through autoclaving, contamination by the presence of fungal structures during irrigation, or by other means, such as the movement of insects. The mycorrhizal colonization of control plants likely took place due to the long period of maintenance of seedlings in the greenhouse (about one year). Similar results have been reported by Costa et al. ([11]), who observed MC in roots of H. speciosa plants with no application of inocula in fumigated soil. Costa & Melloni ([13]) also observed MC in Olea europaea seedlings in an autoclaved and uninoculated substrate.
Our findings indicate that H. speciosa and B. gaudichaudii show higher growth when inoculated in a soil adequately supplied with P (100 mg dm-3). The results of the analysis of the soils used as substrate for the production of seedlings (Tab. 2) indicate that the soil fertility was relatively low for the production of seedlings of nutrient-demanding species. However, the species studied have low nutritional requirements, which is common in species from the Cerrado. In addition, the application of nutrient solution (without P) may have provided better plant growth. In general, the response of plants to the association with AMF is higher for soils with low supply of P ([29]). Therefore, it is expected that when planted in soils with low P supply, such as those from Cerrado ([37]), the seedlings of H. speciosa and B. gaudichaudii will present even better response than that observed in the present study.
H. speciosa seedlings are highly dependent on mycorrhizae (MD) in soil disinfected and with a low supply of P (P ≤ 3 mg dm-3 - [11]). Moreover, Costa et al. ([11]) found a reduction in the response of plants when increasing the doses of P and further reported negative MD values when applying higher P doses (>93 mg dm-3) to disinfected soil inoculated with G. albida and G. etunicatum. This finding highlights the role of soil fertility, especially P, in the response of plants to the mycorrhizal association.
One of the primary benefits of mycorrhizae to plants consists of improving the absorption of nutrients, particularly phosphorus. In some cases, the effect of symbiosis can be replaced by fertilization, especially with P ([44]). The benefits generated by the AMF depends on several factors, such as the species and ecotype of the symbiontic fungus and the genotype of the plants ([50]). An example of this AMF-related specificity was found by Costa et al. ([10]), who observed that H. speciosa plants inoculated with G. etunicatum were not superior to those of the control treatment regarding SDM production. On the other hand, plants inoculated with G. albida presented an increase of 158.53% for the production of shoot biomass. The authors demonstrated that mycorrhizal inoculation shortened the production time of the seedlings by approximately 30 days, thus reducing the cost of production at the nursery.
In the present study, the application of the AMF inoculum together with the SPP (T4) had larger values of H, D, and volume of the root system (VR) of H. speciosa plants compared to T3. Larger increments in H and D of seedlings in response to AMF was also reported by Samarão et al. ([45]) who evaluated the initial growth of Anonna muricata seedlings. On the other hand, Caldeira et al. ([8]) observed an opposite trend when evaluating the influence of AMF on the initial growth of three tree legumes native to Brazil (Adenanthera pavonina, Mimosa guilandenae, and Enterolobium schomburgkii).
It is important to point out that the absence of a positive response in the growth of seedlings inoculated with AMF does not involve damage to the seedlings in their initial growth ([52]). Indeed, in some cases morphological variables related with growth, such as H, D and dry mass, may not be affected. However, improvements in nutrition, resistance to diseases and pests, among other aspects, can be observed.
The importance of mycorrhizal inoculation goes beyond the nutritional benefits generated in plants. When the roots are colonized by AMF, the root system is better protected from pathogens ([50]). This has been confirmed by several studies, where the action of the AMF provided a reduction in the damage caused by Meloidogyne incognita ([51]) and Fusarium oxysporum f. sp. herbemontis ([12]).
Regarding the seedling growth, Samarão et al. ([45]) pointed out that the efficiency of the inoculated fungi depends on their ability to compete with other AMF already present in the soil. Furthermore, a positive effect of the application of AMF inocula in the non-sterilized soil has been reported, as the inoculation generated a positive response in terms of seedling growth and nutrition ([45]).
Studies evaluating the effect of AMF on plant growth are usually performed using a single species of AMF as inoculum, especially on sterilized substrates. These results are usually obtained in the absence of competition among AMF species in the soil. However, in natural environments it is common to observe a better response of the symbiosis when introducing a greater diversity of microorganisms in the soil ([20]).
The positive effect of using a mix of native AMF species in the recovery of an area degraded by mining in the Northeast region of Brazil was reported by Souza et al. ([52]). The authors observed that the mixture of native AMF were more efficient than an exotic AMF isolate (Acaulospora longula) in promoting the growth of Guazuma ulmifolia seedlings at 14 months after planting, especially when the seedlings received less amount of organic fertilizer via manure. They detected only 6 of the 10 species of AMF inoculated in the nursery after one year since seedling establishment in the field, suggesting a difference in the adaptation and persistence of these microorganisms to the environmental conditions the degraded site. Thus, the inoculation of different symbiotic microorganisms, which possibly occurred using the SPP, may become more advantageous for the seedlings.
The effect of microorganisms on growth dynamics and biomass allocation in plants can be better assessed by analyzing the relationships between several morphological attributes of seedlings, such as H/D, H/SDM and SDM/RDM. Lower values of H/D and SDM/RDM are desirables as they indicate plants more robust and adapted to adverse situations. These relationships can predict the chances of seedling survival after planting ([27]). The same observation was made by Navarro et al. ([36]) in a study on the importance of morphological characteristics and the survival of seedlings of forest species cultivated in Mediterranean climate regions.
The increase in SDM/RDM in B. gaudichaudii in response to the native AMF (T3) in comparison to the mix of isolate AMF (T2) likely indicates that the native AMF promoted greater acquisition of soil resources. This result suggests that native AMF can produce a greater amount of hyphae and explore a greater volume of soil than the fungi G. decipiens, R. clarus, and S. heterogama. Further, being much smaller than the roots, fungal hyphae absorb a greater amount of water and nutrients with lower energy cost for the host plants, as compared with roots. As a consequence, host plants allocate more C to the symbiont and less to their root system ([44]), thus resulting in a higher SDM/RDM ratio.
Gomes & Paiva ([27]) stated that the SDM/RDM ratio is an intrinsic characteristic of each species, which does not depends on the site. However, Coelho et al. ([9]) found that the substrate composition significantly affected the SDM/RDM ratio in seedlings of Heteropteris aphrodisiaca. The authors reported SDM/RDM values of 1.88 and 0.59 for the substrates with the highest and lowest fertility level, respectively. This result is due to the greater allocation of C in the roots as an efficient strategy of nutrient search in poor soils. Freitas et al. ([22]) also found a maximum SDM/RDM ratio (1.34) in Dipteryx alata seedlings in response to the application of high doses of CaCO3 and MgCO3. The authors explained this result suggesting the an increase in base saturation of the substrate causes greater availability of P, which in turn determines a lower development of the root system of plants.
The survival of seedlings of forest species in the field is affected by their allometric characteristics and the relationships between their morphological attributes. Zida et al. ([54]) evaluated the field performance of seedlings of two species of the African savannah (Acacia macrostachya and Pterocarpus erinaceus) grown in the nursery in relation to their morphological characteristics. The authors observed that the total height of seedlings did not influence the plant survival rate, while the low shoot/root ratio (SDM/RDM < 1.0) at planting ensures their better performance during dry periods. For environments like those in the Cerrado, characterized by irregular rainfall and long periods of water deficit, the evaluation of the above parameter may be relevant for favoring seedling survival in adverse environments ([28]).
The lower C allocation to the roots of host plants promoted by AMF could theoretically involve a reduced water uptake. However, the ability of AMF to improve plant water status has been previously observed in wheat plants (Triticum aestivum) cultivated under water stress ([33]). The lower incidence of water stress in inoculated plants is attributed to the finer penetration of hyphae into the soil, which ensure an increased water uptake and, consequently, greater photosynthetic efficiency of the host plants ([33]). Therefore, the ability of AMF to improve plant water status can be essential in recovery projects aimed to establish native seedlings in the Cerrado.
The use of soil from the parent trees as substrate for seedling cultivation may entail the unintended introduction of extraneous microorganisms other than AMF with beneficial or detrimental effects on plant growth. Therefore, further in-depth investigations are needed on the microbial community living in this source of inocula and their effects on plants in the field, whether for commercial and/or environmental purposes.
Conclusion
The addition of rhizospheric soil from the parent plants (SPP) or the inoculation with native AMF improves the initial growth of H. speciosa and B. gaudichaudii seedlings in the nursery. We recommend the application of these symbiont microorganisms in the substrate of cultivation aimed at obtaining seedlings for environmental restoration purposes and/or commercial plantations. Our data demonstrated that symbiont microorganisms are significantly beneficial to seedling growth of two species native to Brazilian Cerrado, being the SPP an excellent and easy accessible source of these inocula. Such findings may help seedling producers consciously use this natural resource as a way to improve their forest nurseries, especially in low-tech production systems.
Acknowledgements
GMA: conceptualization, methodology, formal analysis, investigation, data curation, writing (original draft, review, editing), project administration; HNP and MCMK: term, conceptualization, methodology, software, validation, formal analysis, investigation, resources, visualization, supervision, project administration and funding acquisition; SD de P, BDG and G de MA: formal analysis, data curation, writing (original draft, review, editing).
The authors would like to acknowledge the Coordination for the Improvement of Higher Education Personnel (CAPES), the National Council for Scientific and Technological Development (CNPq), and the Research Support Foundation of the State of Minas Gerais (FAPEMIG).
References
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Haroldo Nogueira de Paiva 0000-0002-8891-5373
Samuel Dutra de Paula 0000-0002-3934-8317
Bruna Duque Guirardi 0000-0002-6003-2755
Departamento de Engenharia Florestal, Universidade Federal de Viçosa, Viçosa, Minas Gerais (Brazil)
Departamento de Microbiologia/BIOAGRO, Universidade Federal de Viçosa, Viçosa, Minas Gerais (Brazil)
Departamento de Engenharia Agrícola, Universidade Federal de Viçosa, Viçosa, Minas Gerais (Brazil)
Corresponding author
Paper Info
Citation
Abreu GM, Paiva HN, Megumi Kasuya MC, Paula SD, Guirardi BD, Araújo GM (2022). Soil of the parent plant and AMF mix improve Cerrado’s seedlings growth in forest nurseries. iForest 15: 197-205. - doi: 10.3832/ifor3833-015
Academic Editor
Maurizio Ventura
Paper history
Received: Mar 30, 2021
Accepted: Mar 01, 2022
First online: May 28, 2022
Publication Date: Jun 30, 2022
Publication Time: 2.93 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2022
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 8500
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 3963
Abstract Page Views: 2584
PDF Downloads: 1517
Citation/Reference Downloads: 0
XML Downloads: 436
Web Metrics
Days since publication: 1375
Overall contacts: 8500
Avg. contacts per week: 43.27
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2022): 2
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Soil fauna communities and microbial activities response to litter and soil properties under degraded and restored forests of Hyrcania
vol. 14, pp. 490-498 (online: 11 November 2021)
Research Articles
Changes in the properties of grassland soils as a result of afforestation
vol. 11, pp. 600-608 (online: 25 September 2018)
Research Articles
Impact of deforestation on the soil physical and chemical attributes, and humic fraction of organic matter in dry environments in Brazil
vol. 15, pp. 465-475 (online: 18 November 2022)
Research Articles
Phytopathogenic fungi in forest nurseries of Middle Siberia
vol. 13, pp. 507-512 (online: 05 November 2020)
Research Articles
Wood-soil interactions in soil bioengineering slope stabilization works
vol. 2, pp. 187-191 (online: 15 October 2009)
Research Articles
Influences of forest gaps on soil physico-chemical and biological properties in an oriental beech (Fagus orientalis L.) stand of Hyrcanian forest, north of Iran
vol. 13, pp. 124-129 (online: 07 April 2020)
Review Papers
The soil-conscious forestry and the forbidden apple
vol. 17, pp. 252-268 (online: 16 August 2024)
Research Articles
Effects of altitudinal gradients on leaf area index, soil microbial biomass C and microbial activity in a temperate mixed forest ecosystem of Northwestern Turkey
vol. 10, pp. 334-340 (online: 15 December 2016)
Research Articles
Soil stoichiometry modulates effects of shrub encroachment on soil carbon concentration and stock in a subalpine grassland
vol. 13, pp. 65-72 (online: 07 February 2020)
Research Articles
The effect of clear-cut age on soil organic carbon and nitrogen indices in Scots pine (Pinus sylvestris L.) stands
vol. 18, pp. 146-153 (online: 09 June 2025)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword