Ectomycorrhizal diversity in a mature pedunculate oak stand near Morović, Serbia
iForest - Biogeosciences and Forestry, Volume 16, Issue 6, Pages 345-351 (2023)
doi: https://doi.org/10.3832/ifor4362-016
Published: Nov 22, 2023 - Copyright © 2023 SISEF
Research Articles
Abstract
Pedunculate oak is among the most economically important deciduous forest tree species in Europe and is also a host for many important ectomycorrhizal (ECM) fungi. The aim of this study was to analyse the ECM fungal community in a mature pedunculate oak stand near Morović, Serbia in spring and autumn. ECM fungi were determined by combining morpho-anatomical characterization of ectomycorrhizas with molecular analysis based on PCR amplification of the internal transcribed spacer region of fungal nuclear ribosomal DNA. The number of ECM fungal taxa and the number of different categories of fine roots were counted, diversity indices were calculated, and ECM fungi were classified into exploration types. Twenty-one ECM fungal taxa were recorded in the studied mature pedunculate oak stand, 19 in spring and 13 in autumn. ECM communities consisted of one dominant taxon and a larger number of rare taxa. Lactarius quietus was the most abundant ECM fungus in both seasons which made association with more than half of ECM root tips. At the stand near Morović, contact exploration type (ET) dominated, short-distance ET was less abundant, while medium-distance fringe ET and long-distance ET were rare in both seasons. The most pronounced difference between seasons is recorded in the number of ECM fungal taxa. The number of ECM fungal taxa and diversity indices recorded in the studied pedunculate oak stand were lower or similar compared to values obtained in stands of oak species across Europe.
Keywords
Ectomycorrhizal Fungi, Quercus robur L., Morpho-Anatomical Characterization, ITS Region
Introduction
Pedunculate oak (Quercus robur L.) belongs to the most economically important forest tree species in Europe because of its high-quality wood ([14], [15], [22]). Its acorns are a valuable source of food for many animals giving it a great ecological role ([15]). Furthermore, this species is heliophilous, and light-demanding which enables the regeneration of many other tree species and thus enriches forest biodiversity ([15]). Pedunculate oak occurs in most European countries ([14], [15]). In the Republic of Serbia, the area under the pedunculate oak covers 32,400 ha ([7]) located in the valleys of the Sava, Danube, and Morava rivers, while the best quality pedunculate oak forests are situated in the area Ravni Srem, Western Serbia ([37]).
Ectomycorrhizal (ECM) fungi have a great influence on the establishment, growth, and survival of trees in most temperate ecosystems because they provide trees with water and nutrients in return for carbohydrates ([42]). Forest trees of the same and different species are connected among themselves by a common mycelial network formed by ECM fungi which have an especially important role in forest regeneration, succession, and resistance against different stress factors ([41]). Thus, mycorrhizas play an essential role in the stability of forest ecosystems which is especially important under environmental stress ([29], [44]). Since stress tolerance and adaptation of ectomycorrhiza to different environmental conditions depend on the species of ECM fungus, knowledge about the structure of ECM fungal community can provide valuable information about the physiology of forest trees ([25]). The latest findings of Anthony et al. ([5]) showed that tree growth in conifer and broadleaved forests across Europe was strongly correlated with the composition of the ECM fungal community.
Oaks are hosts of many ECM fungi. In the DEEMY database there are 63 descriptions of ectomycorrhizas on Quercus spp. ([4]). Especially species-rich is the genus Russula containing 14 described ECM fungi, while the genus Tuber (truffles) contains nine ECM fungi, some of which being highly valuable as food ([20]). Moreover, members of the genus Quercus sp. and particularly Q. robur are known to be among the best host plants for all the valuable European Tuber species ([18]).
Ectomycorrhizas on temperate oaks were analysed across European countries ([45]), in Ireland ([35]), south England ([8]), France ([12]), Central Germany ([40]), Austria ([23]), and Poland ([10], [36]).
There is not much data about the diversity of ECM fungi on oaks in the Republic of Serbia. Initial studies were performed in sessile oak stands from Fruška Gora ([30]) and in the young pedunculate oak stand near Morović ([32]). The aim of this study was to analyse the ECM fungal community in mature pedunculate oak stand near Morović, Serbia in two seasons spring and autumn.
Materials and methods
Sampling site and procedures
Sampling was conducted in 145-year-old pedunculate oak (Quercus robur L.) stand under shelterwood cutting procedure. The stand was situated in forest administration “Morović” under management of Public Enterprise “Vojvodinašume” (44° 58′ 02.3″ N, 19° 10′ 56.2″ E; elevation 86 m a.s.l.), where some of the best pedunculate oak forests of Republic of Serbia are located. Other woody species, present with a minor share, are Acer campestre L. and Acer tataricum L. According to meteorological records from the nearby station of Sremska Mitrovica over the period 1991-2020, the mean annual temperature in the area is 11.8 °C and the average annual rainfall is 617.1 mm. According to the average monthly sum of precipitation in this area, the months with the most and the least precipitations were June and February, respectively (⇒ http://www.hidmet.gov.rs/ciril/meteorologija/stanica_sr.php?moss_id=13266).
Physical and chemical properties were determined in the surfice layer of the soil (upper 30 cm). The following soil characteristics were analyzed: particle-size distribution (%) by the international B-pipette method with preparation in sodium pyrophosphate; determination of soil textural classes based on particle-size distribution using Atteberg classification; CaCO3 percentage (%) was measured volumetrically by using Scheibler’s calcimeter; and pH in H2O was determined with electrometric method using a combined electrode on Radiometer pH meter. Carbon and nitrogen content were determined with CHN element analyzer (Vario® EL III, Elementar Analysensysteme, Germany), while the content of humus was measured by the method of Turin. All analyses were performed in the laboratory of Soil Science at the Institute of Lowland Forestry and Environment in Novi Sad applying the methodology described by Galić et al. ([16]).
Soil sampling was performed on 29th May 2019 (spring) and 1st October 2019 (autumn) using a soil corer with a total volume of 274 ml and length of 18 cm . In both seasons, ten soil samples were taken at about 1 m from the target tree trunk. Soil samples were stored in a refrigerator at 4 °C for up to three months. Before analyses each sample was submerged overnight in tap water to loosen the soil structure. All fine roots were carefully washed from the soil and divided into vital ECM root tips, or old, nonturgescent, and nonmycorrhizal roots using a dissecting stereomicroscope Olympus SZX10® (Olympus Corp., Tokyo, Japan) with magnifications 10-63× (light source: Olympus Highlight 3100, daylight filter). Vital ECM root tips were categorized into different morphotypes of ectomycorrhizas based on their morphological and anatomical characteristics using a dissecting microscope and a microscope (Olympus BX53®) with magnifications 100-1000×.
Morphotypes of ectomycorrhizas were described following the methodology given by Agerer ([1]) and Kraigher ([24]). When it was possible, a fungal partner was determined by comparison of obtained descriptions with published descriptions in Agerer ([3]) or Agerer & Rambold ([4]).
Morphotypes of ectomycorrhizas were also classified into the exploration types as proposed by Agerer ([2]). All categories of fine root tips were quantified by counting under the dissecting microscope.
Molecular identification of ectomycorrhizal fungi
Molecular identification of fungus from ECM root tip was based on PCR amplification of internal transcribed spacer (ITS) region of fungal nuclear rDNA. Genomic DNA was extracted from 2-5 ECM root tips of every ECM morphotype using a DNeasy® Plant Mini Kit (Qiagen, Hilden, Germany). Reactions of amplification were performed using ITS 1F ([17]) and ITS 4 primer pair ([48]) in Eppendorf Master cycler (Eppendorf AG, Hamburg, Germany). The PCR mixture for each sample was composed of 2.5 µL of 10× Gold Buffer, 2 µL of deoxynucleotide triphosphates (0.2 mM each), 0.6 µL of each primer (10 µM each), 2 µL of MgCl2 (2.0 mM), 15 µL of sterile distilled water, 0.3 µL of Taq polymerase (5 U µL-1), and 2 µL of a DNA extract. Thermal cycling conditions were as follows: initial denaturation and polymerase activation at 95 °C for 5 min; 13 cycles at 94 °C for 45 s, 55 °C for 55 s and 72 °C for 45 s; 13 cycles at 94 °C for 45 s, 55 °C for 55 s and 72 °C for 120 s; 12 cycles at 94 °C for 45 s, 55 °C for 55 s and 72 °C for 180 s and a final extension at 72 °C for 10 min. After purification using the QIAquick PCR® purification kit (Qiagen, Valencia, CA, USA) amplified DNA fragments were sent for sequencing to Macrogen Europe BV (Amsterdam, Netherlands). The ECM fungi were determined at the level of species, genus, or family by comparing the obtained sequences to those deposited in GenBank ([33]) and UNITE ([34]) database. The threshold value applied to differentiate the different OTUs based on ITS sequence similarity was 97%.
Data analysis
Diversity indexes were calculated per sample and per season (i.e., by pooling the ECM community data) following the formulas given by Atlas & Bartha ([6]) and Taylor et al. ([46]): (i) species richness index (d) = (S-1)/log10N, where S is the number of ECM fungal taxa and N is the number of all mycorrhizal tips; (ii) Shannon-Weaver’s diversity index (H) = C/N (N logN - Σ ni log ni), where C=2.3, N is the number of all mycorrhizal tips and ni is the number of mycorrhizal tips of an individual ECM fungal taxon; (iii) Evenness (e) = H/logS, where H is the Shannon-Weaver’s diversity index and S is the number of ECM fungal taxa; (iv) Equitability (J) = H/Hmax, where H is the Shannon-Weaver’s diversity index and Hmax is the theoretical maximum H assuming that each ECM fungal taxon was equally abundant; (v) Berger-Parker’s evenness index (BP) = 1 - (Nmax/N), where Nmax is the number of mycorrhizal tips of the most frequent ECM fungal taxon and N is the number of all mycorrhizal tips.
Data from an individual soil sample was used as a statistical unit. Student’s t-test and Mann-Whitney’s U test were used to analyse seasonal differences for the measured parameters. t-test was used to test the significance of differences between spring and autumn in the number of ECM fungal taxa; vital ECM root tips; old, non-turgescent, and non-mycorrhizal roots; total number of fine root tips; percentage of vital root tips, and abundance of exploration types (ET). In order to fit the normal distribution, data were transformed as follows: count data were transformed by square root transformation ([9]), while percentage values were transformed by arcsine transformation using the Bliss formula ([43]). The Mann-Whitney U test was used to test the significance of differences in diversity indexes. All statistical analyses were performed using the package STATISTICA® ver. 12 (StatSoft Inc., Tulsa, OK, USA).
Results
The chemical and physical characteristics of analyzed soil samples from the mature stand of pedunculate oak showed high content of total clay, slightly acidic pH (6.31) and concentration of carbon, nitrogen and humus of 2.26%, 0.126% and 3.12%, respectively (Tab. 1). According to its granulometric content, soil can be classified in the texture class clay loam, while soil type is chernozem gleic. The obtained C/N ratio is favourable and leads toward nitrogen mineralization.
Tab. 1 - Physico-chemical properties of the soil from the mature pedunculate oak (Quercus petrea L.) stand near Morović, NW Serbia.
| Soil Property | Value |
|---|---|
| Total sand (%) | 30.4 |
| Total clay (%) | 69.6 |
| Texture class | Clayey-loam |
| pH in H2O | 6.31 |
| CaCO3 (%) | 1.26 |
| Humus (%) | 3.12 |
| Carbon (%) | 2.26 |
| Nitrogen (%) | 0.13 |
| C/N | 17.93 |
From 20 soil samples taken in the mature stand of pedunculate oak in two seasons, a total of 51,739 fine roots were analysed from which 6.488 represented vital ECM root tips.
Total values of measured root parameters and diversity indices had higher values in spring compared to autumn (Tab. 2). The most pronounced difference was recorded in the number of ECM fungal taxa. Average values of all analysed parameters were also higher in spring than in autumn, but differences were not statistically significant (Tab. 2). However, p-value of t-test for the number of ECM fungal taxa obtained in two seasons was close to 0.05, which could indicate a certain trend in the decrease of this parameter in autumn compared to spring. In both seasons oak trees had a very low share of vital ECM root tips.
Tab. 2 - Comparison of total and average values (± standard error) of number of ectomycorrhizal fungal taxa, vital ectomycorrhizal root tips, old, non-turgescent and non-mycorrhizal roots, total fine roots, % of vital ectomycorrhizal roots and diversity indexes in the mature pedunculate oak (Quercus petrea L.) stand near Morović (NW Serbia) in spring and autumn. (a): p-values after Student’s t-test; (b): p-values after the Mann-Whitney’s U test.
| Parameter | Spring | Autumn | p-value | ||
|---|---|---|---|---|---|
| Total per site |
Average per soil sample |
Total per site |
Average per soil sample |
||
| Number of ectomycorrhizal fungal taxa | 19 | 4.5 ± 0.5 | 13 | 2.9 ± 0.7 | 0.055 a |
| Number of vital ectomycorrhizal root tips | 4002 | 400.2 ± 197 | 2486 | 248.6 ± 101 | 0.442 a |
| Number of old, nonturgescent and nonmycorrhizal root tips | 27570 | 2757 ± 896 | 17681 | 1768 ± 408 | 0.402 a |
| Total number of fine roots | 31572 | 3157.2 ± 966 | 20167 | 2016.7 ± 460 | 0.381 a |
| % of vital ectomycorrhizal root | 13 | 13 ± 4 | 12 | 11 ± 3 | 0.565 a |
| Species richness index (S) | 4.99 | 1.59 ± 0.17 | 3.53 | 0.91 ± 0.25 | 0.064 b |
| Shannon-Weaver index (H) | 1.91 | 1.09 ± 0.14 | 1.51 | 0.69 ± 0.20 | 0.186 b |
| Evenness (e) | 1.49 | 1.71 ± 0.16 | 1.36 | 1.33 ± 0.32 | 0.970 b |
| Equitability (J) | 0.64 | 0.74 ± 0.07 | 0.59 | 0.58 ± 0.14 | 0.970 b |
| Berger-Parker index (BP) | 0.47 | 0.40 ± 0.07 | 0.38 | 0.32 ± 0.09 | 0.345 b |
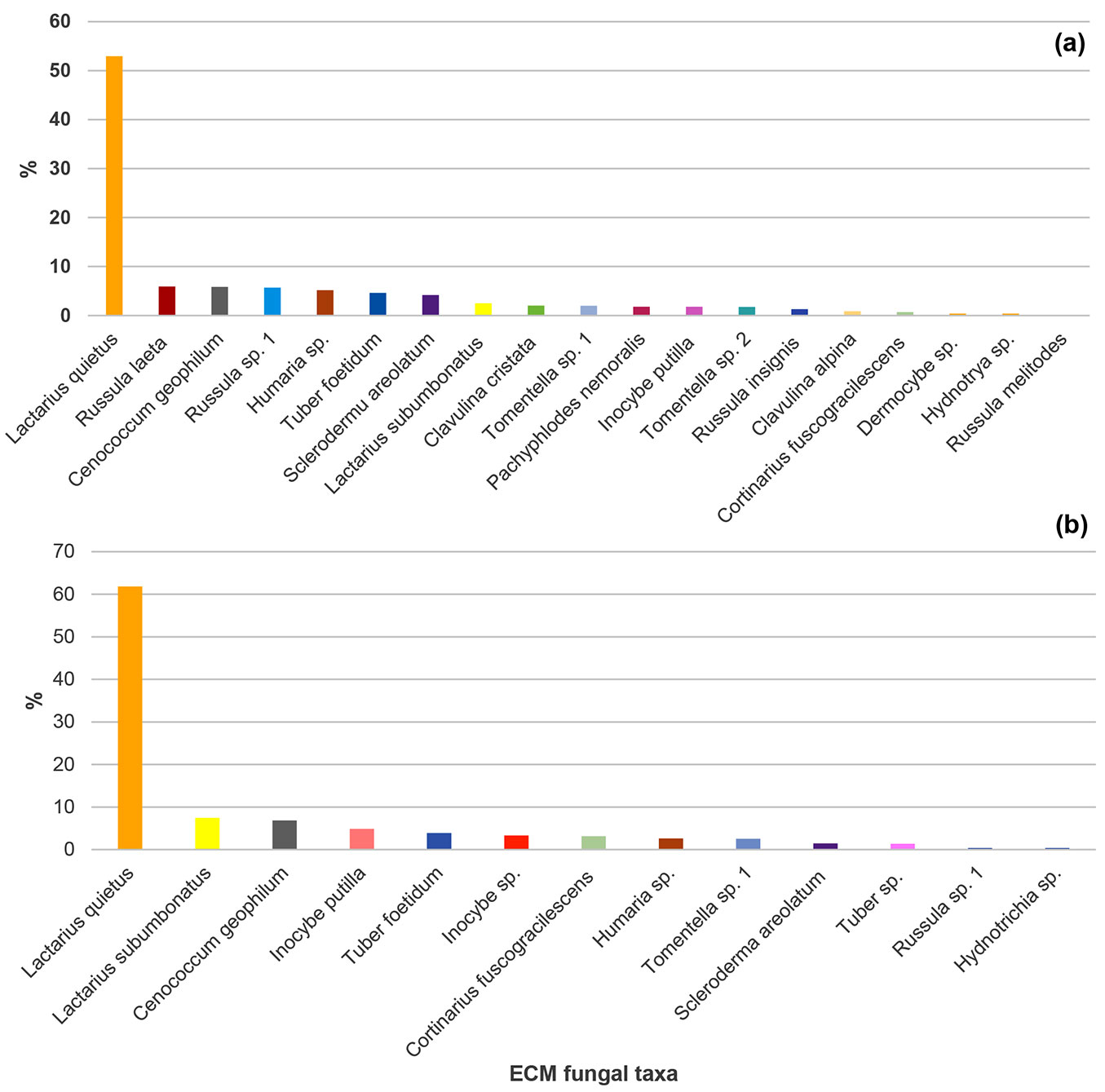
Twenty-one ECM fungal taxa were recorded in the studied mature pedunculate oak stand in the two analysed seasons. Nineteen ECM fungal taxa were recorded in spring (14 Basidiomycota and five Ascomycota), while 13 ECM fungal taxa were observed in autumn (eight of Basidiomycota and five of Ascomycota). Thirteen ECM taxa were identified to the species level and eight to the genus level (Fig. 1). Eleven ECM fungi occured in both seasons: Cenococcum geophilum Fr., Cortinarius fuscogracilescens A. Favre, Inocybe putilla Bres., Lactarius quietus (Fr.) Fr., Lactarius subumbonatus Lindgr., Scleroderma areolatum Ehrenb., Tuber foetidum Vittad., Humaria sp., Hydnotrya sp., Russula sp., and Tomentella sp. Following ECM fungi were recorded only in spring: Clavulina cristata (Fr.) J.Schroet., Pachyphlodes nemoralis Hobart, Bona & Conde, Russula insignis Quél., Russula laeta Jul. Schäff., Clavulina alpina, Dermocybe sp., Russulamelitodes and Tomentella sp. 1, while Inocybe sp. and Tuber sp. were observed exclusively in autumn (Fig. 1).
Fig. 1 - Relative abundance of ectomycorrhizal fungal taxa (based on the number of ectomycorrhizal root tips belonging to the particular ectomycorrhizal fungal taxon in relation to all ectomycorrhizal root tips) in a mature pedunculate oak stand near Morović (NW Serbia) in spring (a) and autumn (b).
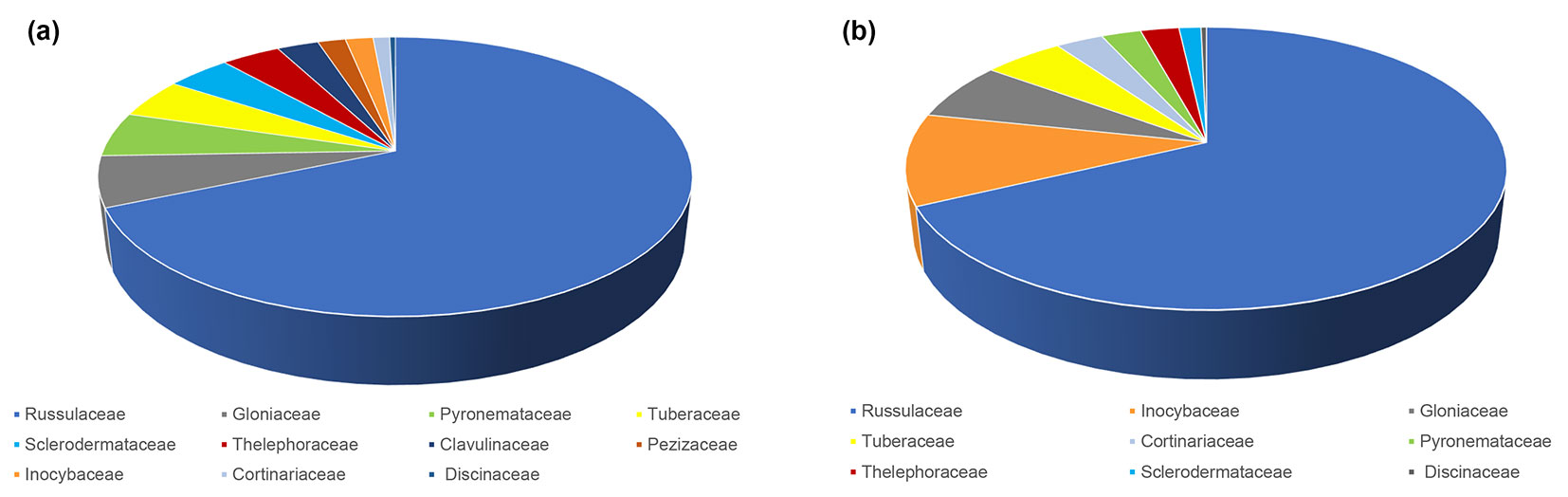
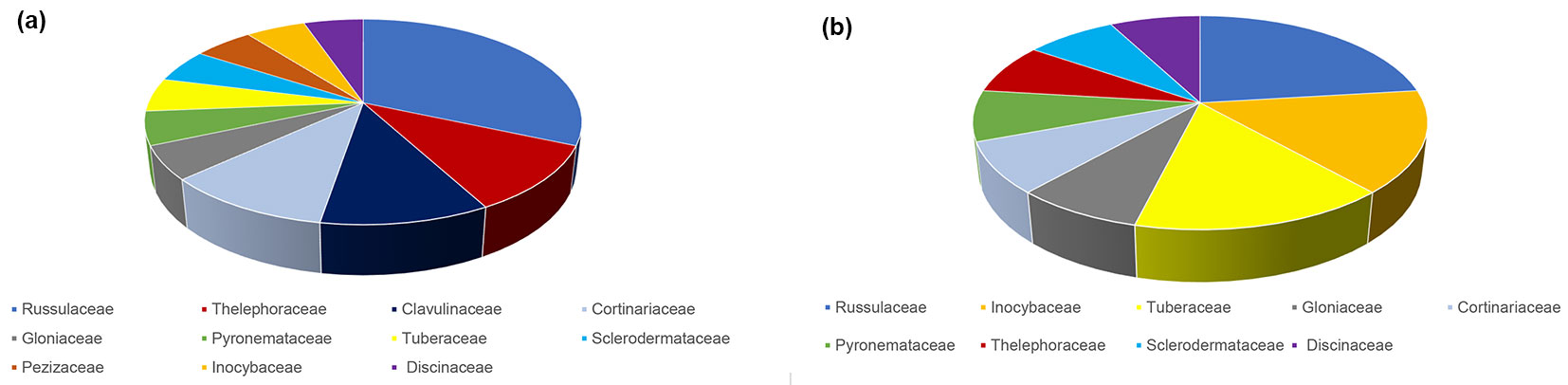
The most abundant ECM fungus in both seasons was L. quietus which made association with more than half of all ECM root tips, while all other ECM fungal taxa were evidently less abundant. R. laeta, the second most abundant in spring was not found in autumn, while L. subumbonatus was the second most abundant ECM fungus in autumn. C. geophilum was the third most abundant fungal taxon in both seasons (Fig. 1). Family Russulacae had the highest relative abundance and species richness in both analysed seasons (Fig. 2 and Fig. 3).
Fig. 2 - Relative abundance of taxonomic families of ectomycorrhizal fungi based on the number of ectomycorrhizal root tips belonging to a particular family in relation to all ectomycorrhizal root tips in mature pedunculate oak stand near Morović (NW Serbia) in spring (a) and autumn (b).
Fig. 3 - Species richness of taxonomic families of ectomycorrhizal fungi based on the number of ectomycorrhizal taxa belonging to a particular family in relation to all ectomycorrhizal taxa in mature pedunculate oak stand near Morović (NW Serbia)in spring (a) and autumn (b).
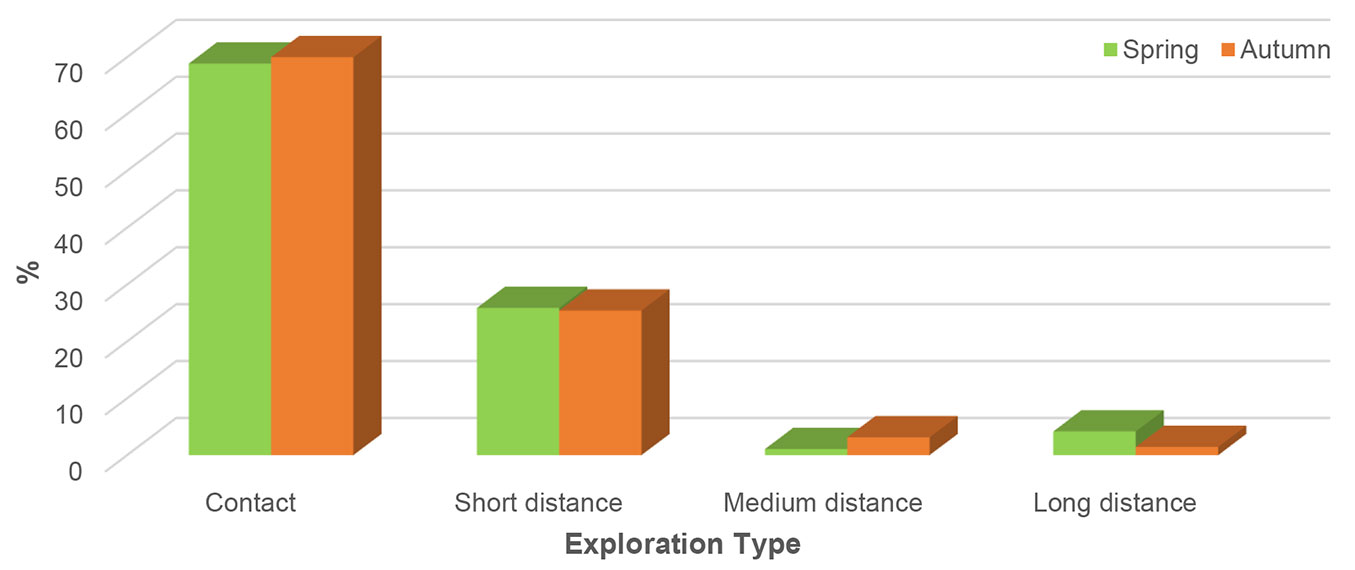
There was no difference in the number of ECM exploration types (ET) between spring and autumn. In both seasons, contact ET was dominant, which was associated with almost 70% of all vital ECM root tips, and followed by short distance ET. Contrastingly, medium- and long-distance ETs were scarce (Fig. 4).
Fig. 4 - Relative abundance of ectomycorrhizal exploration types in mature pedunculate oak (Quercus robur L.) stand near Morović (NW Serbia) in spring and autumn.
Discussion
In a mature pedunculate oak stand from the site near Morović in spring and autumn, 19 and 13 ECM fungal taxa were recorded, respectively. In total, 21 ECM fungal taxa were observed and analysed in the pedunculate oak stand for two seasons. Our results are similar to the findings of Milović et al. ([32]) who recorded 18 ECM fungal taxa in summer in the young pedunculate oak stand from the same area. Similarly,17 and 12 ECM taxa were determined in two sessile oak stands from Fruška Gora ([30]). Furthermore, in three declining pedunculate oak stands in western Poland, the number of ECM fungal taxa ranged from 11 to 15 ([10]). Similarly, in a 150-year-old pedunculate oak forest in north-eastern Poland, fungal species richness ranged from 12 to 14 taxa ([36]). On sessile oak from the Taurus mountains in Central Germany, 18 ECM fungal taxa were recorded ([40]), while on pedunculate and sessile oaks from Ireland, 21 ECM taxa were determined ([35]). However, across three pedunculate oak-dominated woodlands in southern England, the average species richness was in the range of 17-45 ([8]). Furthermore, in oak plots across European countries, the average richness of ECM fungi during one season was 55, ranging from 24 fungal taxa in the Netherlands to 83 in Romania ([45]). A 15 month-study on temporal changes in the ECM community of a temperate oak forest in northeast France revealed 75 fungal taxa ([12]). A 3 year-investigation of the diversity of ECM morphotypes in pedunculate and sessile oaks in Austria, revealed 46 and 38 ECM morphotypes ([23]). The different number of ECM fungal taxa obtained in these studies could be explained by different age classes of oak stands and different sampling intensities (i.e., different numbers or/and size of samples and different number of examined seasons).
On average, 4.5 and 2.9 ECM fungal taxa were recorded in soil samples from the mature pedunculate oak stand in spring and autumn, respectively. This is slightly lower in comparison to the young pedunculate oak stand from the same locality, where six ECM fungal taxa were recorded per soil sample ([32]). The recorded values of fungal taxa per soil sample in this study are comparable to two sessile oak stands from Fruška Gora, where three ECM fungal taxa were found on average ([30]). On the other hand, our results are considerably lower than those recorded on oaks in Austria ([23]) where on average nine morphotypes were found.
Root colonization of mature pedunculate oak trees with vital ECM fungi was very low (11-13%), lower than in young pedunculate oak stand at the same locality (19% - [32]) and in pedunculate oak trees from the old-growth forest in Poland (23-27% - [36]). A considerably low number of ECM fungal taxa recorded in the mature pedunculate oak stand near Morović (21) could be related to low levels of oak root colonization with vital ECM fungi.
Values of the Shannon-Weaver index obtained in soil samples from the mature pedunculate stand in spring and autumn (1.09 and 0.69) were considerably lower in comparison to young pedunculate oak stand from the same area near Morović (1.44 - [32]), to vital (1.2-1.3) and declining (1.3-1.5) sessile and pedunculate oak trees in Austria ([23]), and in a Quercus ilex stand 3 years after fire in Spain (1.1-1.4 - [13]). However, the values of Shannon-Weaver index obtained in this study were comparable to those recorded in two pedunculate oak stands from Fruška Gora (0.69 and 0.87 - [30]).
The number of ECM fungal taxa, number of vital ECM root tips, number of old, nonturgescent and nonmycorrhizal root tips, the total number of fine roots, % of vital ECM root tips, and diversity indices in mature pedunculate oak (Quercus robur L.) stand from site near Morović were higher in spring compared to autumn, which is in accordance to the findings of Milović et al. ([31]) in a mature poplar plantation. In the A1 soil horizon (0-5 cm) of a temperate oak forest in France, the maximum species richness of ECM fungi was recorded in September of the first year of the study, but in September of the next year, the same parameter had a minimum value ([12]). It might be assumed that factors affecting the richness of ECM fungi could vary from year to year. Furthermore, in Poland in temperate oak forest soil, Voríšková et al. ([47]) found that the fungal community had been affected by season. The most important factors contributing to the observed variations were litter decomposition and allocation of photosynthate.
ECM communities of the examined mature oak stand near Morović consisted of one dominant and many rare fungal taxa. Many previous studies showed that ECM communities have few dominant and many rare fungal taxa ([45], [30], [32]). ECM fungus Lactarius quietus dominated ECM fungal community in mature pedunculate oak stand in both seasons and made more than half of all vital ECM root tips. According to Courty et al. ([12]) and Suz et al. ([45]), L. quietus is an oak specialist present during all the year with fluctuating abundance. Although belonging to contact ET category, known by scarce extraradical mycelium, L. quietus can behave saprotrophically, using soil organic matter as substrate in the situation when C demand is high and photoassimilates are not available ([11]). Also, this ECM fungus is found as an indicator species which represents ECM fungal community cluster associated with low-tree growth in broadleaf forests (European beech and mixed oak - [5]). However, in the young pedunculate oak stand near Morović, where ECM fungi Entoloma sp., Thelephoraceae sp., Russula cf. odorata, Russula lilacea and Tomentella sp. 1 made associations with most ECM root tips, L. quietus was not observed ([32]).
The considerable difference in ECM fungal community between young and mature pedunculate oak stand near Morović could be explained by the effect of stand age. It is well known that tree age has a strong effect on the ECM community because older stands have different litter quality and quantity, as well as different dynamics of nutrients and water compared to younger trees ([26]). Accordingly, there is an ordered succession of early-stage fungi with late-stage fungi, and these two groups seemed to have different abilities to form mycorrhizas on roots growing in soils with accumulations of recalcitrant leaf litter ([27]). Lactarius quietus is considered as a middle late-stage fungus ([21]) which could explain its absence from young oak stand.
In the mature pedunculate oak stand, the family Russulacae had the highest relative abundance and species richness in both analysed seasons, which is consistent with the results of Barsoum et al. ([8]) and Suz et al. ([45]). In the temperate oak forests across Europe, nearly 45% of mycorrhizas belonged to the family Russulaceae ([45]) but in our study, almost 70% of all vital ECM roots belonged to this family.
Classification of ectomycorrhizas on ETs connects fungal morphological traits such as the amount and differentiation of extraradical mycelium, with their ecology ([2]). Each exploration type is defined by functional traits regarding its ability to store carbon (C) and take up and translocate nutrients ([19]).
There are two main ECM strategies for growth and nitrogen acquisition. One focuses on the uptake of labile nitrogen forms such as amino acids, ammonium, and nitrate, and the other focuses on insoluble, complex organic resources ([19]). Mycorrhizas with contact, short- and medium-distance smooth ETs use labile, mainly inorganic nitrogen (N). On the other hand, medium-distance fringe and mat, and long-distance ETs are rich in the extraradical mycelium and have medium and long-distance hydrophobic rhizomorphs. Due to hydrolytic exoenzymes, they have access to insoluble and nonlabile substances such as proteins, but they are more C demanding ([19], [28]).
Rosinger et al. ([38]) investigated patterns in ECM diversity, community composition, and ETs in beech, pine, and spruce forests across Europe and noted that contact and short-distance ETs had higher mean abundance compared to medium-distance and long-distance ETs. Contact and short-distance ETs of ECM fungi had mainly broad environmental ranges, they preferentially take up N in the forms of nitrate and ammonium and facilitate humus build-up which leads to high C sequestration in soils ([38]). Furthermore, Bzdyk et al. ([10]) noted that the abundance of contact ET was positively correlated with soil organic matter (C:N ratio and organic carbon content), while the abundance of short-distance ET was closely related to calcium and phosphorus content and pH. According to Suz et al. ([45]) in European forests N pollution and geography are the main factors that structured the abundance of ETs in soil. They found a positive response of contact ECM to N-related variables (throughfall N deposition, N in organic and mineral horizon, and soil solution nitrate).
In mature pedunculate oak stand, contact ET of ECM fungi dominated in both seasons, followed by short distance ET. Since the analysed stand was under shelterwood cutting procedure, canopy opening might provide favourable conditions for increased mineralization. Therefore, the soil at the investigated site contains a sufficient amount of labile inorganic nitrogen to be inhabited by ECM taxa belonging to contact and short-distance ETs, which are considered to be carbon cost-effective in an environment rich in inorganic nitrogen ([19], [45]).
In the young pedunculate oak stand from the same locality, short-distance ET were dominant, followed by MD smooth-distance, and contact ET ([32]). Since the composition of ETs of ECM fungi from young and mature pedunculate oak stands are different, it can be assumed that the composition of ETs of the ECM fungal community changes by the tree age. This is in accordance with previous findings that older stands have a different composition of litter and different dynamics of nutrients compared to younger trees ([26]) which might favour different ETs.
Conclusions
This work presents the results of the first study on the ECM fungi diversity of a mature pedunculate oak stand in Serbia. During two seasons, 21 ECM fungal taxa were recorded, described, and identified mostly with molecular methods. Nineteen ECM taxa were observed in spring and thirteen in autumn. ECM communities consisted of one dominant taxon and a large number of rare taxa. The most abundant ECM fungus in both seasons was Lactarius quietus which made association with more than half of ECM root tips analyzed. At the study site, contact exploration type was dominant in both seasons, short-distance ET was less abundant, while medium-distance fringe ET and long-distance ET were rare. The most pronounced difference between spring and autumn is observed in the total number of ECM fungal taxa. Number of ECM fungal taxa and diversity indices recorded in studied pedunculate oak stand were lower or similar compared to values obtained in stands of oak species across Europe.
Acknowledgements
The study was supported by the project 451-03-47/2023-01/200197 financed by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia. Edmund O. Bauer, USDA Forest Service, Northern Research Station, Institute of Applied Ecosystem Studies (USA) is gratefully acknowledged for revising the manuscript for English style and grammar.
References
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Branislav Kovačević 0000-0002-9125-0659
Milan Drekić 0000-0002-8189-7993
Andrej Pilipović 0000-0002-9458-0581
Saša Pekeč 0000-0002-3803-2978
Lazar Kesić 0000-0003-2643-9727
Milutin Dilas 0000-0003-1324-9545
Velisav Karaklić 0000-0003-1264-9934
Zoran Galić 0000-0002-0748-9203
University of Novi Sad, Institute of Lowland Forestry and Environment, Antona Cehova 13, 21000 Novi Sad (Serbia)
Corresponding author
Paper Info
Citation
Milović M, Kovačević B, Drekić M, Pilipović A, Pekeč S, Kesić L, Dilas M, Karaklić V, Galić Z (2023). Ectomycorrhizal diversity in a mature pedunculate oak stand near Morović, Serbia. iForest 16: 345-351. - doi: 10.3832/ifor4362-016
Academic Editor
Daniela Baldantoni
Paper history
Received: Apr 11, 2023
Accepted: Sep 20, 2023
First online: Nov 22, 2023
Publication Date: Dec 31, 2023
Publication Time: 2.10 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2023
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 7929
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 3665
Abstract Page Views: 2507
PDF Downloads: 1412
Citation/Reference Downloads: 3
XML Downloads: 342
Web Metrics
Days since publication: 828
Overall contacts: 7929
Avg. contacts per week: 67.03
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2023): 1
Average cites per year: 0.33
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Ectomycorrhizal fungal community in mature white poplar plantation
vol. 14, pp. 540-547 (online: 26 November 2021)
Research Articles
Ectomycorrhizal fungal community associated with autochthonous white poplar from Serbia
vol. 9, pp. 330-336 (online: 12 November 2015)
Research Articles
Fungal and bacterial communities in a forest relict of Pinus pseudostrobus var. coatepecensis
vol. 16, pp. 299-306 (online: 09 November 2023)
Research Articles
Mapping fungi from below ground: online genetic resources and ectomycorrhizal geographic distributions
vol. 4, pp. 252-255 (online: 13 December 2011)
Research Articles
Geographic determinants of spatial patterns of Quercus robur forest stands in Latvia: biophysical conditions and past management
vol. 12, pp. 349-356 (online: 05 July 2019)
Research Articles
Potential spread of forest soil-borne fungi through earthworm consumption and casting
vol. 8, pp. 295-301 (online: 26 August 2014)
Review Papers
Soil fungal communities across land use types
vol. 13, pp. 548-558 (online: 23 November 2020)
Research Articles
Concordance between vascular plant and macrofungal community composition in broadleaf deciduous forests in central Italy
vol. 8, pp. 279-286 (online: 22 August 2014)
Research Articles
Naturally regenerated English oak (Quercus robur L.) stands on abandoned agricultural lands in Rilate valley (Piedmont Region, NW Italy)
vol. 4, pp. 31-37 (online: 27 January 2011)
Research Articles
Ectomycorrhizae of Norway spruce from its southernmost natural distribution range in Serbia
vol. 12, pp. 43-50 (online: 10 January 2019)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword