Ectomycorrhizae of Norway spruce from its southernmost natural distribution range in Serbia
iForest - Biogeosciences and Forestry, Volume 12, Issue 1, Pages 43-50 (2019)
doi: https://doi.org/10.3832/ifor2729-011
Published: Jan 10, 2019 - Copyright © 2019 SISEF
Research Articles
Abstract
Norway spruce (Picea abies Karst.) reaches its southernmost limit in the mountainous regions of south Serbia and Bulgaria. The species is a regionally important timber species for the wood industry and a significant host for various ectomycorrhizal fungi, including edible species. We analysed ectomycorrhizal community and fine root parameters of high continental / subalpine Norway spruce stands at three sites (Stara planina, Kopaonik, Tara) located in protected areas in Serbia. In addition, we assessed the potential effects of altitude and growing season on the ectomycorrhizal diversity and fine root parameters. Using standardised sampling in combination with morpho-anatomical and molecular identification of ectomycorrhizae, we recorded 29 different anatomorphotypes. None of the identified fungi belonged to commercial edible fungal species. Compared to other Norway spruce ectomycorrhiza studies in central Europe, sites in Serbia exhibited lower species diversity and different dominant species composition, with Cenococcum spp. and Russula spp. as the dominant ectomycorrhizal fungi. A number of ectomycorrhizal types and the value of the species richness index differed between Stara planina and Tara in the autumn, but the influence of site and season on the studied diversity indices was not significant. The total number of fine roots increased in the spring, while percentage of vital ectomycorrhizal root tips increased in the autumn. This study was the first examination of Norway spruce ectomycorrhizal communities at the edge of the natural geographical range of the species.
Keywords
Ectomycorrhiza, Picea abies Karst., Community Structure, Diversity, Fine Roots
Introduction
Norway spruce (Picea abies Karst.) is economically important, being the main species in the Boreal and subalpine conifer forests, distributed from Central Europe to Northern and Eastern Europe. The species reaches its southernmost natural distribution in the Dinaric Alps, the Balkan Mountains, and the Carpathian Mountains ([12]).
In Serbia, Norway spruce is the most represented coniferous tree species with a forest gene pool making up 5.2% of the total volume (National Forest Inventory of Republic of Serbia - [9]). As in other southern alpine areas, Norway spruces is distributed above the beech area on high mountains and mountain ranges with cold and humid climate or in frost sinkholes ([20], [8]). The local populations are likely to be relicts from the last glaciation period ([29], [35]).
Besides its importance as a quality wood source, Norway spruce is known to host several ectomycorrhizal species ([5]) that are renowned for their culinary value, for example Boletus edulis Bull., Cantharellus cibarius Fr. and Hydnum rufescens Pers. These and other ectomycorrhizal fungi are commonly collected by locals and either consumed or sold at regional markets ([11]).
Norway spruce forests of Europe have been thoroughly studied for ectomycorrhizal diversity ([44]). Ectomycorrhizal communities in Norway spruce populations were well studied from the Central and Southern Alps, namely Slovenia ([26]), Austria ([45]) and Germany ([7]), as well as in the Carpathian mountains for Central European populations ([34]) and the Northern populations in the boreal region ([14], [32], [33]).
The isolated populations of Norway spruce on mountains in Serbia remain unexplored for their ectomycorrhizal community. To bridge this knowledge gap, we selected sites at three protected areas at mountains where spruce reaches its southernmost distribution range: Stara planina, Kopaonik and Tara, located in Southeast, South and Southwest Serbia, respectively. The study focused on the diversity of ectomycorrhizal fungi at Norway spruce natural sites and on potential influences of two contrasting seasons (spring and autumn) and three different sites on ectomycorrhizal communities of spruce.
Methodology
Sites and sampling procedures
The sampling sites were selected in spruce stands located in protected areas in Serbia. Sites were selected aiming at covering the distribution patches of spruce at its southernmost natural distribution range on mountains in Serbia ([40]). Detailed characteristics of the selected areas are provided in Tab. 1.
Tab. 1 - Coordinates of sites, altitude, climate, mean annual temperature and precipitation ([36]), soil type (Z. Galić, personal communication), mean temperature in June 2014 and September 2013 (Z. Galić, personal communication) and tree species composition at Stara planina, Kopaonik and Tara sites. (*): mean annual temperature and precipitation data shown for nearest measuring station in Dimitrovgrad and Zlatibor for Stara planina and Tara, respectively.
| Site | Stara planina | Kopaonik | Tara |
|---|---|---|---|
| Coordinates | 43°10′ 34.6″ N 22°43′ 25.0″ E |
43°18′ 16.8″ N 20°50′ 54.3″ E |
43°55′ 03.8″ N 19°25′ 51.6″ E |
| Altitude (m a.s.l.) | 950 | 1490 | 1060 |
| Climate | Temperately continental submontanum, montanum | Temperately continental montanum, subalpine | Temperately continental montanum |
| Mean annual temperature (°C) * | 10.0 | 3.6 | 7.7 |
| Mean temperature in June 2014 (°C) | 14.5 | 11.0 | 12.6 |
| Mean temperature in September 2013 (°C) | 12.8 | 9.1 | 9.3 |
| Mean annual precipitation (mm) | 624.7 | 984.4 | 1017.3 |
| Soil type | Brown soil | Ranker | Brown soil |
| Species | Natural forest of Fagus sylvatica, Picea abies and Abies alba spontaneously occuring in minor parts | Natural Picea abies forest without regeneration |
Natural mixed forest of Fagus sylvatica, Picea abies and Abies alba with regeneration |
| Age of spruce trees (years) | 10-20 | 50-70 | 40-50 |
Soil sampling (four per site) was performed in the absence of snow cover, in September 2013 and June 2014, resulting in eight samples per site. A standardised soil corer with 4-cm diameter and 18-cm length (total volume 274 ml) was used for soil core sampling at 0.5 m from the tree trunks ([26]). At mixed sites, the areas with pure Norway spruce were targeted for sampling. When possible, soil cores were taken from locations with trees of different age to obtain potentially wider diversity of ectomycorrhizal community.
Soil samples were stored at 4 °C for up to 6 weeks. Prior to analyses, each sample was submerged in cold tap water to loosen the soil structure. All roots were carefully washed from soil. Using a binocular (Kruss GmbH, Hamburg, Germany) with magnifications 10-45× (light source: Olympus Highlight 3100, daylight filter), fine roots were separated into vital ectomycorrhizal root tips, old and non-turgescent fine roots, or non-mycorrhizal vital fine roots.
The ectomycorrhizal species were identified in a two-step procedure combining morphological and anatomical characterisation of ectomycorrhizal root tips to a level of an individual anatomorphotype. Each anatomorphotype was further analysed by molecular analysis of nuclear rDNA ITS region.
Microscopic characteristics of ectomycorrhizal root tips were assessed using an Olympus BX 51® (Olympus Corp., Tokyo Japan) with magnifications 100-2000×. Anatomorphotypes of ectomycorrhiza were identified by comparison with published descriptions in Agerer et al. ([4]), Agerer ([3]), or Agerer & Rambold ([5]), following the methodology given by Agerer ([1]) and Kraigher ([25]).
Ectomycorrhizal types were also classified into the exploration types based on the presence and abundance of emanating elements as proposed by Agerer ([2]). All vital ectomycorrhizal root tips, old and non-turgescent fine roots and non-mycorrhizal vital fine roots were counted under a binocular. Total number of fine roots was obtained by summing all of these categories of roots.
Coarse roots in each soil sample were checked for tree roots species confirmation following the anatomical characteristics of wooden parts ([30]). All extraneous coarse roots with attached fine roots were eliminated from further analysis.
Molecular identification of ectomycorrhizal fungi
Molecular confirmation of fungal partners in ectomycorrhiza using molecular methods was based on a PCR amplification of fungal nuclear rDNA ITS region from each separated anatomorphotype. This molecular marker is currently considered as the best for fungi barcoding and differentiation at the species level ([24]). Total genomic DNA was extracted from ethanol-stored ectomycorrhizal root tips using a Plant DNeasy® Mini Kit (Qiagen, Hilden, Germany). If DNA extraction of representative ectomycorrhizal root tips of some anatomorphotype was not successful and morpho-anatomical identification was insufficient to determine the ectomycorrhizal fungus, this ectomycorrhizal type remained unidentified and was labelled as “unknown” type.
Amplifications were performed with ITS 1f ([17]) and ITS 4 primer pair ([46]). PCR reactions were optimized for the quantity of DNA extract and annealing temperature to give the best product in the reaction. Amplification reaction was performed in GeneAmp® PCR System 9700 (Applied Biosystems, Foster City, CA, USA) according to the procedure explained by Sulzbacher et al. ([43]). Negative controls with no fungal DNA were run for each experiment to check for any contamination. The PCR mixture for one sample was composed of 5 µl of 10× Gold Buffer, 4 µl of deoxynucleotide triphosphates (0.2 mM each), 1.2 µl of each primer (0.32 µM each), 4µl of MgCl2 (2.0 mM), 30.3 µl of sterile distilled water, 0.3 µl of Taq polymerase (0.03 U µl-1), and 4 µl of DNA extract. Thermal cycling conditions were as follows: initial denaturation and polymerase activation at 95 °C for 5 min; 13 cycles at 94 °C for 45 sec, 55 °C for 55 sec and 72 °C for 45 sec.; 13 cycles at 94 °C for 45 sec, 55 °C for 55 sec and 72 °C for 120 sec; 12 cycles at 94 °C for 45 sec, 55 °C for 55 sec and 72 °C for 180 sec and a final extension at 72 °C for 10 min.
Amplified DNA was separated and analysed as described by Grebenc et al. ([19]). Amplified DNA fragments were separated and purified from the agarose gel using the Wizard SV® Gel and PCR Clean-Up System® (Promega Corporation, Madison, WI, USA) and sent for Sanger sequencing to Macrogen Korea (Seoul, Korea). Sequencher® ver. 5.1 (Gene Codes Corporations, Ann Arbor, MI, USA) was used to identify the consensus sequence from the two strands of each isolate. Species, genus, or family of ectomycorrhizal fungi were determined by comparing the sequences to those deposited in GenBank ([31]) and UNITE ([19]) databases.
Data analysis
To compare diversity at sample, site, and season level, Species richness (d), Shannon Weaver diversity index (H), Evenness (e) and Equitability (J) were calculated following Atlas & Bartha ([6]), while the Berger-Parker evenness index (BP) was calculated according to Taylor et al. ([44]).
Data of an individual soil sample was used as statistical unit. The two-way ANOVA and Fisher’s LSD test were used to analyse seasonal and site differences for the measured parameters. In order to obtain normal distribution of data sets for these tests, square root transformation was performed ([10]). For a number of ectomycorrhizal types, vital ectomycorrhizal root tips, and total fine roots, arcsine transformation was performed using the Bliss formula ([42]) for the percentage of vital root tips. Statistical analyses were performed using the software package STATISTICA® ver. 12.0 (StatSoft, Tulsa, OK, USA).
Results
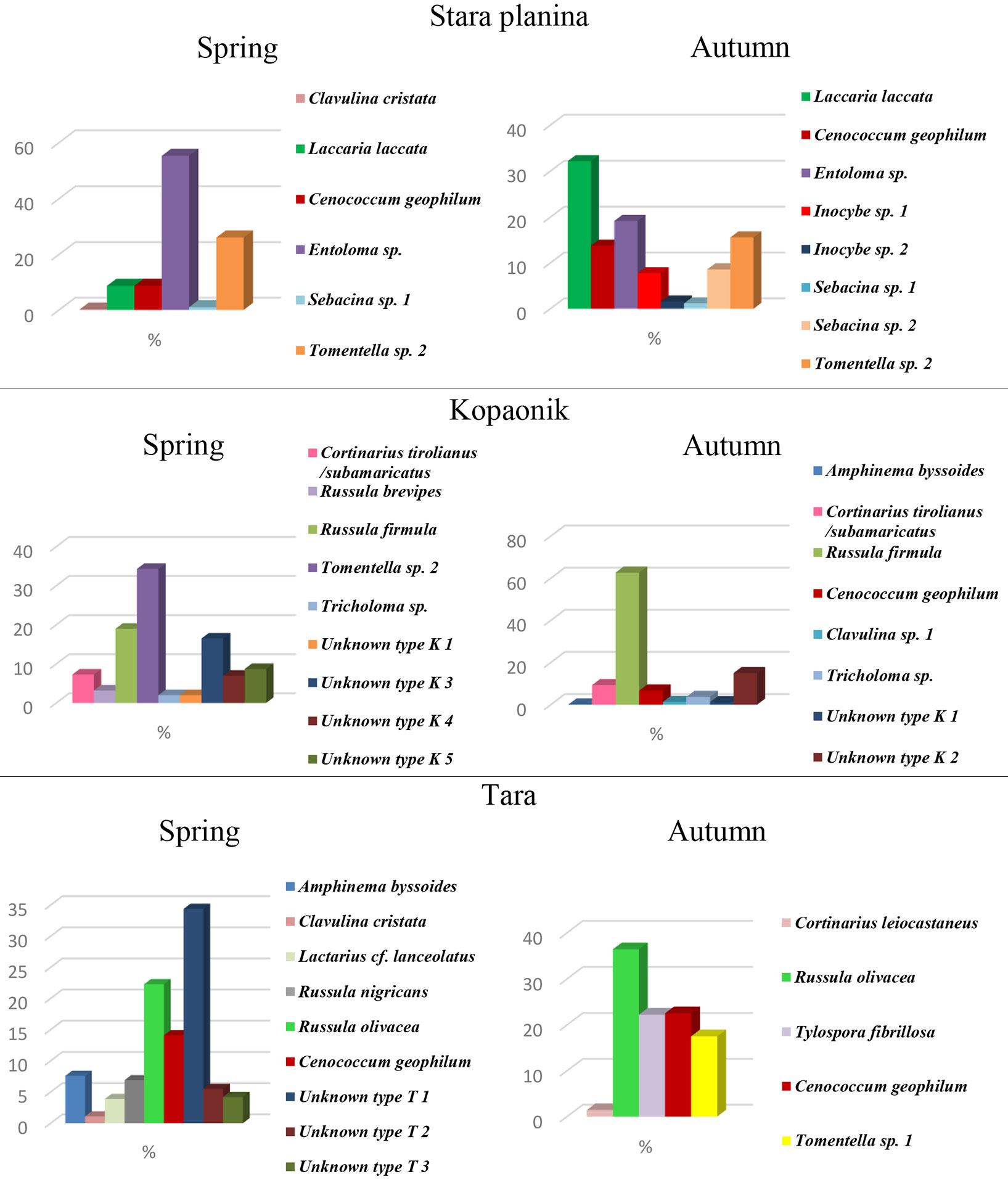
Overall, we found 29 different types of ectomycorrhizae at the three selected sites, i.e., 9 at Stara planina, 13 at Kopaonik, and 12 at Tara (Fig. 1). Six ectomycorrhizal types were characteristic for Stara planina, 9 for Tara and 10 for Kopaonik. Only the ectomycorrhizal type determined as Cenococcum geophilum was found in all the three examined sites. Tomentella sp. 2 was common at Stara planina and Kopaonik, while Amphinema byssoides was common at Kopaonik and Tara, and Clavulina cristata at Stara planina and Tara (Fig. 1). Entoloma sp. and Tomentella sp. 2 were abundantly present at Stara planina in the spring, while in the autumn Laccaria laccata, Entoloma sp. and Tomentella sp. 2 dominated. Ectomycorrhizal community from Kopaonik had Tomentella sp. 2 and Russula firmula as the major constituents in the spring, while the latter made up about 60% of all ectomycorrhizal root tips in the autumn. Tara in the spring was dominated by Unknown type T1 and Russula olivacea, while in the autumn the most abundant were Russula olivacea, Cenococcum sp. and Tylospora fibrillosa. Only 11 types of ectomycorrhizae were recorded at the studied sites (Stara planina 5, Kopaonik 4 and Tara 2) in both seasons (Fig. 1).
Fig. 1 - Relative abundance of ectomycorrhizal types at examined sites Stara planina, Kopaonik, and Tara in the spring and autumn.
The lowest total number of ectomycorrhizal types was recorded for Tara in the autumn (5), while the highest number (9) was recorded in the spring for the same site and for Kopaonik. The lowest number of vital ectomycorrhizal root tips per site was counted for Tara in the autumn (735), while the highest number was counted for Stara planina in the spring (2060). Also, the highest total number of fine roots and the lowest percentage of vital ectomycorrhizal root tips (8%) were counted at Stara planina in the spring. On the other hand, the highest percentage of vital ectomycorrhizal root tips was recorded for Kopaonik in the autumn (46% - Tab. 2).
Tab. 2 - Summarised values for total number of ectomycorrhizal types, vital ectomycorrhizal root tips, total number of fine roots, and percentage of vital ectomycorrhizal root tips on spruce from different sites in Serbia in two examined seasons (based on 4 soil cores per site and per season).
| Variable | Stara planina | Kopaonik | Tara | |||
|---|---|---|---|---|---|---|
| spring | autumn | spring | autumn | spring | autumn | |
| Total number of ectomycorrhizal types | 6 | 8 | 9 | 8 | 9 | 5 |
| Total number of vital ectomycorrhizal root tips | 2060 | 1273 | 1463 | 1469 | 893 | 735 |
| Total number of fine roots | 28175 | 4239 | 5274 | 3174 | 4347 | 2683 |
| % of vital ectomycorrhizal root tips | 8 | 32 | 28 | 46 | 41 | 27 |
Mean number of ectomycorrhizal types per soil sample was significantly higher for Stara planina in the autumn (4.75) compared with Tara in the same season (2.5 - Tab. 3). Number of vital ectomycorrhizal root tips did not significantly differ between seasons and among sites. Samples from Stara planina in the spring had the highest total number of fine roots and the lowest percentage of vital ectomycorrhizal roots compared to that from other sites. Value of Species richness index significantly differed between Stara planina and Tara in the autumn (1.507 vs. 0.629, respectively), but other diversity indices were not significantly different between seasons nor among sites (Tab. 3). Effects of site and season were significant for total number of fine roots and percentage of vital ectomycorrhizal root tips, while the site × season interaction was significant only for total number of fine roots (Tab. 4).
Tab. 3 - Mean number of ectomycorrhizal (ECM) types, vital ECM root tips, total number of fine roots, percentage of vital ECM root tips, and diversity indices on spruce from different sites in Serbia in the spring and autumn, based on 4 soil samples. Differences among values of a particular variable marked with the same letter are not significant (p > 0.05), according to Fisher’s LSD test.
| Variable | Sites by Season | Sites | Seasons | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stara planina | Kopaonik | Tara | Stara planina |
Kopaonik | Tara | Spring | Autumn | ||||
| spring | autumn | spring | autumn | spring | autumn | ||||||
| Mean number of ECM types | 3.5 ab | 4.75 b | 3.75 ab | 3.00 ab | 3.25 ab | 2.5 a | 4.12 a | 3.37 a | 2.87 a | 3.50 a | 3.42 a |
| Number of vital ECM root tips | 515 a | 318.7 a | 365.7 a | 369.7 a | 223.2 a | 226.5 a | 416.8 a | 367.7 a | 224.8 a | 367.9 a | 304.9 a |
| Total number of fine roots | 7043.7 b | 1547 a | 1327 a | 806.5 a | 1186.7 a | 671.7 a | 4295.3 b | 1066.7 a | 929.2 a | 3185.8 b | 1008.4 a |
| % of vital ECM root tips | 8.7 a | 27.2 ab | 29.4 ab | 45.8 b | 23.0 b | 36.1 b | 17.9 a | 37.5 b | 29.5 ab | 20.4 a | 36.3 b |
| Species richness index (d) | 0.925 ab | 1.507 b | 1.087 ab | 0.826 ab | 0.950 ab | 0.629 a | 1.216 a | 0.955 a | 0.785 a | 0.988 a | 0.982 a |
| Shanon Weaver index (H) | 0.736 a | 1.216 a | 1.024 a | 0.649 a | 0.872 a | 0.581 a | 0.976 a | 0.835 a | 0.725 a | 0.875 a | 0.815 a |
| Evenness (e) | 1.158 a | 1.748 a | 1.843 a | 1.239 a | 1.695 a | 1.007 a | 1.453 a | 1.540 a | 1.350 a | 1.563 a | 1.333 a |
| Equitability (J) | 0.503 a | 0.756 a | 0.800 a | 0.538 a | 0.736 a | 0.437 a | 0.630 a | 0.670 a | 0.590 a | 0.681 a | 0.579 a |
| Berger-Parker index | 0.319 a | 0.544 a | 0.413 a | 0.270 a | 0.417 a | 0.309 a | 0.432 a | 0.340 a | 0.364 a | 0.382 a | 0.375 a |
Tab. 4 - F-test of two-way ANOVA for number of ectomycorrhizal types, vital ectomycorrhizal root tips, total number of fine roots, and percentage of vital ectomycorrhizal root tips. (*): p < 0.05.
| Variable | Factors | ||
|---|---|---|---|
| Sites (A) |
Seasons (B) |
Interaction (A×B) |
|
| Mean number of ectomycorrhizal types | 1.417 | 0.050 | 1.262 |
| Number of vital ectomycorrhizal root tips | 1.428 | 0.385 | 0.320 |
| Total number of fine roots | 16.891* | 16.600* | 6.348* |
| % of vital ECM root tips | 3.616* | 6.485* | 0.156 |
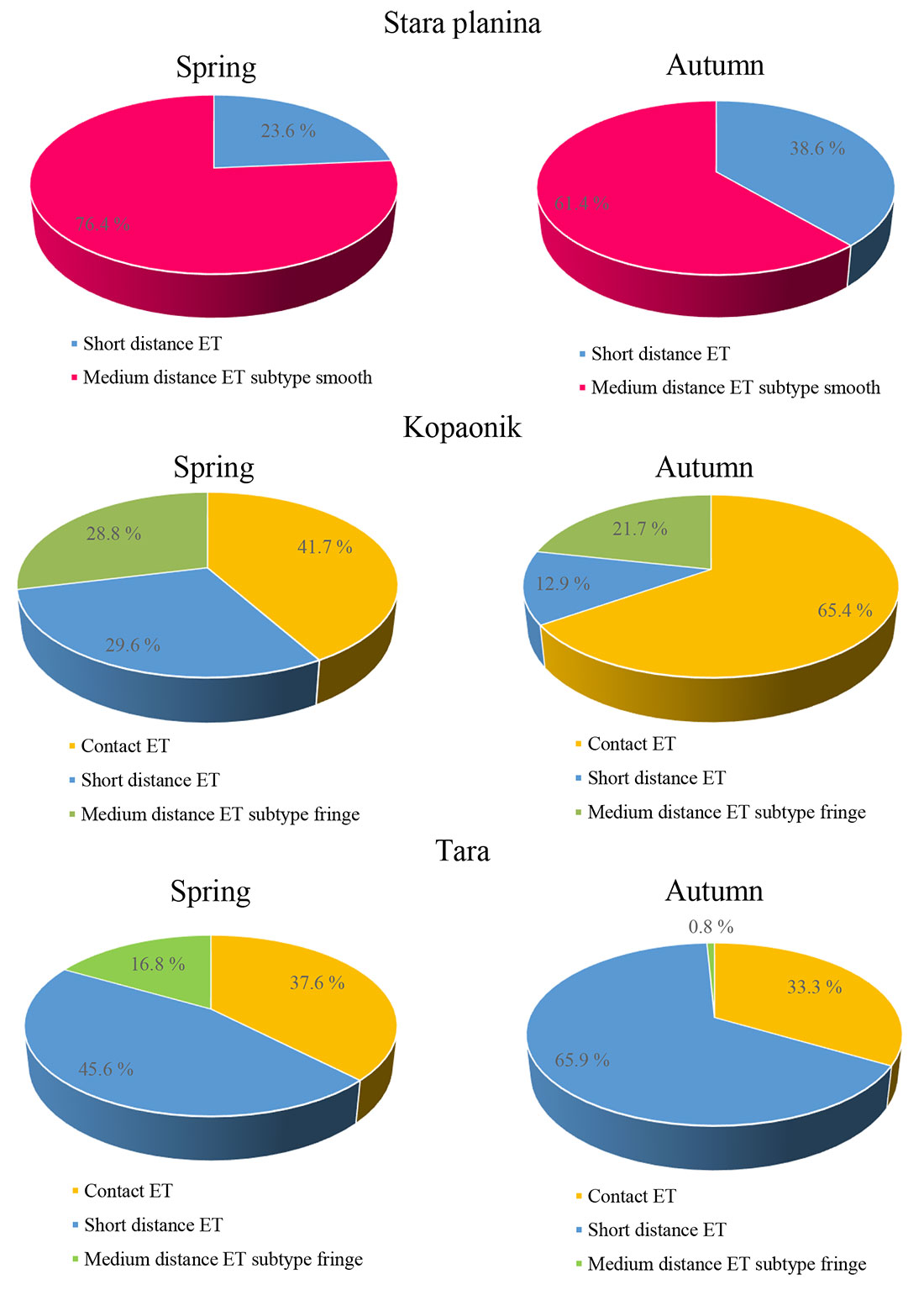
Presence and abundance of exploration types (ET) differed among the investigated spruce stands. Stara planina was dominated by a medium distance exploration type (subtype smooth) with abundance that decreased in the autumn compared to the spring. On the other hand, a number of short distance exploration types increased from the spring to the autumn (Fig. 2a). At Kopaonik the contact exploration type was the most abundant ET in both seasons but its number increased in the autumn while the abundance of the other ET types (short distance ET and medium distance ET fringe subtype) decreased relative to the spring. (Fig. 2b). At Tara, the short distance exploration type was the most numerous ET in both seasons and its abundance increased in the autumn. Abundance of contact ET was similar in the studied seasons while the number of medium distance ET (subtype fringe) decreased in the autumn compared to the spring (Fig. 2c).
Fig. 2 - Mean abundance of exploration types (ET) at examined sites Stara planina (top), Kopaonik (middle) and Tara (bottom) in the spring and autumn.
Abundance of contact ET and medium distance ET (both smooth and fringe subtypes) were significantly influenced by site, while the season had no significant impact (Tab. 5).
Tab. 5 - F-test of two-way ANOVA for exploration types (ET). (*): p < 0.05.
| Variable | Factors | ||
|---|---|---|---|
| Sites (A) |
Seasons (B) |
Interaction (A×B) |
|
| Contact ET | 8.175* | 0.213 | 0.35 |
| Short distance ET | 2.205 | 0.184 | 0.455 |
| Medium distance ET-subtype fringe | 9.203* | 2.374 | 0.966 |
| Medium distance ET-subtype smooth | 67.757* | 0.539 | 0.539 |
Discussion
This insight into the ectomycorrhizal diversity and fine root parameters in Serbia is the first example of a Norway spruce ectomycorrhizal community study from the southernmost edge of the species’ range. Although all sites were located in protected, unpolluted areas on mountains, the examined ectomycorrhizal communities differed substantially in their structure and abundance of ectomycorrhizal types.
In general, the most widespread ectomycorrhizal symbiont in the southernmost natural distribution of Norway spruce was Cenococcum geophilum (Ascomycota), likely the most widespread ectomycorrhizal species worldwide ([16]). Although several ectomycorrhizal root tips were sampled from this anatomorphotype, only one type (from Stara planina sampled in the autumn) was successfully DNA-analysed and determined to the genus level. All other ectomycorrhizal tips were identified as Cenococcum geophilum according to their easily distinguishable morphological and anatomical characteristics. C. geophilum was the most frequent ectomycorrhizal type which was found at all studied sites. In the Barbarian Limestone Alps (1050 m a.s.l.) Baier et al. ([7]) also found Cenococcum geophilum Fr. (with a proportion of 25%) as the dominant species in spruce stands. It was known that Cenococcum geophilum and Sebacina spp. successfully cope with high humus contents and large C:N ratios ([7]), while C. geophilum prefers organic layers enriched with organic compounds. The frequent occurrence of C. geophilum in the studied spruce stands can be explained with the high humus content of soil at these sites.
Tomenteloid fungi are also known to be frequent and widespread ectomycorrhizal partners of deciduous and evergreen tree species in the forests of Europe and North America ([18], [13]). In presented study, this group of fungi was represented by at least one tomenteloid ectomycorrhizal type at every site which was always among the most abundant ectomycorrhizal fungi. Thelephoroids and athelioids are important components of the belowground ectomycorrhizal community in most temperate and boreal forests, but their role might be crucial in forest ecosystems exposed to stress ([34], [27]). Their representatives in this study were Tylospora fibrillosa and Amphinema byssoides. Fungal taxa observed by Ostonen et al. ([32]) in Norway spruce forests across the European climate gradient were presented in following lineages: “russula-lactarius”, “tomentella-thelephora”, “amphinema-tylospora”, “piloderma”, “paxillus-gyrodon” and “boletus” as the dominant colonizers most commonly detected in spruce root tips. In our study, members from the groups “russula-lactarius”, “tomentella-thelephora” and “amphinema-tylospora” were found as well. However, representatives of “piloderma”, /paxillus-gyrodon” and “boletus” groups were not recorded during our research. A possible explanation of this difference is that Ostonen et al. ([32]) elucidated ectomycorrhizal fungi in Norway spruce stands in different climates, i.e., from subarctic-boreal to temperate regions in Europe.
Baier et al. ([7]) investigated the ectomycorrhizal community in upper soil horizons of a young Norway spruce stand in the Bavarian Limestone Alps, which was dominated by Cenococcum geophilum, Tomentella, Lactarius and Sebacina, representing altogether 60% of all ectomycorrhizal root tips within the plot. All these genera were also recorded at the sites in Serbia examined in this study, although some of them were less abundant.
From 29 ectomycorrhizal anatomorphotypes recorded at the investigated spruce stands in Serbia, 9 types were found on Stara planina, 13 on Kopaonik and 12 types were recorded on Tara mountain.
The lowest number of ectomycorrhizal types was recorded at Stara planina where Picea abies trees are spontaneously occurring in natural beech forest, while Kopaonik and Tara spruce stands were pure or mixed, respectively. Since Norway spruce trees have been naturally grown at these sites for years, more propagules of ectomycorrhizal fungi were able to form mycorrhizal symbiosis with spruce trees there. Also, Stara planina has lower altitude, less precipitation and higher temperature than the other two sites.
It is worth noting that there were differences in the age and character of spruce trees in forest stands among the different sites. Namely, the Norway spruce stand on Kopaonik was homogenous and 50 to 70 years old, while the Tara forest stand was comprised of 40- to 50-year-old spruce trees mixed with beech and silver fir, and on Stara planina spruce trees were 10 to 20 years old and planted in natural beech forest. There is a well known division between early- and late-stage fungi that form mycorrhizae with tree roots grown in soils with different physical and chemical properties, especially different accumulations of recalcitrant leaf litter ([28]). However, succession is not a process with a strict change of species, but rather species composition complexity is increased with time ([41]). Similarly, the level of species richness of ectomycorrhizal types correlated with the age of spruce trees at Stara planina.
Investigating the diversity of ectomycorrhizae in four mature spruce stands in Poland, Karlinski & Kieliszewska-Rokicka ([22]) distinguished 37 ectomycorrhizal morphotypes in total. They found relatively high mycorrhizal diversity (28 morphotypes) in the upland mixed forest situated on marsh soil. On the other hand, at the mountain plots with practically homogeneous Norway spruce stands, they recorded only 12 and 13 ectomycorrhizal morphotypes. The number of ectomycorrhizal anatomorphotypes we found at Kopaonik (13) and Tara (12) is comparable to their results. Similarly, in a pure spruce stand on the Taunus mountains in Central Germany, Schirkonyer et al. ([39]) recorded 16 mycorrhizal genera and species. On the other hand, sites Kopaonik and Tara differed in forest stand characteristics, namely the spruce forest on Kopaonik was nearly monospecific, while spruce at the Tara site was mixed with Fagus sylvatica and Abies alba. One would expect higher diversity of ectomycorrhizal anatomorphotypes on Tara mountain, but soil conditions and other factors are important as well. Indeed, the influence of plant species diversity and soil conditions on the richness of ectomycorrhizal fungi is well known ([41]).
In a 100-year-old Norway spruce forest in Sweden, Dahlberg et al. ([14]) identified 25 species of ectomycorrhizal fungi, while in three different forest research plots in Slovenia, Kraigher ([26]) recorded 53 ectomycorrhizal types on Norway spruce. Investigating the belowground ectomycorrhizal community in three Norway spruce stands with different degrees of decline in the Czech Republic, Peter et al. ([34]) detected 43 ectomycorrhizal types out of which 25, 20 and 15 species were found in the least, moderately and most damaged forest, respectively. The higher number of ectomycorrhizal types in the aforementioned study could be due to the higher number of samples and the longer period of examination.
Dominance of some species in ectomycorrhizal communities is indicated by the Shannon Weaver index ([27]). The observed values of Shannon Weaver index in the studied sites in Serbia ranged from 0.58 to 1.22, which was much lower than those obtained in mature spruce stands at unpolluted sites in Slovenia (2.2) and Sweden (3.5), respectively, by Kraigher & Petkovšek ([27]). According to the same authors, this index in polluted areas varied greatly and amounted to 0.2-0.7 in Slovenia and up to 3.3 in France and Denmark ([27]).
The most abundant ectomycorrhizal types, representing more than 10% of ectomycorrhizal communities of spruce stands from Stara planina, Kopaonik and Tara were: Laccaria laccata, Russula firmula, R. olivacea, Tylospora fibrillosa, Tomentella sp. 1, Tomentella sp. 2, Entoloma sp., Cenococcum sp., Unknown types K3 and T1; other morphotypes constituted less than 3% of the entire ectomycorrhizal community. Thus, it is clear that the ectomycorrhizal community associated with spruce from the examined sites in Serbia consisted of few abundant and numerous infrequent ectomycorrhizal types, which is in accordance with previous studies ([22], [15], [23]).
Wang et al. ([45]) investigated the ectomycorrhizal community structure on Picea abies at the tree line in the Austrian Alps; they found that at the higher altitude the ectomycorrhizal community was dominated by Cortinarius sp., whereas at the lower elevation site the community was dominated by Russula. Similarly, we found that in spruce stands in Serbia the genus Russula was the most abundant with four anatomorphotypes, while the genera Clavulina, Tomentella, Inocybe, Sebacina and Cortinarius had two types each.
Root parameters
The edge of the southernmost distribution of Norway spruce is under constant external pressures, such as pests and unfavourable conditions due to the warming climate ([21]). These permanent unfavourable conditions are reflected in the average amount of vital ectomycorrhizal root tips in spruce stands in Serbia that were both in autumn (826-1161 dm-3) and spring (1334-2064 dm-3) lower than in mature spruce stands in the Central Alps (4309-6716 dm-3 - [26]).
In this study, the three selected sites did not significantly differ in the number of ectomycorrhizal types and vital ectomycorrhizal root tips nor diversity indices. These results were in accordance with Ostonen et al. ([32]), who found no differences between the mean numbers of ectomycorrhizal root tips per spruce tree across the studied gradient. However, total number of fine roots was higher at Stara planina compared to the other two sites.
Comparison of two seasons
According to previous studies ([15], [23]), we found no significant differences between the spring and autumn in the number of ectomycorrhizal types and vital ectomycorrhizal root tips, as well as in diversity indices. However, the total number of fine roots was greater in the spring compared with the autumn because of the higher number of old, non-turgescent and non-mycorrhizal roots present in the spring, which could be explained by the seasonal influence on fine roots. Many roots die during winter causing an increase in the number of old and non-turgescent roots in spring. On the other hand, during the growing season, the number of vital roots increases and the senescence of roots decreases, resulting in decreased quantities of old and non-turgescent roots in autumn.
Implication for edible fungi
Boletus edulis, Cantharellus cibarius and Hydnum rufescens are well-known commercial ectomycorrhizal species ([11]). Based on suitable plant hosts, ecosystem characteristics, and known species from regional etnomycological habits, these fungi were expected to be found in ectomycorrhizae as well. Yet none of the commonly collected edible species were recorded in ectomycorrhizae at the investigated sites, confirming the well-known evidence that above- and below-ground ectomycorrhizal diversity rarely match ([37]).
Exploration types
Agerer ([2]) found a relationship between exploration types and their potential ecological roles, while Rudawska et al. ([38]) concluded that the abundance of particular exploration types is related to soil chemistry. Baier et al. ([7]) observed that contact- and medium-distance exploration types were associated with soil properties indicative for mineral A-horizon, while short-distance exploration types preferred soil environments rich in humus, which is characteristic for organic layers. Also, they noted that the majority of the ectomycorrhizal fungi preferred organic layers.
In the current study, exploration types characteristic for mineral horizons dominated all tested sites in both seasons. The exception was the short-distance exploration type known to prefer organic layers, which was the most abundant at Tara in the autumn. It is important to highlight that no ectomycorrhizal fungi belonging to long-distance ET were found at all examined sites in both seasons. Abundance of contact- and medium-distance ETs was affected by site. Short-distance ET was present at all sites and the influence of site on its abundance was not statistically significant. Since ectomycorrhizal types belonging to short-distance ET prefer humus-rich environments and its abundance was not influenced by site, it could be assumed that all studied sites have similar characteristics concerning humus content.
Conclusions
In the first study of ectomycorrhiza on spruce in its southernmost natural distribution area, 29 different types of ectomycorrhizae were identified. The ectomycorrhizal communities investigated at Stara planina, Kopaonik and Tara differed in the composition of ectomycorrhizal types and abundance of contact- and medium-distance exploration types, but not in the mean number of ectomycorrhizal types, number of vital ectomycorrhizal root tips, nor diversity indices. Season had no influence on the parameters assessed nor on the abundance of exploration types. In order to obtain a broader picture of ectomycorrhizal communities in Norway spruce forests in Serbia, further research supplemented with metagenomic approaches should be conducted on more sites.
Acknowledgements
This work is a part of the project III 43002 “Biosensing technologies and global systems for continuous research and integrated management of ecosystems”, financed by the Ministry of Education and Science of the Republic of Serbia, the Research programme P4-0107 of the Slovenian Research Agency and STSM under COST Action FP1305 for M. Katanić. Dr. Ronald S. Zalesnyis is gratefully acknowledged for revising the manuscript for English style.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Saša Orlović
Saša Pekeč
Milan Drekić
University of Novi Sad, Institute of Lowland Forestry and Environment, Antona Cehova 13, 21000 Novi Sad (Serbia)
Marko Bajc
Hojka Kraigher
Slovenian Forestry Institute, Večna pot 2, 1000 Ljubljana (Slovenia)
Corresponding author
Paper Info
Citation
Katanić M, Orlović S, Grebenc T, Bajc M, Pekeč S, Drekić M, Kraigher H (2019). Ectomycorrhizae of Norway spruce from its southernmost natural distribution range in Serbia. iForest 12: 43-50. - doi: 10.3832/ifor2729-011
Academic Editor
Alberto Santini
Paper history
Received: Jan 18, 2018
Accepted: Oct 26, 2018
First online: Jan 10, 2019
Publication Date: Feb 28, 2019
Publication Time: 2.53 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2019
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 47008
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 39269
Abstract Page Views: 3747
PDF Downloads: 2986
Citation/Reference Downloads: 3
XML Downloads: 1003
Web Metrics
Days since publication: 2583
Overall contacts: 47008
Avg. contacts per week: 127.39
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2019): 4
Average cites per year: 0.57
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Fine root morphological traits and production in coniferous- and deciduous-tree forests with drained and naturally wet nutrient-rich organic soils in hemiboreal Latvia
vol. 16, pp. 165-173 (online: 08 June 2023)
Research Articles
Fine root production and distribution in the tropical rainforests of south-western Cameroon: effects of soil type and selective logging
vol. 3, pp. 130-136 (online: 27 September 2010)
Research Articles
The effect of soil conditions on submountain site suitability for Norway spruce (Picea abies Karst.) in Central Europe
vol. 16, pp. 210-217 (online: 31 July 2023)
Research Articles
Ectomycorrhizal fungal community associated with autochthonous white poplar from Serbia
vol. 9, pp. 330-336 (online: 12 November 2015)
Research Articles
Dynamics of soil organic carbon (SOC) content in stands of Norway spruce (Picea abies) in central Europe
vol. 11, pp. 734-742 (online: 06 November 2018)
Research Articles
Impacts of Norway spruce (Picea abies L., H. Karst.) stands on soil in continental Croatia
vol. 12, pp. 511-517 (online: 02 December 2019)
Short Communications
Culturable fungi associated with wood decay of Picea abies in subalpine forest soils: a field-mesocosm case study
vol. 11, pp. 781-785 (online: 28 November 2018)
Research Articles
Short-term recovery of fine root carbon stock is inhibited by skid trails in a humid tropical forest
vol. 18, pp. 344-349 (online: 30 November 2025)
Research Articles
A rapid method for estimating the median diameter of the stem profile of Norway spruce (Picea abies Karst) trees
vol. 10, pp. 328-333 (online: 11 February 2017)
Research Articles
Soil CO2 efflux in uneven-aged and even-aged Norway spruce stands in southern Finland
vol. 11, pp. 705-712 (online: 06 November 2018)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword