Effects of substrate and ectomycorrhizal inoculation on the development of two-years-old container-grown Norway spruce (Picea abies Karst.) seedlings
iForest - Biogeosciences and Forestry, Volume 8, Issue 4, Pages 487-496 (2014)
doi: https://doi.org/10.3832/ifor1291-007
Published: Nov 10, 2014 - Copyright © 2014 SISEF
Research Articles
Abstract
The objective of this study was to test the effects of selected peat growth substrates (Agro CS, Gramoflor and Durpeta) and inoculation with commercial ectomycorrhizal inocula (Ectovit and Mycorrhizaroots) on growth, ectomycorrhiza formation, needle nutrients concentration and several physiological parameters of two-years-old containerized Norway spruce seedlings cultivated under standard nursery conditions. The selected substrates differed in origin, composition and nutrient content: Agro CS and Gramoflor were mixtures of various peat types and components with added nutrients, while Durpeta was non-enriched pure peat. Growth parameters of seedlings cultivated in enriched substrates were significantly higher than those grown on the non-enriched substrate. Significant interactions were found between substrate and inoculation treatments. Inoculation with Ectovit stimulated seedling growth in non-enriched substrate but had no effect on seedling parameters in the enriched substrates, and a negative effect on aboveground biomass in Gramoflor. Mycorrhizaroots inoculum significantly decreased shoot to root dry weight ratio, but had no other effect on seedling development. ECM colonization rate of seedlings ranged from 73 to 80%, with no significant effects of the ECM inoculum or growth substrate. DNA analysis revealed a low species diversity of ECM fungi on seedling roots, with a pronounced dominance of the soil-borne ECM species Thelephora terrestris Fr. Chemical analysis of needles and measurement of chlorophyll a fluorescence showed similar trends as seedling growth. Values of chlorophyll a fluorescence parameters and needle N, P, K, Ca and Mg concentrations were higher in both enriched substrates. Ectovit increased (though not significantly) chlorophyll a fluorescence in needles as compared to Mycorrhizaroots- and non-inoculated seedlings, as well as nutrient-uptake (mainly K) in the non-enriched substrate. Our results suggest the importance of the origin and composition of peat-based substrates on the development of container-grown Norway spruce seedlings, while the observed positive effect of the commercial ECM inoculum Ectovit was more probably caused by its physical and chemical properties rather than by its efficiency in promoting ECM fungi symbiosis. The enriched substrates tested appear to be suitable for production of spruce seedlings of acceptable size for outplanting within two growing seasons.
Keywords
Peat Substrate, Ectomycorrhizal Inoculation, Norway Spruce, Container-grown Seedlings, Nutrition, Chlorophyll a Fluorescence, DNA Analysis
Introduction
Norway spruce (Picea abies [L.] Karst.) is ecologically and economically the most important coniferous tree species in Slovakia. Planting stock is predominantly produced as bareroot transplanted seedlings in a 3-5 year nursery rotation. The proportion of container-grown spruce seedlings is still only about 6% of the total production (15 million of spruce seedlings of plantable size annually), though the advantages of container as compared with bareroot stock are well-known, e.g., better root protection, ameliorative and nutrient effects of potting substrate, reduction of transplanting stress on planting site. Other advantages include more effective care for and faster growth of seedlings grown individually in containers (especially under greenhouse conditions) than bareroot seedlings grown in dense spacing in a nursery bed ([47]). The better survival of containerized stock, especially on adverse planting sites ([72], [56], [19]) warrants the increased use of this material and the improvement of nursery practices of container-grown seedlings production.
The development of container-grown seedlings in forest nurseries is affected by many factors. Besides type and size of container ([64], [67]), potting medium plays an important role ([6], [28]). One of the growth media most utilized for growing containerized plants is peat, used either alone or as a main component of peat-based growing mixtures ([39], [70], [28]). Especially light low-humified Sphagnum peat has the required attributes (such as low pH, high cation exchange capacity, low inherent fertility, a proper balance of aeration and water-holding porosity) and provides reasonable growth conditions in the greenhouse ([39], [26]). Nevertheless, an admixture of proper components and fertilizers to pure peat can improve its physical ([26], [27]) and chemical properties ([33], [20]), and as a consequence, promote the growth and physiological quality of seedlings ([6], [5]). In Slovakia, peat is generally used as a growth substrate for production of container-grown seedlings. In recent years, nursery managers began using peat substrates of various composition from foreign producers, mainly from central European and Baltic countries.
Growing substrate and fertilization can also influence the amount and composition of seedlings’ ectomycorrhizae in forest nurseries ([24], [60], [54], [70]). In certain circumstances, ECM symbiosis can increase water and nutrient uptake, tolerance to adverse soil conditions or reduce the effects of soil-borne pathogens on trees ([43], [37], [15]). Successful ECM inoculation of seedlings in the forest nursery can promote their subsequent adaptation and growth on the planting site ([22], [48]). Several types of natural and laboratory produced inocula and application techniques have been used for seedling inoculation ([43], [45], [55]). Production and application of ECM inocula on a commercial scale are increasingly expanding worldwide. Several authors were concerned about the effects of inoculation by ECM fungi on ectomycorrhiza formation, physiology and/or growth of Norway spruce seedlings in aseptic ([31], [62], [69]) and operational conditions ([40], [41], [71], [53], [54], [11]). Reported results are inconsistent; positive effects of application of ECM fungi were not found in all cases. In Slovakia, laboratory produced mycelial inocula of ECM fungi were applied and their effects estimated in small nursery experiments ([53], [54], [57]). Commercial ECM inoculation on a larger scale has been recently carried out in the state-owned nurseries in Slovakia, but the performance of inoculation on seedling development has not been estimated.
The objective of this study was to test the effects of selected peat growth substrates and inoculation with commercial ectomycorrhizal inocula Ectovit and Mycorrhizaroots on growth, ectomycorrhiza formation, needle nutrients concentration and several physiological parameters of two-years-old container-grown Norway spruce (Picea abies Karst.) seedlings cultivated under standard operational conditions.
Material and methods
Experimental design
The experiment was carried out in a greenhouse and outdoor bed under operational conditions in a state-owned nursery located in northern Slovakia (49° 06′ 27″ N, 19° 44′ 54″ E, altitude 830 m a.s.l.). Norway spruce seedlings were grown in plastic trays containing 81 cavities (Plantek 81F, Lännen Plant Systems - BCC Oy, Säkylä, Finland - each cavity 85 cm3, depth 73 mm, top 41×41 mm, 549 cavities m-2) filled with three substrates (Agro CS RS2 - Agro CS a.s., ÄŒeská Skalice, Czech Republic; Gramoflor Cocofibre G-SG - Gramoflor GmbH & Co. KG, Vechta, Germany; Durpeta - UAB Durpeta, Kupiškio, Lithuania). Two commercial ectomycorrhizal inocula treatments were applied (Ectovit®, Symbiom s.r.o., Lanškroun, Czech Republic; and MycorrhizaRoots®, Lebanon Turf, Lebanon, Pennsylvania, USA), along with one non-inoculated control. The experiment was arranged as a completely randomized block design of 9 combinations of main effects (3 substrate × 3 inoculation treatments) repeated three times (3 blocks). One repetition of each treatment combination consisted of 4 trays (324 cavities), thus the experiment consisted of 324 × 3 (substrate) × 3 (inoculation) × 3 (block) = 8.748 seedlings (containers) in total.
Substrates, commercial ectomycorrhizal inocula and inoculation
The main component of all substrates was peat. The substrates were different in origin (peatbog), degree of decomposition, structure, additives composition, nutrient content and other properties. The distinctive difference among the substrates was that Agro CS and Gramoflor were diverse in composition and artificially-nutrient-enriched, while Durpeta was non-enriched pure natural peat. Composition of Agro CS substrate (RS2 for conifers): white peat Profi 0-20 mm 50%, Unguri peat 5-10 mm 20%, black peat 0-20 mm 20%, coconut fibre 10%, Bentonit 15 kg m-3, Multicote 4M (NPK 18-6-12% + Mg 2% + micro-elements) 2 kg m-3, PG Mix (macro- and micro-elements set) 1 kg m-3, Fibazorb 0.1 l m-3. Composition of Gramoflor substrate (Cocofibre G-SG): white peat from north Germany (digging in peat-block form) 0-20 mm 70%, peat fibre 20-40 mm 15%, coarse coconut fibre 15%, clay 10% of weight (30 kg m-3), Kompakt (NPK 21-7-14% + micro-elements) 0.8 kg m-3, Radigen (micro-elements set) 100 g m-3, Triplephosfat 46% 200 g m-3, keratin 3 kg m-3, detergent 900 ml m-3. Durpeta substrate was a natural dark (brown) low-bog type peat without any kind of additives, middle segmented (decomposed), fibre size 0-20 mm, harvested in the Šepeta peatbog, northeastern Lithuania. The substrates were analyzed for basic chemical parameters prior to inoculation (Tab. 1). Analysis of physical properties of the substrates was not done.
Tab. 1 - Analytical parameters of peat-based substrates (n=1 combined sample) used for cultivation of container-grown Norway spruce seedlings inoculated with commercial ectomycorrhizal inocula Ectovit and Mycorrhizaroots.
| Substrate | pHH2O | C (%) |
N (%) |
C/N | P (mg kg-1) |
K (mg kg-1) |
Ca (mg kg-1) |
Mg (mg kg-1) |
|---|---|---|---|---|---|---|---|---|
| AgroCS | 4.35 | 35.2 | 0.90 | 39 | 42 | 932 | 2276 | 964 |
| Gramoflor | 4.62 | 33.3 | 0.89 | 37 | 48 | 879 | 2632 | 835 |
| Durpeta | 3.36 | 46.9 | 0.95 | 49 | 37 | 1010 | 1648 | 640 |
Ectomycorrhizal inoculum Ectovit contains mycelium of four ECM fungi (Cenococcum geophilum, Hebeloma velutipes, Laccaria proxima and Paxillus involutus) and basidiospores of two fungi (Pisolithus arrhizus and Scleroderma citrinum). The spores are dispersed in a peat-based carrier together with ingredients supporting the development of ectomycorrhizae (humates, ground minerals, extracts from sea organisms) and naturally degradable particles of a water-retaining gel. Ectovit was applied as slurry (gel) that was prepared by mixing fungal mycelium with dry components of inoculum (including fungal spores and a powder hydrogel) and an adequate amount of water. The slurry was thoroughly mixed with the growing substrates at a ratio 1:5 (v:v). The containers were filled with this mixture immediately before sowing. The inoculum-substrate mixture contained 0.32 ml of mycelium in each cavity. The amount of spores in Ectovit was not known. Viability of mycelium of all four fungi entrapped in Ectovit was determined by cultivating pure fungal cultures on agar BAF medium ([46]) in sterile conditions. No additional tests for inoculation efficiency of both inocula used were carried out.
MycorrhizaRoots contains spores of ECM fungi Pisolithus tinctorius (1 600 000 dry spores per gram), four species of Rhizopogon (80 000 spores g-1 of each species), two species of Scleroderma (40 000) and two species of Laccaria (16 000), plus vasiculo-arbuscular fungi Glomus (8 species) and Gigaspora margarita (the proportion of all spores in inoculum was 23.3% of the inoculum weight), humic acid (28.9%), cold water kelp extracts (18.0%), ascorbic acid (vitamin C - 12.3%), amino acids (glycine - 8.5%), myo-Inositol (3.5%), maltodextrin (2.25%), thiamine (vitamin B1 - 2.0%), alpha-tocopherol (vitamin E - 1.0%) and surfactant (0.25%). Substrates in the containers were inoculated with a water solution of Mycorrhizaroots (0.8 g 1-1) to wet the root zone thoroughly (approximately 3 l m-2) 2 weeks after seedling emergence and once more in the middle of July (approximately in the middle of the first growing season). Irrigation was suspended for a period to avoid run-off of the inoculum.
Seeds and seedlings
Seeds of Norway spruce were collected from a source in northen Slovakia (selected mature stand certified for seed collection according to Slovakia rules, altitude 900 m a.s.l., certificate number SK-1049/2010). Seeds were washed in tap water, then sterilized 15 min in 30% H2O2, rinsed repeatedly with distilled water, dried at room temperature and then treated with powder fungicide Dithane M-45 (Rohm and Haas, Vienna, Austria), at 1% of seed weight. In the middle of April, immediately after filling containers with substrates, one seed was sown in each cavity and covered with a thin layer of perlite. Trays were placed 15 cm above the greenhouse floor on pallets. Seedlings were grown under natural light and ambient temperatures. Maximum air temperature was controlled during hot days by ventilation. The substrates were irrigated (1-2 times per day) to maintain adequate humidity (approximately 50-70% according to germination and growing phase). Air humidity was not controlled.
Starting since three weeks after the beginning of their emergence, seedlings were supplied twice per week with 0.3% solution of fertilizer (Superex®, NPK 19-4-20% + microelements Mg, B, Co, Cu, Fe, Mn, Mo, Zn, S, Kekkilä Oy, Vantaa, Finland) for the first 3 weeks, and with 0.45% solution of Superex for the next 8 weeks. Fertilizer was applied through the nursery irrigation system. Fungicides Dithane® (Rohm and Haas, Vienna, Austria) and Novozir® (Dow AgroSciences, Lauterbourg, France) were applied alternately at 10-day intervals until the greenhouse polyethylene cover was removed, and then at 2-week intervals until the end of August to avoid damping off and other pathogen diseases. The greenhouse polyethylene cover was removed 8 weeks after seedling emergence. At the end of the first growing season (end of October), seedlings were placed on an open bed (hardening area) for overwintering and remained there throughout the second growing season. The trays were packed with sawdust around the sides and underneath to prevent frost damage. The substrate surface was also covered with a thin layer of sawdust. The seedlings were manually weeded and irrigated as needed and fertilized with 2% solution of Superex® via irrigation system during the second growing season. The time of fertilizer application after the greenhouse cover was removed varied according to the incidence of rainfall.
Sampling, measurements and data analyses
In August of the second growing season, chlorophyll a fluorescence of needles was measured on 12 seedlings from each treatment combination and block (3 seedlings per tray, 324 seedlings totally) using a chlorophyll fluorimeter (Handy Pea fluorimeter, Hansatech Ltd, Kings Lynn, UK). Needles were irradiated by one-second-long saturating pulse (2000 µmol m-2 s-1) after a 30-min long dark adaptation period. The following parameters were measured: F0 (initial fluorescence - all reaction centers are open), Fm (maximum fluorescence - all reaction centers are closed), Fv = Fm - F0 (variable fluorescence), Fv/Fm (maximal photochemical efficiency of PSII), Tfm (time of reaching maximum fluorescence), Area (area above the fluorescence curve), RC/ABS (density of reaction centers), Fv/F0 (competitive non-photochemical processes in PS II in dark-adapted state), Vj (relative variable fluorescence at time 2 ms after start of light pulse) and PI (Performance index for the photochemical activity - [8]).
After the second growing season (end of October), 20 dormant seedlings from each treatment combination and block (5 seedlings per tray, overall 540 seedlings) were randomly selected for estimation of ectomycorrhiza formation, growth, and chemical analysis (nutrient concentration) of the photosynthetic tissue (needles). Stem height, root collar diameter (RCD), and shoot and root dry weights (SDW and RDW, respectively - 48 h at 80 °C) were recorded for each seedling. Total dry weight (TDW) and ratio of shoot and root dry weights (SDW/ RDW) were calculated.
Number of root tips, morphology and percentage of ectomycorrhizae were estimated on four randomly selected seedlings for each treatment combination and block. Seedlings were stored at 4 °C and analyzed within two days after lifting. The root system was removed free of soil and gently washed in tap water. Root tips were examined and counted on the long (10-15 cm approx.) lateral roots of each seedling at ×10-40 magnification by a dissecting microscope. Root tips were determined as ectomycorrhizae and classed into ECM morphotypes on the basis of gross morphological characteristics such as ramification, shape, color, outer mantle characteristics, presence of hyphae and rhizomorphs ([1], [2]). To confirm the formation of ectomycorrhizae, suspected short roots were cut repeatedly and examined by light microscopy (magnification ×400-800). Root cross-sections were cut and stained with cotton blue before microscopic observation. Presence of a complete fungal mantle (hyphae entirely surrounded a periphery of the section), irrespective of its thickness, and a Hartig net with hyphae intercellulary penetrating at least two layers of cortical cells, were considered as evidence of ectomycorrhizae ([54]).
The number of ectomycorrhizae within each ECM morphotype along with the non-mycorrhizal root tips were counted for each seedling. The percentage of each ECM morphotype was determined as a percent portion of the number of ectomycorrhizae of respective morphotype from the total number of root tips (all ectomycorrhizae + non-mycorrhizal root tips). Total ECM colonization was calculated as a sum of percentages of the ECM morphotypes.
On the basis of distinctive specific morphological characteristics, one of three distinguished ECM morphotypes was identified on a species level, though molecular analysis of such morphotype was not carried out. Due to the absence of reliable identifying morphological features and mutual morphological similarity, it was not possible to visually identify the other two ECM morphotypes. Their identification was done by sequencing of the ITS region of ribosomal DNA. Twenty samples of ectomycorrhizae from each visually unidentified morphotype were selected for molecular identification.
PCR reactions aimed at amplifying the nuclear ITS ribosomal region were conducted using the fungi-specific primers ITS1F (5′ - CTT GGT CAT TTA GAG GAA GTA A - 3′; [23]) and the basidiomycetes-specific ITS4B (5′- CAG GAG ACT TGT ACA CGG TCC AG - 3′; [23]). The PCR reactions were conducted in a reaction mix of 20 µl containing: 10 µl of (2×) KAPA2G Robust HotStart ReadyMix® (Kapa Biosystems, Boston, USA); (50 ng µl-1) extracted DNA 5 µl; (10µM) mixture of primers 0.8 µl; (8µg µl-1) BSA 2 µl; 2.2 µl of ultrapure H2O. PCR amplification was performed by initial denaturation for 3 min at 95 °C (1 cycle), 95 °C for 15 s, 50 °C for 1 min, 72 °C for 15 s (35 cycles) and 72 °C for 10 min (1 cycle). PCR reactions were accomplished using a Perkin Elmer GeneAmp 9700® thermal cycler. The quality of the resulting PCR product was evaluated by gel electrophoresis. Sequencing of the PCR product was done by the laboratory of the Elizabeth Pharmacon company (Brno, Czech Republic).
Sequence analysis, alignment and the creation of contigs were performed using the SEQUENCHER® 5.2.4 software (Gene Codes Corporation, Ann Arbor, MI, USA) and CLUSTALW2 ([66]). Sequences with a high degree of similarity were aligned together into contigs. The sequences classified into one homology group were compared with sequences of known origin stored in the NCBI database (National Center for Biotechnology Information, US National Library of Medicine, Bethesda, Maryland, USA) in the BLAST algorithm ([3]). Taxomony units were identified according to Landeweert et al. ([38]) using the identity thresholds described in the work by Hedh et al. ([25]).
Chemical analyses of the substrates and needles were carried out on one combined sample for each substrate and substrate × inoculation combination, respectively, in the laboratory of National Forestry Center, Zvolen, Slovakia. For needle analysis, total C and N were determined by dry combustion (900-1250 °C) and subsequent oxidation-reduction reactions in a CN analyzer FLASH EA 1112. Concentrations of the other elements were determined after digestion of the ground material in a high pressure microwave in concentrated HNO3. Measurements of K, Ca and Mg were taken using an inductively coupled plasma atomic absorption spectrometer (Thermo iCE 3000) and P spectrophotometrically (PHARO 300) from the HNO3 extract by the ammonium molybdate method. The same methods were used for substrate analysis, except for the preparation of extracting solution by Mehlich method before P, K, Ca and Mg detection by ICP-AES. The pH was determined electrometrically in a 1 substrate : 10 water suspension.
The experiment was a three-way classification (substrate, inoculation, block). Experimental plot was one replication of substrate × inoculation × block combination (4 trays = 324 containers). The growth data, chlorophyll a fluorescence data and percentages of ectomycorrhizae were analyzed by two-way analysis of variance (ANOVA) followed by a Tukey’s test (P<0.05) to test for differences among treatments. To reduce the high variation in the occurrence of ectomycorrhizae within the experimental plots, percentages of ectomycorrhizae were arcsin-transformed prior to statistical analysis. When a significant interaction between the test factors was detected, significance of differences between mean values of parameter of corresponding treatment combination was tested by one-way ANOVA (P<0.05). ANOVA was carried out using the SAS® statistical package for PC (SAS Institute Inc., Cary, NC, USA).
Results
Seedling growth
Preliminary estimation of seedling growth (after the first growing season) showed a significant influence of both factors tested (substrates and inocula). The enriched substrates (Agro CS and Gramoflor) and inoculation with Ectovit significantly improved mean values of all measured growth variables as compared with the other related treatments (data not shown). Significant substrate × inoculation interaction for stem height and RCD was also detected.
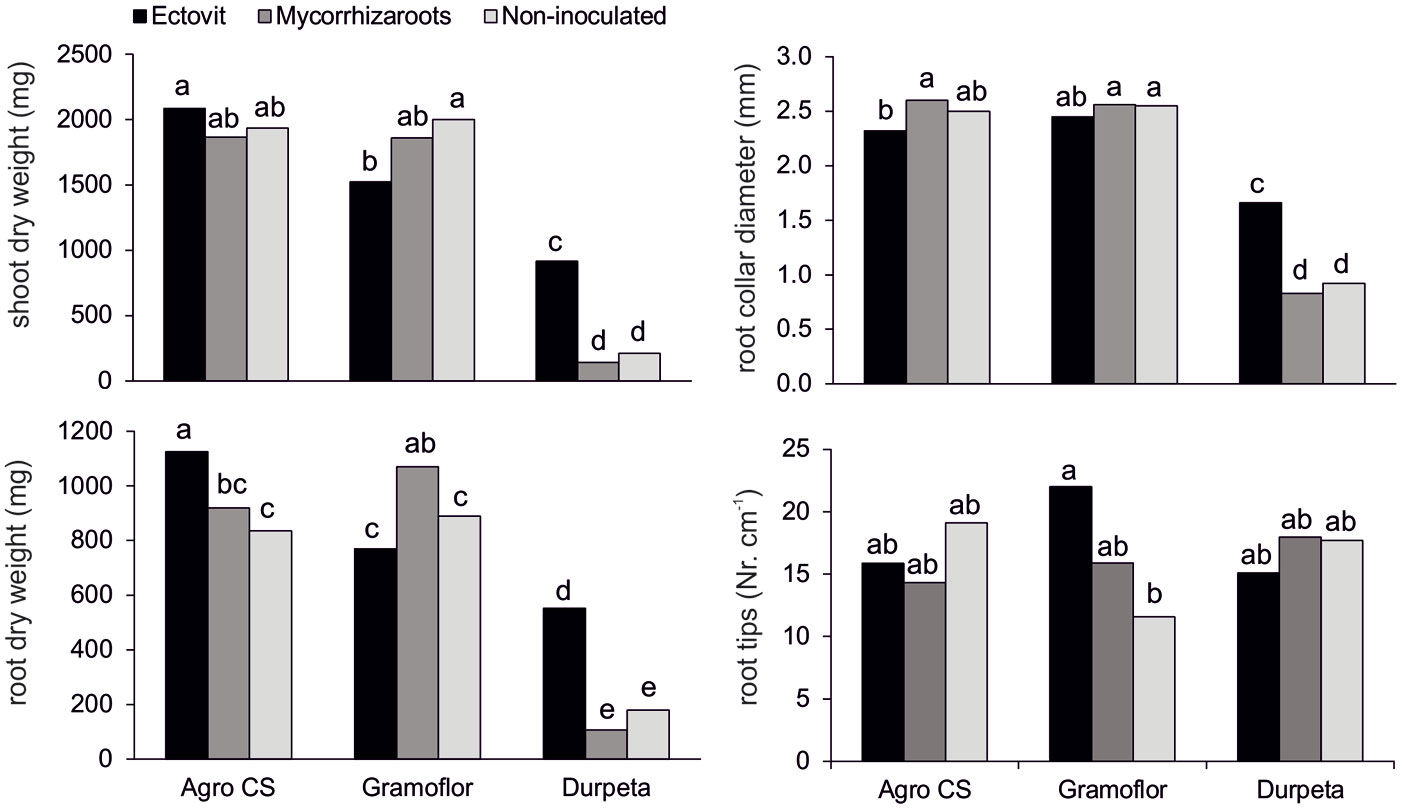
At the end of the second growing season, growth substrate significantly affected all measured growth variables (P<0.05), except the number of root tips, while ECM inoculation influenced RDW and SDW/RDW ratio only (Tab. 2). Seedlings grown on enriched substrates (Agro CS and Gramoflor) showed mean values for all growth variables (except the number of root tips) significantly higher than those grown on non-enriched substrate (Durpeta). Mean values of growth variables of the seedlings grown in the most affecting substrate (AGRO CS or Gramoflor depending on variable) were 2.2-fold (RCD) up to 4.6-fold (SDW) higher than those of seedlings grown in the Durpeta substrate (Tab. 3). Significantly higher values of SDW and TDW were also detected for seedlings grown in Agro CS, as compared with Gramoflor (9% and 8%, respectively). ECM inoculum Ectovit also promoted seedling growth, even though differences between treatments were not significant for most of the growth variables analyzed. Indeed, seedlings inoculated with Ectovit yielded a root biomass significantly higher (29%) than those non-inoculated. SDW/RDW of Mycorrhizaroots-inoculated seedlings was significantly lower than those non-inoculated. No other significant differences in seedling growth among inoculation treatments were found.
Tab. 2 - Analysis of variance (F- and P-values) of the effects of substrate and ectomycorrhizal inoculation on growth parameters and ectomycorrhizal colonization of 2-years-old container-grown Norway spruce seedlings. Degrees of freedom: substrate 2; inoculation 2; block 2; substrate × inoculation 4; error for substrate and inoculation 4; error for substrate × inoculation 8; residual 513; total 539; degrees of freedom for number of root tips and ECM colonization: residual 81; total 107.
| Variable | Substrate | Inoculation | Substrate × Inoculation |
|||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Root collar diameter | 821.19 | 0.001 | 6.16 | 0.060 | 131.25 | 0.001 |
| Stem height | 7853.66 | 0.001 | 5.98 | 0.063 | 86.32 | 0.001 |
| Shoot dry weight | 2387.26 | 0.001 | 1.94 | 0.258 | 19.07 | 0.001 |
| Root dry weight | 392.50 | 0.001 | 10.04 | 0.028 | 63.98 | 0.001 |
| Total dry weight | 1854.64 | 0.001 | 2.98 | 0.161 | 28.06 | 0.001 |
| Shoot/root dry weight | 13.29 | 0.017 | 18.28 | 0.010 | 3.51 | 0.062 |
| Number of root tips | 0.02 | 0.983 | 0.26 | 0.781 | 4.21 | 0.046 |
| Ectomycorrhizal colonization | 4.80 | 0.087 | 4.49 | 0.095 | 1.02 | 0.451 |
Tab. 3 - Growth parameters (mean ± standard error) of 2-years-old container-grown Norway spruce seedlings grown on different substrates inoculated with commercial ectomycorrhizal inocula Ectovit and Mycorrhizaroots. Different letters within substrate and inoculation treatments indicate statistically different values.
| Treatment | Type | Root collar diameter (mm) | Stem height (cm) | Shoot dry weight (mg) | Root dry weight (mg) | Total dry weight (mg) | Shoot/root dry weight | Root tips (n cm-1) |
|---|---|---|---|---|---|---|---|---|
| Substrate | Agro CS | 2.47±0.04a | 20.67±0.30a | 1962±52a | 960±28a | 2922±74a | 2.16±0.05a | 16.44±1.33 |
| Gramoflor | 2.52±0.04a | 20.73±0.44a | 1798±65b | 910±32a | 2708±92b | 2.09±0.05a | 16.49±1.44 | |
| Durpeta | 1.14±0.04b | 7.87±0.36b | 426±41c | 280±24b | 703±64c | 1.57±0.08b | 16.91±1.33 | |
| Inoculation | Ectovit | 2.14±0.05a | 17.31±0.43a | 1509±60a | 816±35a | 2326±92a | 1.94±0.04ab | 17.64±0.86 |
| Mycorrhizaroots | 1.99±0.08a | 15.92±0.70a | 1281±81a | 694±42ab | 1976±120a | 1.77±0.05b | 16.06±0.77 | |
| Non-inoculated | 1.99±0.07a | 15.93±0.65a | 1382±84a | 634±33b | 2018±115a | 2.10±0.09a | 16.13±0.86 |
The results of the ANOVA indicated that a significant (P<0.05) interaction occurred between substrate and inoculation treatments (Tab. 2). Ectovit-inoculated seedlings grown in non-enriched substrate had mean values of growth variables significantly higher (except for SDW/RDW) than those of Mycorrhizaroots and the non-inoculated control. In contrast, they had the same (or in some cases even significantly lower) parameter values when grown in enriched substrates (with exception of RDW in Agro CS - Fig. 1). A depressive effect of Ectovit on seedling growth was evident (except for RCD) especially in Gramoflor. Contrastingly, a positive effect of this inoculum was observed on the number of root tips in this substrate (Fig. 1). Mean values of treatment combinations for stem height and TDW are not shown here, because differences among combinations and their significance broadly overlap those observed for SDW (Fig. 1).
Fig. 1 - Shoot dry weight, root collar diameter, root dry weight and number of root tips of 2-years-old container-grown Norway spruce seedlings cultivated in different peat-based substrates inoculated with commercial ectomycorrhizal inocula Ectovit and Mycorrhizaroots. Different letters indicate significantly different values between substrate and ectomycorrhizal inoculum combinations (P < 0.05).
Ectomycorrhiza estimation
In total, over 46 000 spruce root tips were visually evaluated for ECM colonization. The overall colonization rate ranged from 73 to 80%, regardless of analyzed treatments (Tab. 4).
Tab. 4 - Percentage of ectomycorrhizal morphotypes and total ectomycorrhizal colonization (mean ± standard error) of 2-years-old container-grown Norway spruce seedlings grown in different substrates inoculated with commercial ectomycorrhizal inocula Ectovit and Mycorrhizaroots.
| ECM morphotype |
Substrate | Inoculation | ||||
|---|---|---|---|---|---|---|
| Agro CS | Gramoflor | Durpeta | Ectovit | Mycorrhizaroots | Non- inoculated |
|
| I | 5 ± 1.4 | 8 ± 2.3 | 5 ± 1.0 | 8 ± 1.7 | 7 ± 2.3 | 1 ± 1.5 |
| II | 70 ± 2.4 | 64 ± 4.1 | 66 ± 3.1 | 66 ± 3.4 | 67 ± 4.8 | 68 ± 4.4 |
| III | 5 ± 1.3 | 2 ± 0.9 | 2 ± 0.8 | 3 ± 0.8 | 3 ± 4.1 | 4 ± 3.9 |
| Total ECM colonization | 80 ± 2.3 | 74 ± 3.6 | 73 ± 2.9 | 75 ± 2.8 | 77 ± 4.4 | 73 ± 4.3 |
Regarding the morphological diversity of inspected fine roots, three ECM morphotypes were distinguished: (I) black color, monopodial-pinnate ramification, cylindrical shape, densely grain shiny mantle with emanating hyphae; (II) dark brown, monopodial-pinnate ramification, cylindrical shape, shiny or smooth mantle; (III) light orange brown, monopodial-pinnate or monopodial-pyramidal ramification, bent to sinuous shaped, shiny cottony mantle. All treatments revealed almost the same relative abundance of ECM morphotypes. The most common morphotype (64-70%) was morphotype II, while the other two morphotypes did not exceed 8%. The relative abundance of non-mycorrhizal short roots ranged from 20 (Agro CS) to 27% (Durpeta). Neither substrate or inoculation nor treatment interaction had significant effects on ECM colonization.
Based on specific morphological features of the ectomychorrizae, morphotype I was identified as Cenococcum geophilum Fr. Its relative frequency roughly corresponded to the average occurrence of morphotype I (6% of visually evaluated root tips).
Out of the total amplified DNA samples of morphotypes II and III, 27 good-quality ITS sequences (68%) were obtained. On the basis of their homology, the analyzed sequences were clustered into four homology groups (≥ 95% homology) which should represent individual taxa. Using the BLAST software, we compared the aligned sequences of each homology group with samples of known origin at the NCBI database. A significant alignment (99-100%) was found, allowing the identification of each homology group at the species level. Two ectomycorrhizal species were identified by the sequence of amplified ITS region (Tab. 5). The most common fungus was Thelephora terrestris (Ehr.) Ft. (78% of the obtained ITS sequences), followed by another ECM fungus Laccaria proxima (Boud.) Pat. (15%). Additionally, a saprophyte species such as Bjerkandera adusta (Willd.: Fr.) P. Karst. was detected (4%), as well as the yeast Cryptococcus podzolicus (Babeva & Reshetova) Golubev (5% of the obtained ITS sequences).
Tab. 5 - Fungal species identified from the ectomycorrhizal root tips of 2-years-old Norway spruce seedlings. (Vm): visual morphotyping; (Sq): DNA sequencing.
| Group | Fungal species | Method of identification |
Accession Nr. | BLAST match ID |
Identity (%) |
|---|---|---|---|---|---|
| ECM symbiont |
Thelephora terrestris | Sq | KJ886932 | JQ711980.1 | 99 |
| Laccaria proxima | Sq | KJ918763 | JX907813.1 | 100 | |
| Cenococcum geophilum | Vm | - | - | - | |
| Non-ECM species | Bjerkandera adusta | Sq | KJ918764 | KC176354.1 | 99 |
| Cryptococcus podzolicus | Sq | KJ918765 | FR716534.1 | 99 |
The molecular identification of extracted samples revealed a morphotype-taxon inconsistency for morphotypes II and III. Based on results of the DNA analysis, a clear correspondence between the identified fungus and morphotype was not found. In particular, T. terrestris was recognized within both morphotypes (II and III), while the L. proxima was detected in morphotype III only. The most common species T. terrestris almost completely dominated within all treatments regardless of the inocula application. Despite the rather scarce abundance of C. geophilum and L. proxima, both these species were identified in the artificially Ectovit- and Mycorrhizaroots-inoculated treatments.
Needle macro-elements concentration
Tab. 6 reports the concentration at the end of the experiment of the macro-elements analyzed in the needles of inoculated and non-inoculated seedlings grown in the three tested substrates. Needle nutrients concentration followed a similar trend as seedling biomass development. In general, seedlings grown in the enriched substrates (in the case of N and K in particular in Agro CS) had higher concentrations of macronutrients as compared with seedlings grown in non-enriched substrate, though the only major difference detected was for Ca concentration. Inoculation treatments in enriched substrates had no distinctive influence on seedling nutrition (at least needle nutrients concentration), with exception of N in Agro CS. The highest N level was found for non-inoculated seedlings in Agro CS, and less markedly for the other two substrates. In the non-enriched substrate, needle concentrations of P, K and Ca in Ectovit-inoculated seedlings were higher than those in Mycorrhizaroots-inoculated (12%, 53% and 9%, respectively) and particularly in non-inoculated seedlings (17%, 77% and 12%, respectively). Ectovit was very efficient mainly in K-uptake in non-enriched substrate; K concentration in this treatment combination almost reached the K level in Agro CS and was higher than in Gramoflor substrate. Magnesium was the element with the most balanced needle concentration regardless of substrate and inoculation treatments.
Tab. 6 - Concentration of macro-elements in needles (n=1 combined sample) of 2-years-old container-grown Norway spruce seedlings grown on substrates Agro CS, Gramoflor and Durpeta inoculated with commercial ectomycorrhizal inocula Ectovit and Mycorrhizaroots.
| Substrate × Inoculum combination |
C (%) |
N (%) |
P (mg kg-1) |
K (mg kg-1) |
Ca (mg kg-1) |
Mg (mg kg-1) |
|---|---|---|---|---|---|---|
| Agro CS×Ectovit | 49.8 | 1.25 | 1703 | 7713 | 4885 | 1133 |
| Agro CS×Mycorrhizaroots | 51.1 | 1.68 | 1821 | 7625 | 5358 | 1260 |
| Agro CS×Non-inoculated | 52.9 | 1.98 | 1674 | 7413 | 5417 | 1352 |
| Gramoflor×Ectovit | 51.2 | 1.34 | 1713 | 6338 | 5849 | 1198 |
| Gramoflor×Mycorrhizaroots | 50.5 | 1.38 | 1839 | 7145 | 5657 | 1255 |
| Gramoflor×Non-inoculated | 51.0 | 1.46 | 1735 | 6502 | 5720 | 1350 |
| Durpeta×Ectovit | 51.5 | 1.06 | 1527 | 7483 | 2924 | 1104 |
| Durpeta×Mycorrhizaroots | 48.6 | 1.00 | 1359 | 4885 | 2681 | 1030 |
| Durpeta×Non-inoculated | 52.6 | 1.08 | 1309 | 4232 | 2608 | 1069 |
Chlorophyll a fluorescence
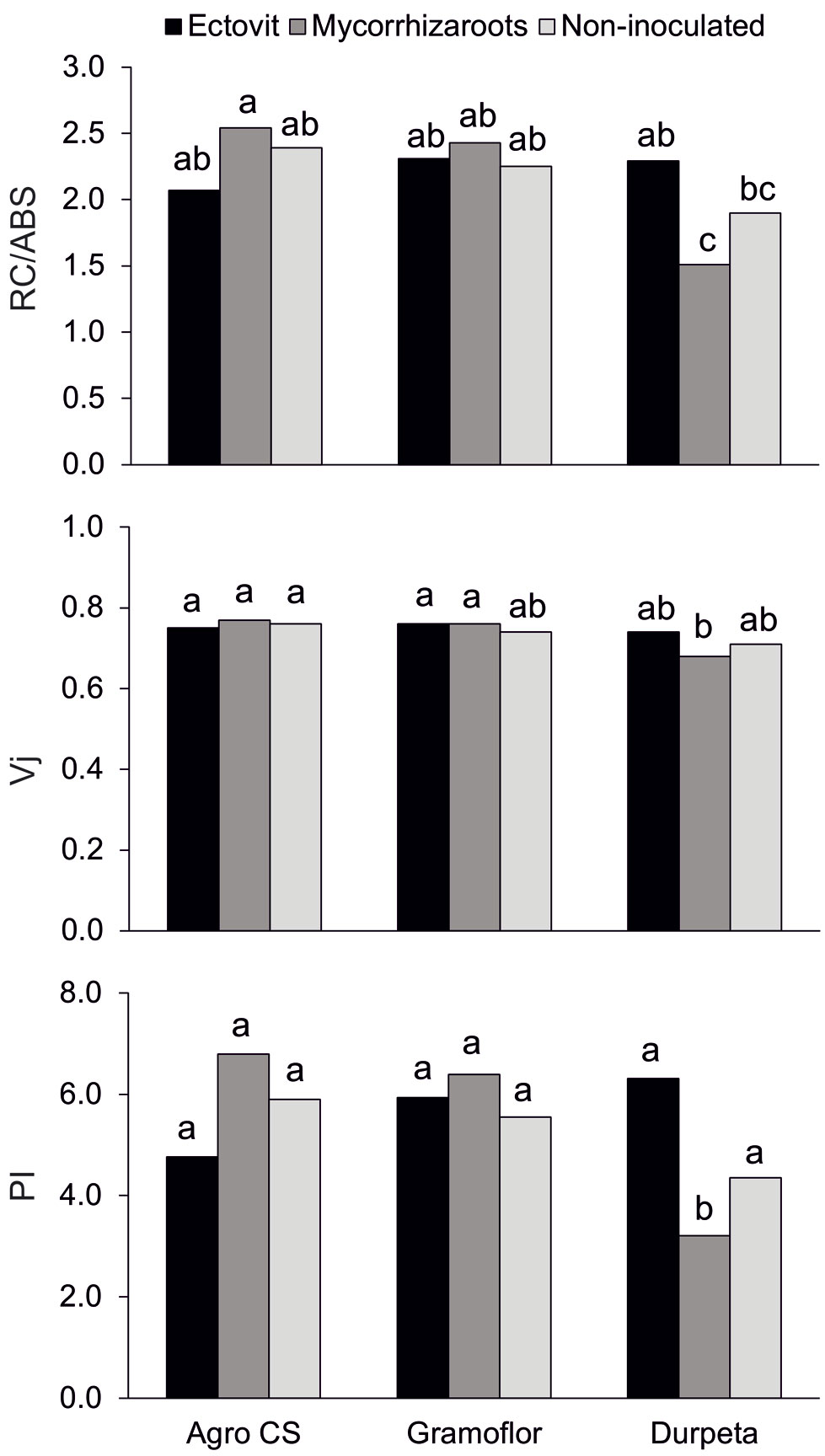
As for chlorophyll a fluorescence parameters, a pattern similar to that observed for growth variables was observed, though most differences were not significant (Tab. 7). Seedlings grown in enriched substrates had means of the fluorescence parameters analyzed higher than seedlings grown in non-enriched substrates, except for variables Fv/Fm and Fv/F0. Significant differences in Tfm, RC/ABS and Vj were also found among substrates (Tab. 7, Tab. 8). Higher values of fluorescence parameters were observed in Ectovit-inoculated seedlings as compared with Mycorrhizaroots- and non-inoculated seedlings, though their differences were not statistically supported. Significant interactions (P<0.05) between substrate and inoculation treatments were found (Tab. 7). In the non-enriched substrate, values of RC/ABS and PI parameters were significantly higher in Ectovit- than in Mycorrhizaroots-inoculated seedlings (Fig. 2), whereas there were no significant differences between inoculation treatments within both enriched substrates. Moreover, seedlings inoculated with Mycorrhizaroots grown in the enriched substrates had values of RC/ABS, Vj and PI parameters significantly higher than those grown in non-enriched substrate; the other two inoculation treatments did not differ between substrates (Fig. 2).
Tab. 7 - Analysis of variance (F- and P-values) of the effects of substrate and commercial ectomycorrhizal inoculation on chlorophyll a fluorescence parameters of 2-years-old container-grown Norway spruce seedlings. Degrees of freedom: substrate 2; inoculation 2; block 2; substrate × inoculation 4; error for substrate and inoculation 4; error for substrate × inoculation 8; residual 297; total 323.
| Parameter | Substrate | Inoculation | Substrate × Inoculation |
|||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
F0
|
4.97 | 0.082 | 0.93 | 0.467 | 0.24 | 0.911 |
Fm
|
2.75 | 0.178 | 1.68 | 0.295 | 0.91 | 0.501 |
Fv
|
0.56 | 0.612 | 2.84 | 0.171 | 2.74 | 0.105 |
Fv/Fm
|
0.68 | 0.556 | 4.51 | 0.094 | 2.38 | 0.138 |
Tfm
|
9.09 | 0.033 | 0.20 | 0.825 | 0.39 | 0.812 |
Area
|
6.79 | 0.052 | 0.33 | 0.739 | 3.41 | 0.066 |
RC/ABS
|
9.98 | 0.028 | 0.63 | 0.578 | 9.31 | 0.004 |
Fv/F0
|
0.38 | 0.707 | 3.96 | 0.113 | 2.81 | 0.100 |
Vj
|
8.45 | 0.037 | 0.71 | 0.544 | 6.07 | 0.015 |
PI
|
3.01 | 0.159 | 1.70 | 0.292 | 6.20 | 0.014 |
Tab. 8 - Chlorophyll a fluorescence parameters (mean ± standard error) of 2-years-old container-grown Norway spruce seedlings grown in different peat-based substrates inoculated with commercial ectomycorrhizal inocula Ectovit and Mycorrhizaroots. (F0): initial fluorescence; (Fm): maximum fluorescence; (Fv) = Fm-F0, variable fluorescence; (Fv/Fm): maximal photochemical efficiency of PSII; (Tfm): time of reaching maximum fluorescence; (Area): area above the fluorescence curve; (RC/ABS): density of reaction centers; (Fv/F0): competitive non-photochemical processes in PS II in dark-adapted state; (Vj): relative variable fluorescence at time 2 ms after start of light pulse; (PI): performance index for the photochemical activity. Different letters within substrate and inoculation treatments indicate statistically different values.
| Factor | Treatment |
F0
|
Fm
|
Fv
|
Fv/Fm
|
Tfm
|
Area
|
RC/ABS
|
Fv/F0
|
Vj
|
PI
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | Agro CS | 0.29±0.02a | 0.89±0.03a | 0.60±0.01a | 0.71±0.01a | 543±14ab | 33945±1480a | 2.33±0.06a | 2.98±0.10a | 0.76±0.01a | 5.83±0.31a |
| Gramoflor | 0.26±0.02a | 0.84±0.03a | 0.59±0.01a | 0.72±0.01a | 560±14a | 35243±2193a | 2.33±0.05a | 3.13±0.09a | 0.75±0.01ab | 5.95±0.25a | |
| Durpeta | 0.20±0.01a | 0.75±0.02a | 0.55±0.01a | 0.72±0.01a | 464±13b | 25709±1825a | 1.90±0.06b | 2.99±0.08a | 0.71±0.01b | 4.63±0.23a | |
| Inoculation | Ectovit | 0.25±0.02a | 0.87±0.03a | 0.61±0.01a | 0.73±0.01a | 528±14a | 32850±2161a | 2.22±0.05a | 3.16±0.09a | 0.75±0.01a | 5.67±0.24a |
| Mycorrhizaroots | 0.26±0.02a | 0.82±0.03a | 0.56±0.02a | 0.70±0.01a | 520±14a | 30926±1731a | 2.15±0.07a | 2.93±0.10a | 0.74±0.01a | 5.44±0.32a | |
| Non-inoculated | 0.24±0.02a | 0.80±0.03a | 0.56±0.02a | 0.72±0.01a | 518±14a | 30940±1690a | 2.18±0.06a | 3.01±0.09a | 0.74±0.01a | 5.27±0.24a |
Fig. 2 - Chlorophyll a fluorescence parameters RC/ABS, Vj, PI of 2-years-old container-grown Norway spruce seedlings cultivated in different peat-based substrates inoculated with commercial ectomycorrhizal inocula Ectovit and Mycorrhizaroots. Different letters indicate significantly different values between substrate and ectomycorrhizal inoculum combinations (P < 0.05).
Discussion
Our results show that the peat-based enriched substrates Agro CS and Gramoflor clearly provided better conditions for development of container-grown Norway spruce seedlings than the pure-peat substrate Durpeta. Such evidence was supported by the enhanced growth and higher physiological parameters of seedlings grown in enriched substrates, as well as by the higher concentration of macronutrients in their needles. A number of studies confirmed that admixture of well-selected components to the peat can substantially improve physical properties of growth substrates and stimulate seedling growth ([26], [6], [5]). In our experiment, a positive effect on nutrient uptake and biomass production of seedlings could be due to the admixture of several components to the enriched substrates, along with the peat type and the degree of humification. However, despite the asserted nutrient enrichment of the commercial substrates Agro CS and Gramoflor, their concentration in N and K was similar that of the non-enriched Durpeta substrate after chemical analysis. This finding is likely to be due to the diverse origin of substrates and their level of decomposition, leading to differences in nutrient content and physical properties. No direct relationship between the substrate and nutrients concentration in the needles was found. On the contrary, macro-elements in seedling foliage reflected the trend already observed for growth, with higher concentrations found in seedlings on enriched substrates. However, these seedlings showed nutrient concentrations in the needles falling within the range of values recommended by Rikala ([58]), except for Ca which was slightly higher, while Durpeta seedlings showed lower values out of the above range. Nevertheless, a positive effect of Ectovit inoculation on the needle P and K concentrations of Durpeta seedlings was observed. A similar positive influence of Ectovit on needle nutrient concentration of Norway spruce cuttings was described by Repáč et al. ([57]).
Other chemical characteristics may directly affect the availability of macro- and micro-elements, as well as the combined effect of physical and chemical properties of substrates ([61]). Indeed, the acidity or alkalinity of the growth medium is of great importance for the availability of nutrients in the soil solution ([9]). The low pH (3.4) of the Durpeta substrate could have caused a weaker seedling development, while the enriched substrates Agro CS (pH 4.3) and Gramoflor (pH 4.6) had recommended value of pH (4.0-5.0), thus providing optimal conditions for growth of coniferous tree species ([59]). However, some authors reported that the maximum availability of nutrients for seedlings grown on organic substrates is reached at pH > 5.5 ([12], [39]). Furthermore, the high C/N ratio of Durpeta substrate in this study could have caused the immobilization of certain nutrients by microorganisms ([34], [60]), negatively affecting the growth of spruce seedlings.
Positive effects of the ECM inoculation on the development of bareroot and container-grown seedlings have been reported in Norway spruce ([36], [53], [11]) and several pine species ([18], [61]). However, in other studies inoculation did not affect or even reduce seedling growth ([60], [54], [17]). Seedling response to inoculation can be affected by several factors, including the type of inoculum, inoculation pattern, interspecific and intraspecific host-fungus variation, environmental conditions, and seedling production practices ([61], [55]). In our experiment the significant interaction found between substrate and inoculation indicated that seedlings’ development is affected by both factors. Indeed, the inoculation with Ectovit promoted the growth and physiological state of seedlings grown in Durpeta substrate. Similarly, significant interaction substrate × ECM inoculation resulted in enhanced growth of seedlings in Pinus sylvestris ([4]), Pinus pinea ([60]) and Picea abies ([54]).
Several studies showed that growth substrate, especially nutrient content, and ECM inoculation considerably affect SDW, RDW and thereby SDW/RDW in Norway spruce seedlings ([53], [11], [21]). In this experiment, the enriched substrates Agro CS and Gramoflor stimulated the growth of aboveground part of seedlings, while the inoculation increased the root biomass, particularly in Mycorrhizaroots-inoculated seedlings. This finding is in accordance with the results reported by Repáč ([53]) and Flykt et al. ([21]), but is in contradiction with those of Brunner & Brodbeck ([11]). Significantly higher values of SDW/RDW in peat-based substrates, as compared with compost-based substrates, were also reported ([54]). Contrastingly, Quoreshi & Timmer ([52]) and Fan et al. ([20]) did not observe significant effects of fertilization and inoculation on the SDW/RDW ratio. Overall, the lower SDW/RDW observed in this experiment (in particular for Mycorrhizaroots-inoculated seedlings) may contribute to better field performance of seedlings, especially on dry sites ([16], [70]).
Visual inspection of the root systems confirmed the abundant root colonization by ECM fungi. The high root colonization observed in this study (overall 75%, irrespective of the treatment) is common in artificially inoculated seedlings grown under controlled conditions ([35], [24], [21]). As for the morphology of ectomycorrhizae, our results suggest a low degree of morphological diversity among treatments, as most of the root tips were classified in the ECM morphotype II. However, the similarity of morphotypes II and III could have potentially led to the underestimation of the abundance of morphotype III.
The fungal community associated with the analyzed spruce seedlings was rather homogeneous, as expected for early successional stages of ECM ([51]). Successful colonization was detected by three ECM fungi, but most ectomycorrhizae (irrespective of the inoculation) were formed by the soil-borne ECM fungus Thelephora terrestris Fr., while the other two (C. geophilum and L. proxima) did not exceed 15% in abundance. However, the method used for their identification did not allow a direct confirmation of their origin from the applied inocula.
The poor ECM species composition associated with the analyzed spruce roots did not reflect the species-rich mixture contained in the applied biological additives. The low colonization effectiveness of tested commercial inocula was most likely the result of their diminished competitive ability as compared to soil-borne ECM species. Natural colonization of P. abies by soil-borne fungi in nursery conditions was reported by several authors ([65], [68], [50]), especially Thelephora terrestris, regarded as the main competitor of introduced ECM fungi ([10], [24], [29]). Moreover, the application of fungicides and fertilizers during the growing period likely had a negative effect on beneficial ECM fungi ([63]).
A significant effect of hydrogel on physical properties of peat was reported by Heiskanen ([27]). According to Chirino et al. ([13]), addition of hydrogel to peat-based substrates increased water-holding capacity and positively affected shoot height of containerized Quercus suber seedlings. By contrast, no effect of Ectovit on rooting and biomass production of Norway spruce cuttings was found by Repáč et al. ([57]). Moreover, the influence of field inoculation with Ectovit on outplanting performance of Norway spruce seedlings was reported as negligible ([30], [49], [56]).
Measurement of chlorophyll a fluorescence is non-destructive, non-invasive and a reliable tool for assessing the response of forest tree seedlings to different substrate properties and ECM inoculation treatments ([7], [14], [32], [42]). Jamnická et al. ([32]) reported a chronic photoinhibition of European beech seedlings under drought treatment, while no significant changes in maximal quantum efficiency (Fv/Fm) were found as compared to irrigated control after hydrogel application. Mancilla-Leyton et al. ([42]) found significant differences in Fv/Fm between cork oak seedlings grown on nutrient-poor and nutrient-rich substrates. Vodnik & Gogala ([71]) reported higher levels of chlorophyll and carotenoids in needles of Picea abies seedlings inoculated with ECM fungi as compared with the control treatment.
In this study, chlorophyll a fluorescence parameters (mainly RC/ABS, Vj and PI) reflected the observed differences in seedlings’ growth among the substrate and inoculation treatments. Mean values of Fv/Fm (0.70-0.73) indicated a slightly lower level of photosynthesis in the investigated seedlings, as compared with the literature ([44]).
Conclusion
Evaluation of growth and physiological parameters of 2-year-old container-grown Norway spruce seedlings showed different effects of the tested peat substrates (differing in origin, composition and thus in physical and chemical properties) on seedling development. Results indicate that the enriched peat-based substrates tested is a suitable potting substrate for Norway spruce seedlings. Significant effects of the interaction substrate × inoculation on several growth and physiological parameters also indicates a distinctive influence of commercial ECM inocula on seedling development. Ectovit inoculum applied as hydrogel stimulated seedling growth in the non-enriched pure peat substrate. This effect was more likely induced by the particular physical and chemical properties of the inoculum rather than to nutritional or non-nutritional effects of the ECM fungi inoculated. Inoculation did not lead to the formation of specific ectomycorrhizae on seedlings, most probably because of the massive competition of the soil-borne ECM fungus Thelephora terrestris.
The results reported in this study may be useful for the improvement of nursery practices in Norway spruce, aimed at the production of high-quality seedlings with enhanced rooting by ectomycorrhizal fungi.
Acknowledgements
We thank Dr. Alistair Pfeifer for the improvement of English, two anonymous reviewers for their comments on an earlier version of the manuscript, Mrs. Jana Povalačová and Mr. Samuel Kozánek for their technical assistance. This work was financially supported by the Scientific Grant Agency of Ministry of Education SR and Slovak Academy of Sciences (project VEGA 1/521/13).
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Miroslav Balanda
Jaroslav Vencurik
Jaroslav Kmet
Diana Krajmerová
Ladislav Paule
Technical University in Zvolen, T. G. Masaryka 24, SK 960 53 Zvolen (Slovak Republic)
Corresponding author
Paper Info
Citation
Repáč I, Balanda M, Vencurik J, Kmet J, Krajmerová D, Paule L (2014). Effects of substrate and ectomycorrhizal inoculation on the development of two-years-old container-grown Norway spruce (Picea abies Karst.) seedlings. iForest 8: 487-496. - doi: 10.3832/ifor1291-007
Academic Editor
Silvano Fares
Paper history
Received: Mar 17, 2014
Accepted: Aug 14, 2014
First online: Nov 10, 2014
Publication Date: Aug 02, 2015
Publication Time: 2.93 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2014
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 56710
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 46891
Abstract Page Views: 3928
PDF Downloads: 4472
Citation/Reference Downloads: 16
XML Downloads: 1403
Web Metrics
Days since publication: 4131
Overall contacts: 56710
Avg. contacts per week: 96.10
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 9
Average cites per year: 0.82
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Short Communications
Effect of four levels of shade on survival, morphology and chlorophyll fluorescence of Nothofagus alessandrii container-grown seedlings
vol. 8, pp. 638-641 (online: 08 January 2015)
Research Articles
Fertilisation of Quercus seedlings inoculated with Tuber melanosporum: effects on growth and mycorrhization of two host species and two inoculation methods
vol. 10, pp. 267-272 (online: 13 December 2016)
Research Articles
Effect of Funneliformis mosseae on growth, mineral nutrition, biochemical indexes and chlorophyll content of Ziziphus spina-christi seedlings at different salinities
vol. 9, pp. 503-508 (online: 08 December 2015)
Research Articles
Ectomycorrhizal fungal community in mature white poplar plantation
vol. 14, pp. 540-547 (online: 26 November 2021)
Research Articles
Needle traits of understory Silver fir, Norway spruce, and Scots pine in response to increased canopy openness in a birch-dominated stand
vol. 18, pp. 273-282 (online: 14 October 2025)
Research Articles
Substrates and nutrient addition rates affect morphology and physiology of Pinus leiophylla seedlings in the nursery stage
vol. 10, pp. 115-120 (online: 02 October 2016)
Research Articles
Outplanting performance of three provenances of Quillaja saponaria Mol. established in a Mediterranean drought-prone site and grown in different container size
vol. 13, pp. 33-40 (online: 21 January 2020)
Research Articles
Calibration of a multi-species model for chlorophyll estimation in seedlings of Neotropical tree species using hand-held leaf absorbance meters and spectral reflectance
vol. 9, pp. 829-834 (online: 17 May 2016)
Research Articles
The combined effects of Pseudomonas fluorescens CECT 844 and the black truffle co-inoculation on Pinus nigra seedlings
vol. 8, pp. 624-630 (online: 08 January 2015)
Research Articles
Maximizing growth of Acacia confusa through native plant growth-promoting bacterial inoculation and seed pelleting for revegetation in landslide areas
vol. 19, pp. 28-37 (online: 11 January 2026)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword