Ectomycorrhizal fungal community in mature white poplar plantation
iForest - Biogeosciences and Forestry, Volume 14, Issue 6, Pages 540-547 (2021)
doi: https://doi.org/10.3832/ifor3827-014
Published: Nov 26, 2021 - Copyright © 2021 SISEF
Research Articles
Abstract
Ectomycorrhizal communities are rarely studied on seasonal basis, especially in poplar plantations. In this study we analysed the ectomycorrhizal community in a mature twenty-year-old white poplar (Populus alba L.) plantation during four consecutive seasons. Using morpho-anatomical and molecular identification 30 taxa of ectomycorrhizal fungi were recorded of which 15 were identified to the species level, 12 to the genus level, 2 to the family, and one morphotype of ectomycorrhizae remained unidentified. The most abundant among identified ectomycorrhizal fungi were: Inocybe griseovelata, Inocybe splendens, Tuber rufum, and Tomentella sp. 2, which together represented up to 50% of all ectomycorrhizal root tips. The number of ectomycorrhizal fungal taxa and the percentage of vital ectomycorrhizal root tips were highest in winter and spring, respectively. The diversity indices of ectomycorrhizae, number of vital ectomycorrhizal root tips, and total fine roots in the studied poplar plantation did not differ between seasons. Ectomycorrhizal fungi belonging to Inocybaceae family and the short-distance exploration strategy were dominant in all four seasons. On the other hand, the abundance of ectomycorrhizal root tips belonging to the medium-distance exploration strategy type was significantly higher in spring in comparison with autumn and winter.
Keywords
Populus alba L., Ectomycorrhizal Diversity, Morpho-anatomical Characterization, Molecular Identification, Seasons
Introduction
White poplar (Populus alba L.) is a pioneer tree species frequently found in lowlands, in humid areas, along rivers, and in flood land forests. The species has a wide distribution range from the Mediterranean to central Asia ([38]). It has relevant environmental functions, such as prevention of soil erosion and formation of windbreaks, as well as ecological importance for maintaining the high biodiversity of riparian woodland. Poplars are fast-growing species with strong regenerative abilities, whose wood is used for the production of pellets and energy as well as raw material for the pulp and paper industry ([38]). Poplars regularly form dual associations with ectomycorrhizal (ECM) fungi and arbuscular mycorrhizal fungi which are known to prefer different soil conditions ([21], [26]). A detailed review on ectomycorrhiza of Populus was given by Szuba ([48]).
Mycorrhizal fungi essentially contribute to the functioning of the forest ecosystem, biogeochemical processes, and forest productivity. They obtain water and nutrients from the soil and deliver them to the host plant in exchange for photosynthetically-produced carbohydrates ([44]). The ECM fungi are able to access limiting nutrients (usually nitrogen) situated beyond the nutrient depletion zone which surrounds the root system of the host plant ([44]). Also, they can release nitrogen from immobilized sources normally inaccessible to the plant, such as soil organic matter or leaf litter that are accumulated on the forest floor. Additionally, they have an important role in promoting carbon sequestration in soil through increasing carbon storage ([47]). Mycorrhizal fungi link aboveground and belowground components of forest ecosystems through the common mycelial network which increases seedlings’ survival and improves their physiology, as seedlings can receive carbon, nutrients, and water from the older trees ([43]). They can improve plant tolerance to different abiotic stress factors as well as boost plant immunity and increase resistance to pathogens ([44], [35]). Furthermore, mycorrhizas have an important role in forest ecosystems’ stability under climate change, by mitigating the detrimental effects of different unfavorable factors as increased CO2 in the atmosphere, temperature rise, drought, soil acidification, pollutants, pests, diseases, etc. ([35]).
Since the functional compatibility and stress tolerance of ectomycorrhizae depend on both partners, especially on fungus, the information on the ECM community structure can be valuable in studies of forest ecosystems functioning ([31]).
ECM fungal communities on autochthonous white poplars were investigated in a drought-adapted natural forest in the Hungarian Plain in Hungary ([19]), and in a natural poplar stand in Special Nature Reserve “Kovilj-Petrovaradin marshes” in Serbia ([29]). Worldwide ECM fungal communities associated with Populus spp. in nature forest were studied in the north-central Rocky Mountains in the USA ([11]) and in the Loess Plateau in China ([34]). In Serbia ECM fungi were preliminary studied in poplar plantation by Katanić et al. ([23]) and Katanić et al. ([24]). Furthermore, ectomycorrhizas were studied in transgenic poplar plantations ([13], [46]) and transgenic aspen clones released in an experimental field ([20]). ECM communities associated with Populus tremula were investigated at sites contaminated with heavy metals in Austria ([33]) and in the USA ([12]). The community structure of ECM fungi associated with poplar clones growing at differently polluted sites was also investigated in Poland ([22]), while Katanić et al. ([28]) studied ECM community of poplars grown on pyrite tailing contaminated site in Serbia. Communities of ECM fungi were also investigated in a trial with a poplar clone exposed to elevated ozone concentration in Italy ([27]).
Besides preliminary studies of Katanić et al. ([23], [24]) who recorded, described, and determined 15 ECM taxa in a white poplar plantation in two different seasons of two consecutive years, there are not many studies concerning diversity of ECM fungi on white poplar grown in plantations. The aim of our work was to analyse diversity of ECM fungal community in a mature plantation of white poplar grown in its natural distribution area during four consecutive seasons. Additionally, seasonal variation of ECM diversity was analysed starting from the hypothesis that diversity and composition of ECM fungal community shift with the change of seasons.
Materials and methods
Sampling site and procedures
The study site was a 20-year-old white poplar (Populus alba L.) plantation located at the Experimental estate of the Institute of Lowland Forestry and Environment, University of Novi Sad, in the vicinity of Novi Sad, Serbia (45° 17′ 25.5″ N, 19° 53′ 38″ E, 74 m a.s.l.). The studied white poplar plantation is located in the riparian zone along the river Danube which provides a natural habitat for this species. Other woody species present in the plantation with minor share were: Vitis silvestris L., Robinia pseudoacacia L., Acer negundo L., Sambucus nigra L., Rubus idaeus L., Cornus sanguinea L., and Morus sp. Self-seeded poplars, including white poplar, also grow in the vicinity of the experimental field. The average annual precipitation in the area is 647.3 mm, and the average yearly temperature is 11.4 °C, according to the nearby meteorological station in Rimski Šančevi ([41]). Climate is temperate continental. Soil type at the experimental field is sandy loam fluvisol with pH 7.84 and content of carbon and nitrogen of 7.55 and 0.13 ppm, respectively ([25]).
Soil samples were collected at the beginning of each of four seasons present in the temperate zones of the northern hemisphere, namely September 2009 (autumn), December 2009 (winter), March 2010 (spring), and July 2010 (summer). Trees for sampling were randomly selected and marked to be at least 20 m from each other and at least 15 m from the edge of the plantation. The same five trees were sampled each season. A standardized soil corer of 274 ml volume and 18 cm deep was used ([32]) for taking soil core samples at about 1 m from the tree trunk. Two soil samples were taken per tree located on the opposite sides of trees. The total number of samples per season was 10, making overall 40 samples taken in the study. Soil samples were stored at 4 °C for up to one month and one day before analyses were submerged in cold water. All roots found in soil cores were carefully washed from soil in tap water and examined under a dissecting microscope to extract fine roots and to classify them as vital ECM root tips, senescent (SC) ECM root tips and nonmycorrhizal (NM) root tips.
Identification of ectomycorrhizae
The fungal partner in ectomycorrhizae was identified by combining morphological and anatomical characterization of ECM root tips by sequencing the complete internal transcribed spacer (ITS) region of nuclear ribosomal DNA. Ectomycorrhizal root tips were separated into different morphotypes using a dissecting microscope Olympus SZX 12 (magnifications 7.5-64×) and a microscope Olympus BX 51 with magnification 100-2000× (Olympus Corp., Tokyo Japan). Morphotypes of ectomycorrhizae were described and when possible, the fungal partner was identified according to published descriptions ([4], [3], [5]) following methodology given by Agerer ([1]) and Kraigher ([31]). Based on the presence and abundance of emanating elements each morphotype of ectomycorrhizae was classified into the corresponding exploration type ([2]). All ECM tips and other fine roots were counted under the dissecting microscope.
The molecular identification of the fungal partner was based on PCR amplification of ITS region of nrDNA and was performed for each morphotype in every season. Total genomic DNA was extracted from ECM root tips (5-10 tips from the same cluster per extraction) by using a Plant DNeasy® Mini Kit (Qiagen, Hilden, Germany). DNA concentration was not measured. Primer pairs ITS1F ([16]) and ITS4 ([50]) were used for PCR amplification of the complete nuclear ITS region. The PCR mixture for one sample was composed of 5 µl of 10× Gold Buffer, 5 µl of Gene Amp® deoxynucleotide triphosphates (2 mM each), 1 µl of each primer (10 µM each), 5µl of MgCl2 (25 mM), 27.6 µl of sterile distilled water, 0.4 µl of AmpliTaq Gold® polymerase (5 U µl-1), and 5 µl of DNA extract. Amplification reactions were performed in a GeneAmp® PCR System 9700 (Applied Biosystems, Foster City, CA, USA) and thermal cycling conditions were as follows: initial denaturation and polymerase activation at 95 °C for 5 min; 15 cycles at 95 °C for 35 s, 55 °C for 55 s and 72 °C for 45 s; 15 cycles at 95 °C for 35 s, 55 °C for 55 s and 72 °C for 120 s; 10 cycles at 95°C for 35 s, 55 °C for 55 s and 72 °C for 180 s and a final extension at 72 °C for 10 min. Amplified DNA fragments were first separated and purified from the agarose gel using the Wizard SV Gel and PCR Clean-Up® System (Promega Corporation, Madison, WI, USA) and send to Macrogen Korea (Seoul, Korea) for sequencing. ECM fungi were determined at the level of species, genus, or family by comparing the obtained sequence to the sequences deposited in GenBank ([36]) and UNITE ([37]) database. The threshold value applied to differentiate the different OTUs based on ITS sequence similarity was 97%.
Data analysis
After determination of ECM fungal taxa the following parameters were derived: the number of taxa of ECM fungi, number of vital ECM root tips, number of SC ECM and NM root tips, number of all fine roots (summary of vital ECM, SC ECM and NM root tips), percentage of vital ECM root tips (the ratio of the number of vital ECM tips and all fine roots) and abundance of exploration types.
Ectomycorrhizal fungal diversity indices were calculated after conversion of ECM morphotypes into specific fungal taxa for every season per single soil sample and total sampling area. The following formulas given by Atlas & Bartha ([6]) and Taylor et al. ([49]) were used: Species richness index (d) = (S-1)/log10N, where S is the number of ECM fungal taxa and N is the number of all mycorrhizal tips; Shannon-Weaver’s diversity index (H) = C/N(N log N - Σni log ni), where C=2.3, N is the number of all mycorrhizal tips and ni is the number of mycorrhizal tips of an individual ECM fungal taxon; Evenness (e) = H/log S, where H is the Shannon-Weaver’s diversity index and S is the number of ECM fungal taxa; Equitability (J) = H/Hmax, where H is the Shannon-Weaver’s diversity index and Hmax is the theoretical maximum of H assuming that each ECM fungal taxon was equally abundant; Berger-Parker’s evenness index (BP) = 1-(Nmax/N), where Nmax is the number of mycorrhizal tips of the most frequent ECM fungal taxon and N is the number of all mycorrhizal tips.
One-way analysis of variance (ANOVA) and Fisher’s Least Significant Difference (LSD) test based on individual soil samples were used to test the effect of season on the number of taxa of ECM fungi, vital ECM root tips, SC ECM and NM root tips, all fine roots, percentage of vital ECM root tips, and abundance of exploration types. Prior to the statistical analysis of these traits, normal distribution of data sets was obtained by square root transformation of the count data ([7]) and by the arcsin transformation using the Bliss formula for the percentage values ([45]). Kruskal-Wallis ANOVA and Multiple range comparison tests were used to test differences in diversity indices. According to Chao & Chiu ([9]), non-parametric approach is usual in the analysis of variation of diversity indices. Statistical analyses were performed using the package STATISTICA® v. 12.0 (StatSoft, Tulsa, OK, USA).
Based on recorded data, the following parameters were derived: the percentage of taxa of ECM fungi that were present in one, two, three, or four seasons; the percentage of vital ECM root tips belonging to taxa of ECM fungi that were present in one, two, three, or four seasons; relative abundance of ECM fungal families and number of taxa of ECM fungi belonging to particular ECM fungal families.
Results
Fine root parameters and ectomycorrhizal diversity indices
From 40 soil samples in total, 56.101 fine roots were analysed, 17.286 of them representing vital ECM root tips. The highest number of ECM fungal taxa was recorded in winter (22) followed by spring (19), while the lowest number was counted in autumn and summer (17 - Tab. 1). The mean number of all fine roots per individual soil sample ranged from 1281 (4678 dm-3) in autumn to maximum of 1446 (5278 dm-3) in winter. The average number of vital ECM root tips per soil sample was lowest in autumn with 271 (991 dm-3) and highest in spring with 576 (2105 dm-3 - Tab. 2). The ratio of vital ECM root tips in the total number of all fine roots was highest in spring (40.1%) and lowest in autumn (21.2% - Tab. 1).
Tab. 1 - Total number of taxa of ectomycorrhizal fungi, vital ectomycorrhizal root tips, senescent ectomycorrhizal and nonmycorrhizal root tips, total fine roots, percentage of vital ectomycorrhizal root tips in total fine roots and diversity indices counted in white poplar (Populus alba L.) plantation per season.
| Counts | Autumn | Winter | Spring | Summer |
|---|---|---|---|---|
| Number of taxa of ectomycorrhizal fungi | 17 | 22 | 19 | 17 |
| Vital ectomycorrhizal root tips | 2717 | 4596 | 5769 | 4204 |
| Number of senescent ectomycorrhizal and nonmycorrhizal root tips | 10101 | 9862 | 8615 | 10237 |
| Total number of fine roots | 12818 | 14458 | 14384 | 14441 |
| % of vital ectomycorrhizal root tips in all fine roots | 21.20 | 31.80 | 40.10 | 29.10 |
| Species richness index (d) | 4.66 | 5.46 | 4.79 | 4.42 |
| Shannon-Weaver index (H) | 2.40 | 2.36 | 2.60 | 2.02 |
| Evenness (e) | 0.85 | 0.78 | 0.88 | 0.71 |
| Equitability (J) | 1.95 | 1.79 | 2.03 | 1.64 |
| Berger-Parker index (BG) | 0.80 | 0.79 | 0.83 | 0.70 |
Tab. 2 - Season-based average values (± standard error) for number of taxa of ectomycorrhizal fungi, vital ectomycorrhizal root tips, senescent ectomycorrhizal and nonmycorrhizal root tips, total fine roots, percentage of vital ectomycorrhizal root tips in all fine roots and diversity indices calculated per soil sample from plantation of white poplar (Populus alba L.) and compared among seasons with one-way ANOVA and Fisher LSD test or Kruskal-Wallis test (KW) and Multiple range comparison tests. Values marked with the same letter are not significantly different (p>0.05) after Fisher’s LSD test or Multiple range comparison test.
| Parameters | Autumn | Winter | Spring | Summer | - |
|---|---|---|---|---|---|
| - | Fisher’s LSD test | ANOVA p-value |
|||
| Number of taxa of ectomycorrhizal fungi | 4.3 ± 0.4 b | 5.8 ± 0.5 a | 5.4 ± 0.4 ab | 4.0 ± 0.6 b | 0.036 |
| Number of vital ectomycorrhizal root tips | 271 ± 57 a | 459 ± 72 a | 576 ± 135 a | 420 ± 164 a | 0.249 |
| Number of senescent ectomycorrhizal and nonmycorrhizal root tips | 1010 ± 146 a | 986 ± 129 a | 861 ± 159 a | 1025 ± 194 a | 0.876 |
| Total number of fine roots | 1281 ± 159 a | 1446 ± 147 a | 1438 ± 255 a | 1446 ± 321 a | 0.945 |
| % of vital ectomycorrhizal root tips in all fine roots | 22 ± 4 b | 32 ± 4 ab | 39 ± 5 a | 21 ± 5 b | 0.021 |
| - | Multiple range comparison test | KW p-value |
|||
| Species richness index (d) | 1.38 ± 0.2 a | 1.83 ± 0.2 a | 1.65 ± 0.1 a | 1.47 ± 0.2 a | 0.278 |
| Shannon Weaver index (H) | 1.07 ± 0.1 a | 1.32 ± 0.1 a | 1.24 ± 0.1 a | 0.98 ± 0.2 a | 0.211 |
| Evenness (e) | 0.75 ± 0.04 a | 0.75 ± 0.05 a | 0.75 ± 0.04 a | 0.65 ± 0.09 a | 0.784 |
| Equitability (J) | 1.74 ± 0.1 a | 1.73 ± 0.1 a | 1.72 ± 0.1 a | 1.49 ± 0.2 a | 0.756 |
| Berger-Parker index | 0.45 ± 0.1 a | 0.52 ± 0.1 a | 0.53 ± 0.04 a | 0.41 ± 0.1 a | 0.431 |
Diversity indices of ECM fungal taxa all had the highest value in spring, except the species richness index (d) with the highest value in winter. On the other hand, all indices showed the lowest value in summer (Tab. 1). ANOVA results showed that the number of taxa of ECM fungi and the percentage of vital ECM root tips in total fine roots were significantly affected by season (p<0.04 and p<0.03, respectively). According to Fisher’s LSD test, significantly lower values of the number of ECM fungal taxa were recorded in summer and autumn in comparison to winter. Other parameters did not differ significantly among seasons, yet the number of vital ECM root tips was highest in spring, twice as much as in autumn, and the average number of old non-turgescent and nonmycorrhizal fine roots was highest in summer and lowest in spring (Tab. 2). None of the analysed fungal taxa diversity indices differed significantly between the seasons according to Kruskal-Wallis ANOVA.
Ectomycorrhizal fungal community structure
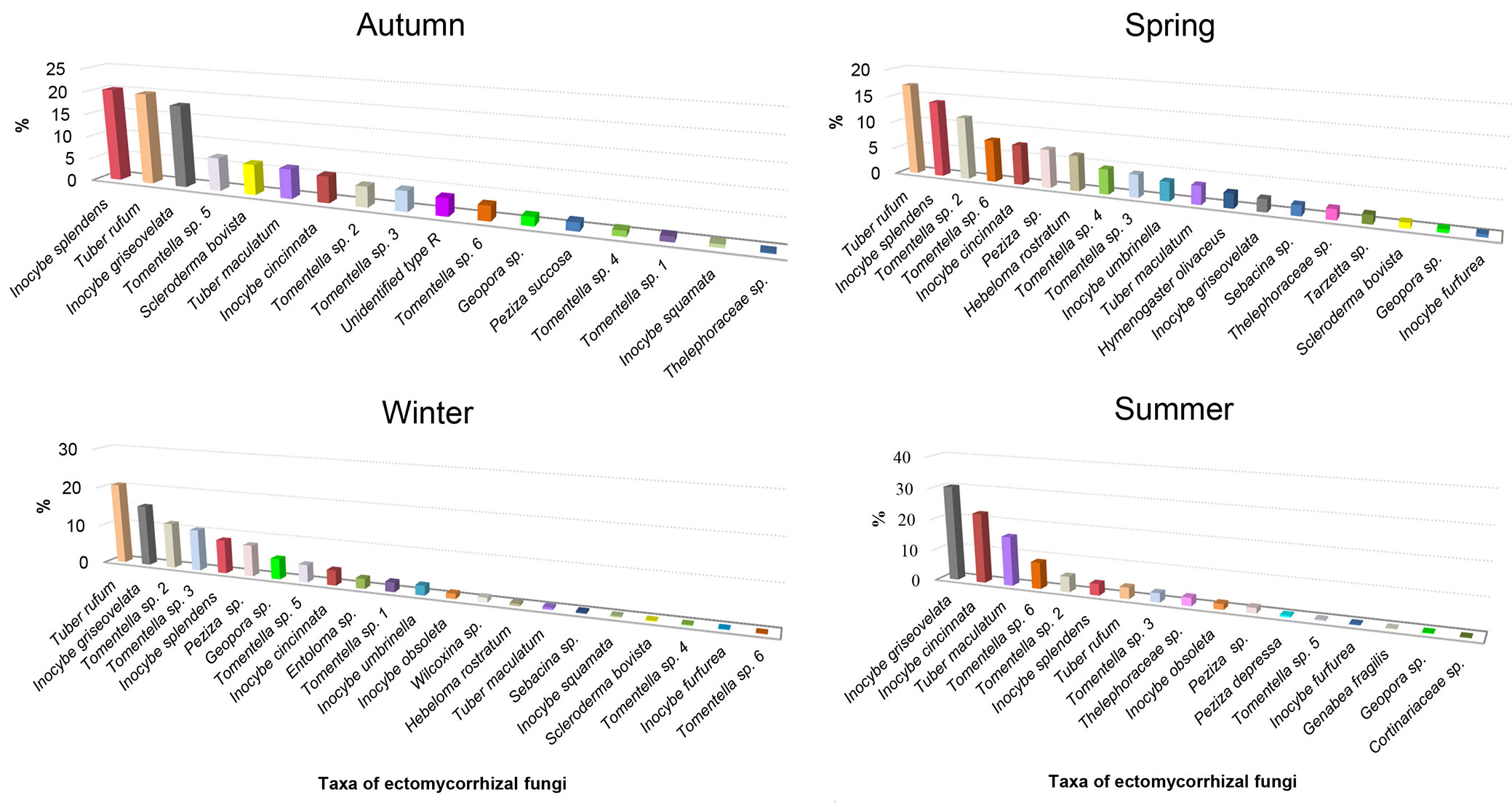
Identification of fungal partner in ectomycorrhizae revealed 30 distinct taxa of ECM fungi, from which 15 were identified to the species level, 12 to the genus level, 2 to the family level, while one morphotype of ectomycorrhizae remained unidentified (Fig. 1, Tab. S1 in Supplementary material).
Fig. 1 - Relative abundance of ectomycorrhizal fungi (based on the number of ectomycorrhizal root tips belonging to particular taxa of ectomycorrhizal fungi in relation to all ectomycorrhizal root tips) in white poplar (Populus alba L.) plantation in four consecutive seasons.
Out of the 30 identified ECM fungi only nine were present in all four seasons (Inocybe cincinnata, Inocybe griseovelata, Inocybesplendens, Tuber rufum, Tuber maculatum, Geopora sp., Tomentella sp. 2, 3, and 6). Several of them were one-season specific, namely Peziza succosa and unidentified type R were recorded only in autumn, Entoloma sp. and Wilcoxina sp. in winter, Hymenogaster olivaceus and Tarzetta sp. in spring, while Genabea fragilis, Cortinariaceae sp. and Peziza depressa were observed in summer (Fig. 1).
The most abundant among the 30 identified ECM fungi were Inocybe griseovelata, Inocybe splendens, Tuber rufum, and Tomentella sp. 2 which represented up to 50% of total ECM root tips, while other types were recorded at lower rate. In winter 22 ECM fungi were recorded. Among them Tuber rufum, Inocybe griseovelata, Tomentella sp. 2, Tomentella sp. 3, and Inocybe splendens colonized more than 60% of all ECM root tips. In spring 19 ECM fungi were found, from which Tuber rufum, Inocybe splendens, Tomentella sp. 2, Tomentella sp. 6, and Inocybe cincinnata dominated the ECM community. In summer 17 ECM fungi were observed but Inocybe griseovalata, Inocybe cincinnata, and Tuber maculatum made up almost 70% of all ECM root tips. In autumn also 17 ECM fungi were observed from which Inocybe splendens, Tuber rufum, Inocybe griseovelata, Tomentella sp. 5, and Scleroderma bovista made up more than 60 % of all ECM root tips (Fig. 1).
Seasonal variation
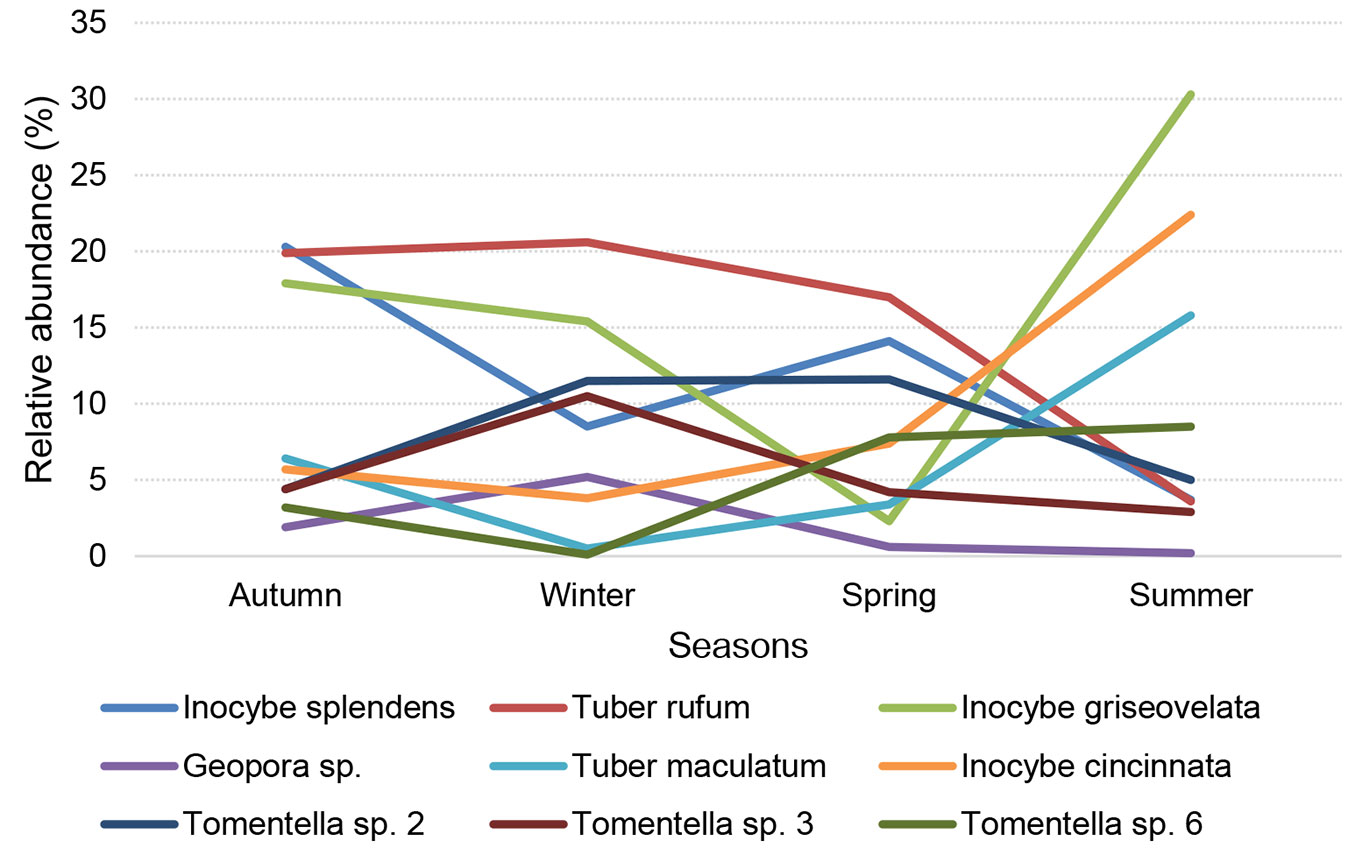
The composition of the ECM community considerably differed among seasons, as well as the abundance of the species that occurred throughout the year (Fig. 2). Tuber rufum was the most abundant ECM fungus during autumn, winter, and spring while in summer its abundance decreased. In summer we recorded an increase in the relative abundance of Inocybe griseovelata, Inocybe cincinnata, and Tuber maculatum which were the most abundant in this season. Inocybe splendens dominated the ECM community in autumn and spring, while its abundance decreased in summer. When the abundance of members of the genus Tomentella was observed seasonally, Tomentella sp. 3 had the highest relative abundance in winter, Tomentella sp. 2 in winter and spring, while Tomentella sp. 6 in spring and summer. Although recorded in all seasons, Geopora sp. had low relative abundance which reached its maximum in winter.
Fig. 2 - Seasonal dynamics of relative abundance of ectomycorrhizal fungal taxa present during all seasons in the white poplar (Populus alba L.) plantation.
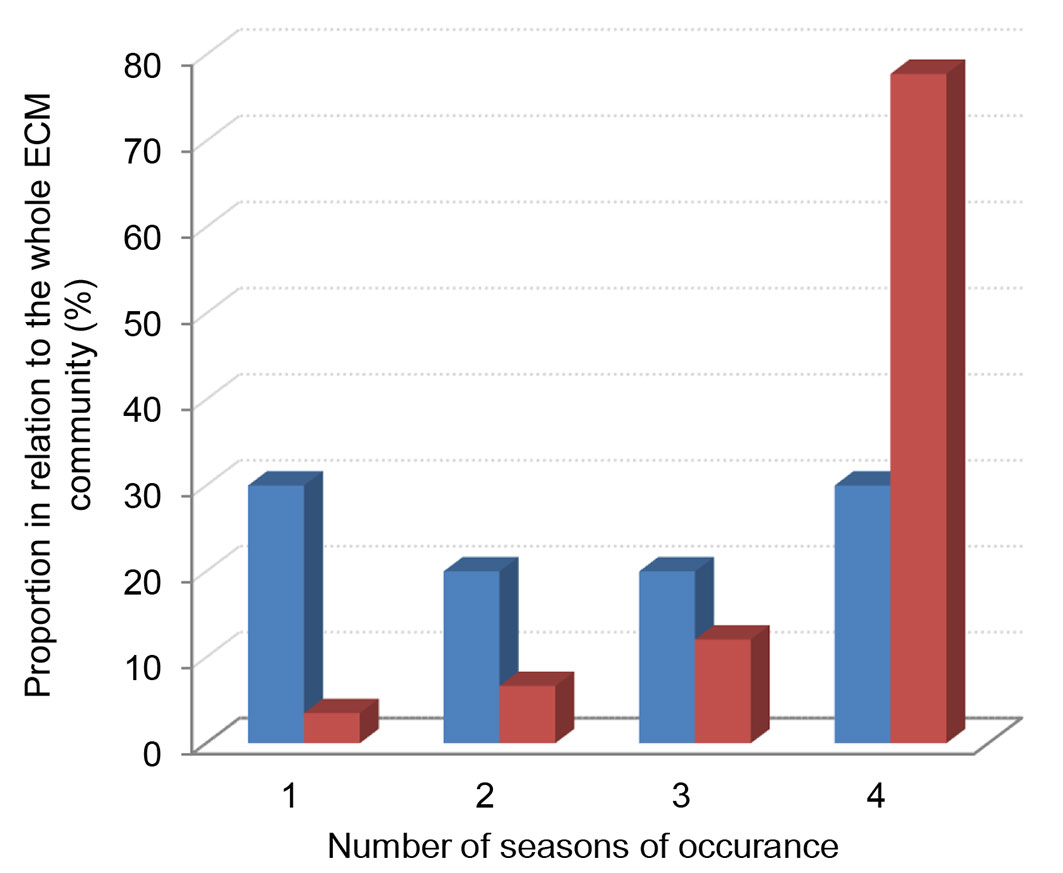
The percentage of ECM fungi recorded in one or more (two, three, or four) seasons did not differ considerably and amounted up to about 30% of the whole ECM community. On the other hand, the proportion of vital ECM root tips belonging to ECM fungi that were present in one, two and, three seasons amounted up to 10% of all ECM tips, while ECM fungi that were present in all four seasons represented 79% of all ECM root tips, indicating that the dominant ECM fungi were present in all four seasons (Fig. 3).
Fig. 3 - Distribution of frequencies of ECM fungal taxa recorded in the plantation of white poplar (Populus alba L.), i.e., sampled once vs. twice vs. three vs. four different seasons, expressed using number of taxa (blue bars) and numbers of ECM root tips (red bars)
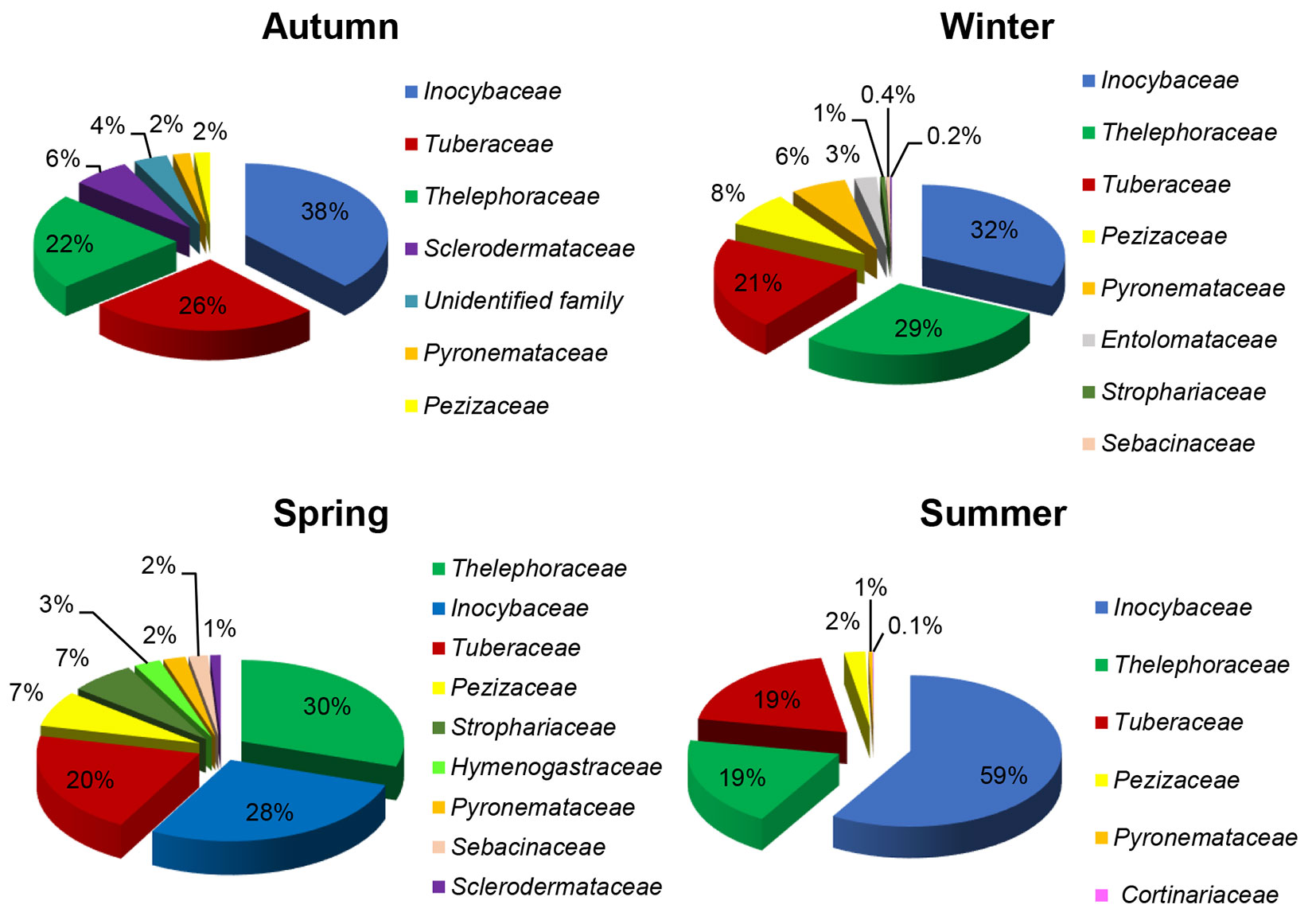
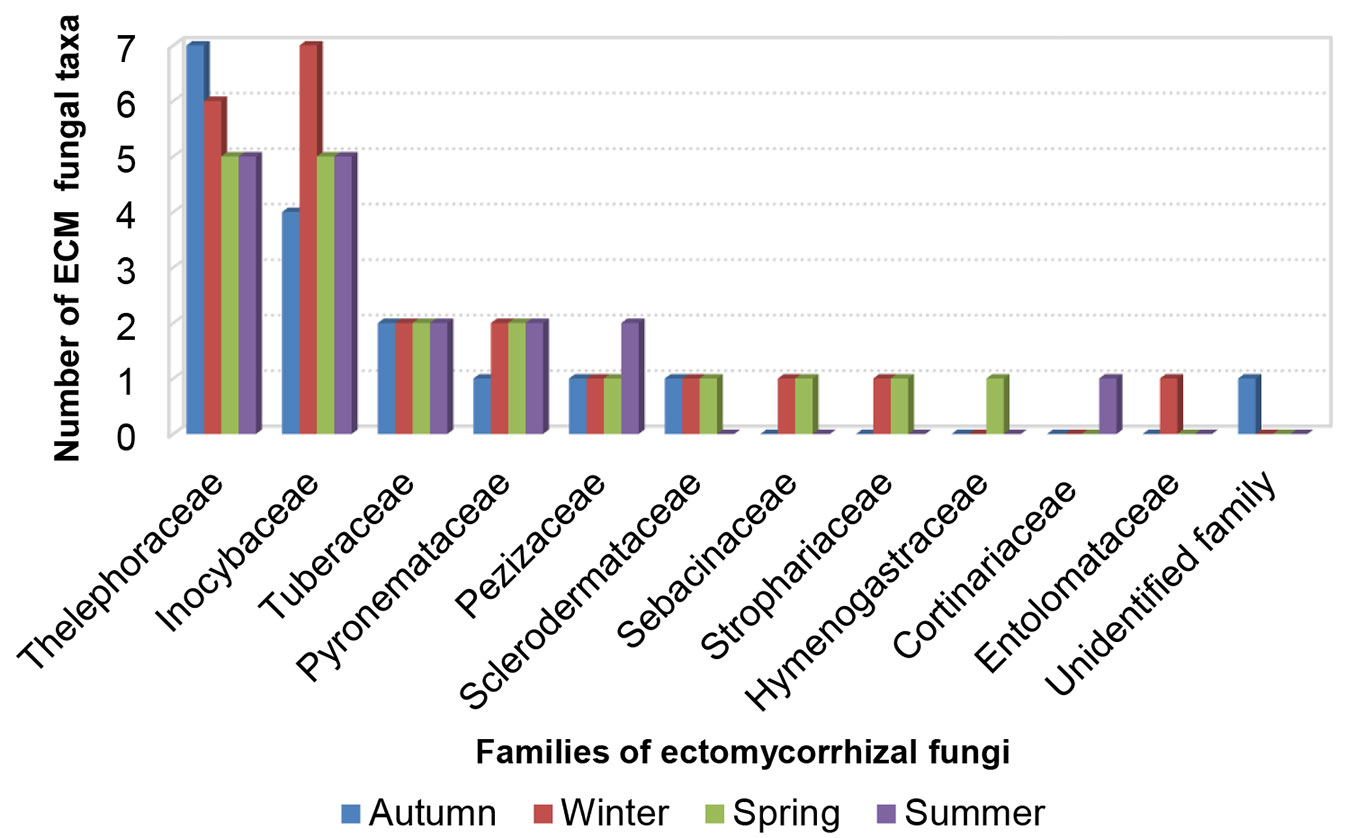
When the abundance of taxonomic families recorded in our study was compared, Inocybaceae stood out as the most abundant family, with percentages ranging from 32% of all root tips in winter up to 59% in summer (Fig. 4). Inocybaceae was followed by Thelephoraceae (ranging from 19% in summer to 30% in spring) and Tuberaceae (from 19% in summer to 26% in autumn - Fig. 4). According to the total number of types of ectomycorrhizae in the studied year, the most taxa-rich family was Inocybaceae. The highest number of taxa per season was recorded in Inocybaceae in winter and Thelephoraceae in autumn (seven), while the majority of other taxonomic families had only a single species recorded per season (Fig. 5).
Fig. 4 - Relative abundance of taxonomic families of ectomycorrhizal fungi based on the number of ectomycorrhizal root tips belonging to particular family in relation to all ectomycorrhizal root tips in white poplar (Populus alba L.) plantation in autumn, winter, spring and summer.
Fig. 5 - Seasonal number of taxa of ectomycorrhizal fungi belonging to particular fungal taxonomic families in the studied white poplar (Populus alba L.) plantation.
Seasonal dynamics of the exploration types
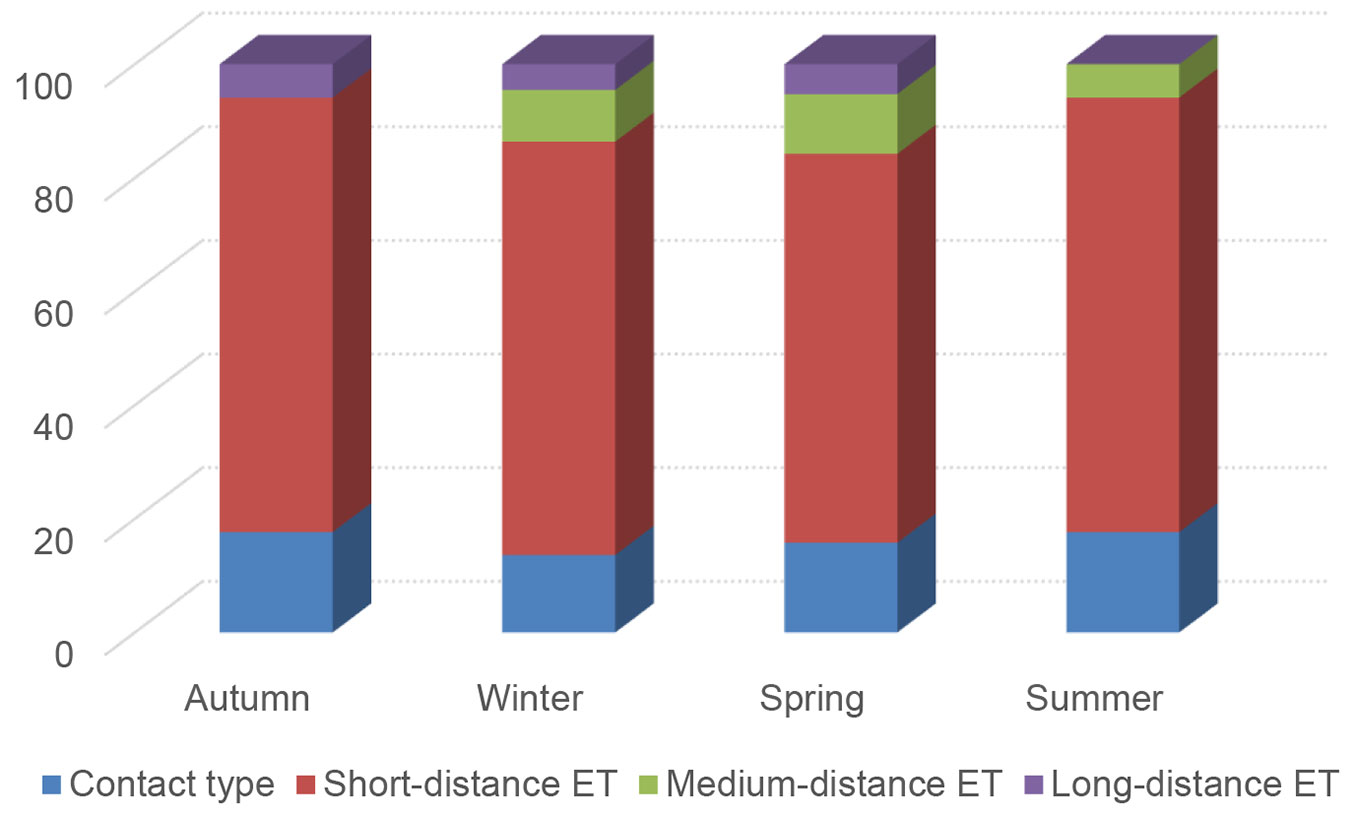
In all seasons, the short distance exploration type dominated the ECM community of white poplars. Medium-distance exploration type had significantly higher abundance in spring than in winter and autumn. Other exploration types did not show significant variability in the number of ECM root tips between examined seasons (Tab. 3). Long-distance exploration type had low abundance in all seasons, and the pick-a-back exploration type was not observed at all (Tab. 3, Fig. 6).
Tab. 3 - Seasonal dynamics of the average relative abundance of ectomycorrhiza exploration types (%, ± standard error) per soil sample. Fisher’s LSD test and one-way ANOVA were used to assess differences among seasons.
| Exploration type | Fisher’s LSD test | ANOVA p-value |
|||
|---|---|---|---|---|---|
| Autumn | Winter | Spring | Summer | ||
| Contact type | 5.5 ± 4.0 a | 10.9 ± 4.0 a | 16.6 ± 7.0 a | 6.6 ± 4.5 a | 0.367 |
| Short-distance | 91.5 ± 4.0 a | 88.2 ± 4.1 a | 71.5 ± 9.8 a | 81.3 ± 10.4 a | 0.331 |
| Medium-distance | 0 b | 0.8 ± 0.8 b | 11.2 ± 6.9 a | 2.0 ± 1.0 ab | 0.030 |
| Long-distance | 3.0 ± 2.0 a | 0.2 ± 0.2 a | 0.6 ± 0.6 a | 0 a | 0.267 |
Fig. 6 - Seasonal dynamics of the percentage of exploration types in the ectomycorrhizae associated with white poplar (Populus alba L.).
Discussion
Only a few studies have been reported on the ECM communities in poplar plantations ([13], [23], [24], [46]) and even less with their seasonal dynamics.
The average amount of vital ECM root tips recorded in mature white poplar plantation (989-2102 dm-3) is comparable to white poplar stand from Special Nature Reserve (1106-1570 dm-3 - [29]) and in poplar stand contaminated by pyrite tailing (2007 dm-3 - [28]). However, the observed values are considerably higher in comparison to the poplar clone exposed to increased ozone concentration (682 dm-3 - [28]). The number of all fine roots per soil volume in our poplar plantation (4675-5278 dm-3) is lower compared to Nature Reserve where this value was 8163-9651 dm-3 ([29]) and in poplar clone sensitive to ozone (10.398 dm-3 - [27]) as well as in poplar stand contaminated by pyrite tailing (12.424 dm-3 - [28]). It could be concluded that in extreme conditions the number of total fine roots tends to increase.
The most pronounced seasonal difference was observed in the number of ECM fungal taxa which was significantly higher in winter compared to summer and autumn. The species richness index in the studied plantation was highest in winter with a tendency to reduce in summer and autumn. Similarly, Buée et al. ([8]) recorded a higher number of ECM morphotypes in winter than in summer. They also concluded that the species structure of the ECM community and the metabolic activity of each morphotype changed depending on the season, temperature, and soil moisture. Furthermore, on willows and birch Hrynkiewicz et al. ([17]) observed a higher number of morphotypes in spring than in autumn. However, in Quercus ilex maximum values of the Species richness and the Shannon diversity index were recorded in autumn by De Roman & De Miguel ([14]). The fine roots dynamics also showed significant seasonal differences. The percentage of vital ECM root tips was highest in spring and lowest in autumn. Similarly, Courty et al. ([10]) recorded the highest number of ECM root tips in spring, while the lowest value was observed in autumn. Further, in an autochthonous white poplar site in a nature reserve ([29]), a higher number of vital ECM root tips were counted in spring compared to autumn, reflecting a potential general effect of temperate climate, where the highest rate of root activity and renewal is in spring. On the other hand, all calculated indices except the species richness were lowest in summer, which could be explained by drought conditions. Similarly, De Roman & De Miguel ([14]) noted that the lowest values of Species richness and Shannon diversity index were in summer. However, in the surface soil layer in a temperate oak forest, Courty et al. ([10]) recorded the maximum Species richness of ECM fungi in September 2004, but in September of the next year this parameter reached a minimum value. It might be assumed that environmental factors that affect a community of ECM fungi could vary from year to year depending on the weather condition. In order to get more information about the seasonal dynamics of the ECM community in white poplar plantations, further studies should include results of more subsequent years.
In the analysed plantation, only about one-third of all types of ectomycorrhizae were present throughout the whole year, while others were infrequent and can be considered as rare. This finding is in accordance with the results of many authors ([20], [14], [29], [40]), who observed that the ECM community is typically species-rich, made up of a small number of abundant species colonizing most fine roots, and a high number of rare species. Abundant species of ECM fungi were present at studied site in all seasons with a different or similar relative abundance, while some rare species were specific for a particular season. This finding is in accordance with Richard et al. ([39]) who found that the differences between seasons generally had no significant effect on the ECM community structure but caused significant changes in the abundance of particular families and species. Although Koide et al. ([30]) assumed that species of lower frequency could not be reliably grouped according to the temporal pattern, they considered temporal partitioning, a mechanism that promotes stable species coexistence, such is the case when species are active at different times of a year which could reduce the likelihood of competitive exclusion.
Among dominant and all-year present ECM fungal genera, which represented over 3/4 of all vital ECM root tips (Inocybe, Tuber, Tomentella), the genus Inocybe was the most abundant with 7 species of ectomycorrhizal fungi, while genera Tomentella and Tuber had 6 and 2 members, respectively. Most of recorded dominant genera can be considered as pioneer species ([18]) and were found in plantation forests ([23], [24], [46], [22]). This observation also supports the study of Krpata et al. ([33]), where the same taxa dominated the ECM community of poplar at an heavy metal polluted site. In an autochthonous white poplar stand in the vicinity of our research site 20 ECM fungal taxa were recorded in spring and autumn. The ECM community was dominated in both seasons by Entoloma sp., Tuber maculatum, Cenococcum geophilum, Tuber rufum and Peziza sp. ([29]). In our study Inocybe griseovelata, Inocybe splendens, Tuber rufum, and Tomentella sp. 2 were present during the whole year and represented about 50% of the total ECM community. Therefore, the observed dominant and year-around recorded/present taxa may be the key for the stability of ECM community, sustaining the stability of white poplar stands in a riparian zone, regardless of stand age or time of the year.
Classification of ectomycorrhizae into exploration types (ETs) connects ECM fungal biology, ecology, and morphology ([2]). ETs were proven to have certain site indication value, particularly related to soil composition and nutrients availability ([42]). In the study of Jakucs ([19]), in two poplars dominated forests grown on sandy soils and adapted to drought, dominant ECM fungi belonged to the long-distance ET, indicating that both mycobionts play a similar ecological role in two plots. In our study, the most abundant ET on white poplars in the examined poplar plantation in all analysed seasons was short-distance ET, followed by contact ET. Similarly, in autochthonous white poplars from a nature reserve Katanić et al. ([29]) recorded short-distance ET as the most abundant, followed by medium-distance ET. In Scots pine, Rudawska et al. ([42]) noted that the abundance of particular ET was related to the soil chemistry. They found that the occurrence of contact ET was related to the high nutrient content, while a high proportion of medium-distance ET with fringes was found only at the site contaminated with heavy metals. At unpolluted sites characterized by a higher level of nitrogen in the soil, Karlinski et al. ([22]) found that the most abundant representatives of the ECM community were species of the genus Tomentella (belonging to contact ET), while at a polluted site they found as the most abundant a medium-distance fringe ET. Also, in association with poplars from pyrite tailings contaminated site, Katanić et al. ([27]) noted the dominance of types of ectomycorrhizae belonging to a medium-distance ET.
The observed dominance of ectomycorrhizae belonging to short-distance exploration strategy in the white poplar plantation over all seasons, as well as in natural white poplar dominated forests, might indicate similar environmental conditions at these sites. Investigating patterns in the distribution of ETs on roots in European beech, pine, and spruce stands across Europe, Rosinger et al. ([40]) found that contact or short-distance ETs of ECM fungi have broad environmental ranges and the highest abundance compared to other ETs. Short-distance ETs costs plant less carbon and were favoured in colder climates where soils were richer in total nitrogen, while long-distance ETs dominated communities were more common in warmer and less fertile environments ([15]). Further, a low proportion of medium-distance and long-distance ETs at the investigated site indicate that nutrients and water were present in sufficient quantities in the vicinity of fine roots, therefore not so many ECM fungi had to explore distant parts of the soil and spend carbon on it. A higher abundance of medium-distance ET observed in spring compared to winter and autumn could be explained by the higher number of vital fine roots observed in the spring. It seems that medium-distance ET with emanating hyphae and rhizomorphs have a higher chance to make contact and to colonize newly formed roots in spring compared to contact and short-distance ETs that are characterized by poorly developed extrametrical mycelium ([2]). Thus, due to higher chances to colonize an increased number of new fine roots in spring, the abundance of ECM fungi belonging to medium-distance ET increased as well.
Conclusions
This work represents the first relatively comprehensive study of the diversity of ECM fungi in white poplar plantation during four consecutive seasons. Thirty ECM fungal taxa were recorded and identified in most cases by molecular methods only, due to lack of descriptions of their ectomycorrhizas in the literature. Although the number of ECM fungal taxa was highest in winter and the percentage of vital ECM root tips was highest in spring, our results showed that the diversity indices of ectomycorrhizae in the studied poplar plantation did not change across seasons. However, the structure of ECM community depended on the season but only in the case of infrequent ECM fungal taxa, while abundant ECM fungal species were present in all studied seasons. Since ECM fungi belonging to the short-distance exploration strategy were dominant in all four seasons, it could be concluded that the functional diversity of ECM fungal community did not change much with seasons.
Thus, we assume that the hypothesis concerning the diversity of ECM fungal community shift with the change of seasons can be rejected, while the effect of seasons on ECM fungal community composition has been confirmed only in the case of infrequent species.
Acknowledgements
HK, SO and MM conceived the study. MM, TG and MB performed research. Data analysis was performed by MM and BK. The manuscript was written by MM, BK and TG and all authors commented and approved the final version of the manuscript.
The study was co-financed by the Slovenian Research Agency through the Research Programme P4-0107 “Forest Biology, Ecology and Technology”, through the Scholarship Ad Futura (OMEGA D.O.O., for MM) and project no. 451-03-9/2021-14/ 200197 financed by the Ministry of Education, Science and Technological Development of the Republic of Serbia.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Saša Orlović 0000-0002-2724-1862
Branislav Kovačević 0000-0002-9125-0659
University of Novi Sad, Institute of Lowland Forestry and Environment, Antona Cehova 13, 21000 Novi Sad (Serbia)
Marko Bajc
Hojka Kraigher 0000-0001-5696-2178
Slovenian Forestry Institute, Večna pot 2, 1000 Ljubljana (Slovenia)
Corresponding author
Paper Info
Citation
Milović M, Orlović S, Grebenc T, Bajc M, Kovačević B, Kraigher H (2021). Ectomycorrhizal fungal community in mature white poplar plantation. iForest 14: 540-547. - doi: 10.3832/ifor3827-014
Academic Editor
Maurizio Ventura
Paper history
Received: Mar 26, 2021
Accepted: Sep 24, 2021
First online: Nov 26, 2021
Publication Date: Dec 31, 2021
Publication Time: 2.10 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2021
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 31940
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 27139
Abstract Page Views: 2346
PDF Downloads: 1911
Citation/Reference Downloads: 2
XML Downloads: 542
Web Metrics
Days since publication: 1536
Overall contacts: 31940
Avg. contacts per week: 145.56
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2021): 2
Average cites per year: 0.40
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Ectomycorrhizal fungal community associated with autochthonous white poplar from Serbia
vol. 9, pp. 330-336 (online: 12 November 2015)
Research Articles
Ectomycorrhizal diversity in a mature pedunculate oak stand near Morović, Serbia
vol. 16, pp. 345-351 (online: 22 November 2023)
Research Articles
Influence of inorganic salts on biomass production, biochemical composition, and bioethanol production of Populus alba
vol. 13, pp. 566-574 (online: 07 December 2020)
Research Articles
Assessment of cadmium tolerance and phytoextraction ability in young Populus deltoides L. and Populus × euramericana plants through morpho-anatomical and physiological responses to growth in cadmium enriched soil
vol. 10, pp. 635-644 (online: 01 June 2017)
Research Articles
Effects of substrate and ectomycorrhizal inoculation on the development of two-years-old container-grown Norway spruce (Picea abies Karst.) seedlings
vol. 8, pp. 487-496 (online: 10 November 2014)
Research Articles
The use of branch enclosures to assess direct and indirect effects of elevated CO2 on photosynthesis, respiration and isoprene emission of Populus alba leaves
vol. 1, pp. 49-54 (online: 28 February 2008)
Research Articles
Ectomycorrhizae of Norway spruce from its southernmost natural distribution range in Serbia
vol. 12, pp. 43-50 (online: 10 January 2019)
Research Articles
Identification and molecular characterization of LTR and LINE retrotransposable elements in Fagus sylvatica L.
vol. 2, pp. 119-126 (online: 10 June 2009)
Review Papers
Genomics of the Dutch elm disease pathosystem: are we there yet?
vol. 8, pp. 149-157 (online: 07 August 2014)
Review Papers
Arbuscular mycorrhizal fungi as a tool to ameliorate the phytoremediation potential of poplar: biochemical and molecular aspects
vol. 7, pp. 333-341 (online: 17 April 2014)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword