Preliminary indications for diverging heat and drought sensitivities in Norway spruce and Scots pine in Central Europe
iForest - Biogeosciences and Forestry, Volume 13, Issue 2, Pages 89-91 (2020)
doi: https://doi.org/10.3832/ifor3216-012
Published: Mar 01, 2020 - Copyright © 2020 SISEF
Short Communications
Abstract
Massive and increasing tree mortality is currently observed in the two conifer species Norway spruce and Scots pine in Central Europe. Consecutive dry years are made responsible for this phenomenon. Leaf trait measurements, in specific leaf osmotic potential (πosm) and leaf water potential at turgor loss (πtlp), indicate that the underlying mechanisms for tree mortality are most likely different between the two species. πtlp of spruce was highly negative, revealing a potentially high drought tolerance of the species. πtlp of Scots pine was less negative, suggesting a higher susceptibility to drought stress. I conclude that the mortality of Norway spruce might be caused by rising temperatures and that the summer temperatures in the past years were beyond the species thermal tolerance threshold. Overall, I want to highlight and enhance the discussion that the search for suitable species for a climate change adapted forest should go in both directions, i.e., species should be chosen to make the forest fit for both increasing drought and heat stress.
Keywords
Tree Mortality, Water Stress, Heat Stress, Physiological Limitations, Conifers
Introduction
Climate-change induced forest mortality is currently a rapidly emerging trend ([1]). This means that many forests have lost their ability to sustain the global biogeochemical cycle or their function in providing vital habitat, and valuable ecosystem services for human communities ([4]). A good understanding of the drivers, general patterns, and severity of changes can help to apply mitigation strategies to reduce economic and cultural consequence ([1]). In Europe, Norway spruce and Scots pine have been the two most important timber species for the forestry sector over centuries (for historic background see [9], [17]). Especially, those two economically very important conifer species have been massively affected by accelerated tree mortality in recent years across Europe ([1]). In the past, the main cause of mortality in Norway spruce was wind disturbance with consecutive disastrous bark beetle outbreaks ([7]). The increasing frequency of summer drought and heatwaves has replaced wind disturbance as the main cause of Norway spruce mortality ([11]). In Central Europe, the massive mortality of Scots pine is a relatively new phenomenon in many areas and came for many forest managers and decision-makers almost unexpected ([15]), especially as Scots pine has been thought to be relatively drought tolerant. Hence, drivers and mechanisms causing tree mortality are not always clear.
This study presents the leaf osmotic potential (πosm) as a tree physiological measure that is directly related to a species drought tolerance. πosm can be translated into the leaf water potential at turgor loss (turgor loss point, πtlp) and represents the permanent wilting point ([2]). The aim of this study was to verify if water limitation explains the accelerated mortality in Norway spruce and Scots pine from a leaf hydraulic perspective and to present a comparison to the regional species pool.
Methods
Study area
The collection of the plant material took place in the surroundings of Ammerndorf in the rural district of Fürth in Middle Frankonia, Germany (49° 24′ 36.0″ N, 10° 49′ 39.3″ E). The forest management history of the district is influenced strongly by Peter Stromer, who initiated a reforestation approach in the year 1368, turning the formerly depleted mixed-species forests (probably Scots pine, birch, and oak) into pure stands of Scots pine (Pinus sylvestris L. - [17]). The area is characterized by a very patchy forest distribution. Approximately 30% of the area is currently stocked with forest and the rest is used for agriculture. The main tree species cultivated in the area are Scots pine, followed by Norway spruce (Picea abies Karst.). The two conifer species make up 80% of the forest area and the other 20% by broadleaved species, with European oak (Quercus robur L.) being the most important species and the invasive black cherry (Prunus serotina Ehrh.) rapidly taking over the understory. The area has experienced various dramatic and cascading die-off events in the last two years following three very dry summers and heatwaves in 2015, 2016 and 2019 ([14]). Bark beetle outbreaks have killed about 30% of the spruce trees and a complex combination of fungi are accelerating the mortality of pine ([15]). In some stands, all pine trees have died, which means that only 20% of the stand is stocked with mainly oaks. To compare the potential drought tolerance of spruce and pine with the local tree community, botanical samples were collected for 32 woody species native to the area (Tab. 1). I assume that this species pool (25th to 75th percentile, see box plots in the figure) would reflect well the historic drought adaptation of the local woody vegetation.
Tab. 1 - List of native woody species sampled during the study.
| Species name |
|---|
| Abies alba |
| Acer campestre |
| Acer platanoides |
| Acer pseudoplatanus |
| Alnus glutinosa |
| Betula pendula |
| Caprinus betulus |
| Cornus sanguinea |
| Corylus avellana |
| Crataegus monogynae |
| Euyonimos europea |
| Fagus sylvatica |
| Frangula alnus |
| Fraxinus excelsior |
| Ilex aquifolium |
| Larix decidua |
| Picea abies |
| Pinus sylvestris |
| Populus tremula |
| Prunus avium |
| Prunus spinosa |
| Quercus patrea |
| Quercus robur |
| Salix alba |
| Salix caprea |
| Sambucus nigra |
| Sambucus racemosa |
| Sorbus aucoparia |
| Taxus bacata |
| Tilia cordata |
| Tilia platyphyllos |
| Ulmus glabra |
Determination of the turgor loss point via osmometry
The water potential at turgor loss point (πtlp) was estimated by measuring the leaf osmotic potential at full hydration (πosm) with a vapor pressure osmometer (VAPRO 5520®, Wescor, Logan, UT, USA - [2]). Three tree individuals per species were sampled. One sun-exposed branch from each individual was collected during the growing season between the 7th and the 12th of July 2019. After cutting the branches from the trees, they were placed in humid and opaque plastic bags and brought to the laboratory as fast as possible. In the laboratory, the branches were cut again underwater at least two nodes distal to the original cut and placed in buckets with water, covered with plastic bags to let rehydrate overnight. The next day, two leaf samples were taken per individual. All collected samples were processed within 24 h after collection. For broadleaved species, fully expanded leaves per branch of broad were collected and a disc was cut out per sample with a 4-mm-diameter cork borer. From the rehydrated branch of the conifers, a couple of needles were used and aligned next to each other to form a larger area, so that a disc could be cut out with similar size to the broad-leaved species. The discs were wrapped in aluminum foil and submerged in liquid nitrogen (LN2) for at least 2 minutes ([2]). The standard 10 μL chamber well of the osmometer was used for the measurements. Before putting the discs into the chamber of the osmometer, the discs were punctured with a dissection needle for about 10 to 15 times to improve evaporation through the cuticle and to reduce equilibration time ([13]). The osmometer was running in the auto-repeat mode and all measurements were recorded until the equilibrium was indicated by an increase between measurements of less than 0.01 MPa (approximately 5 osmometer readings). The osmotic potential at full hydration (πosm) was calculated from the solute concentration value c0 (in mmol kg-1) given by the osmometer, using the following equation (eqn. 1):
where R is the ideal gas constant, and T, the temperature in degrees Kelvin (here 25 °C). Leaf water potential at turgor loss (πtlp) was calculated πosm using the calibration equation established by Bartlett et al. ([2] - eqn. 2):
Results
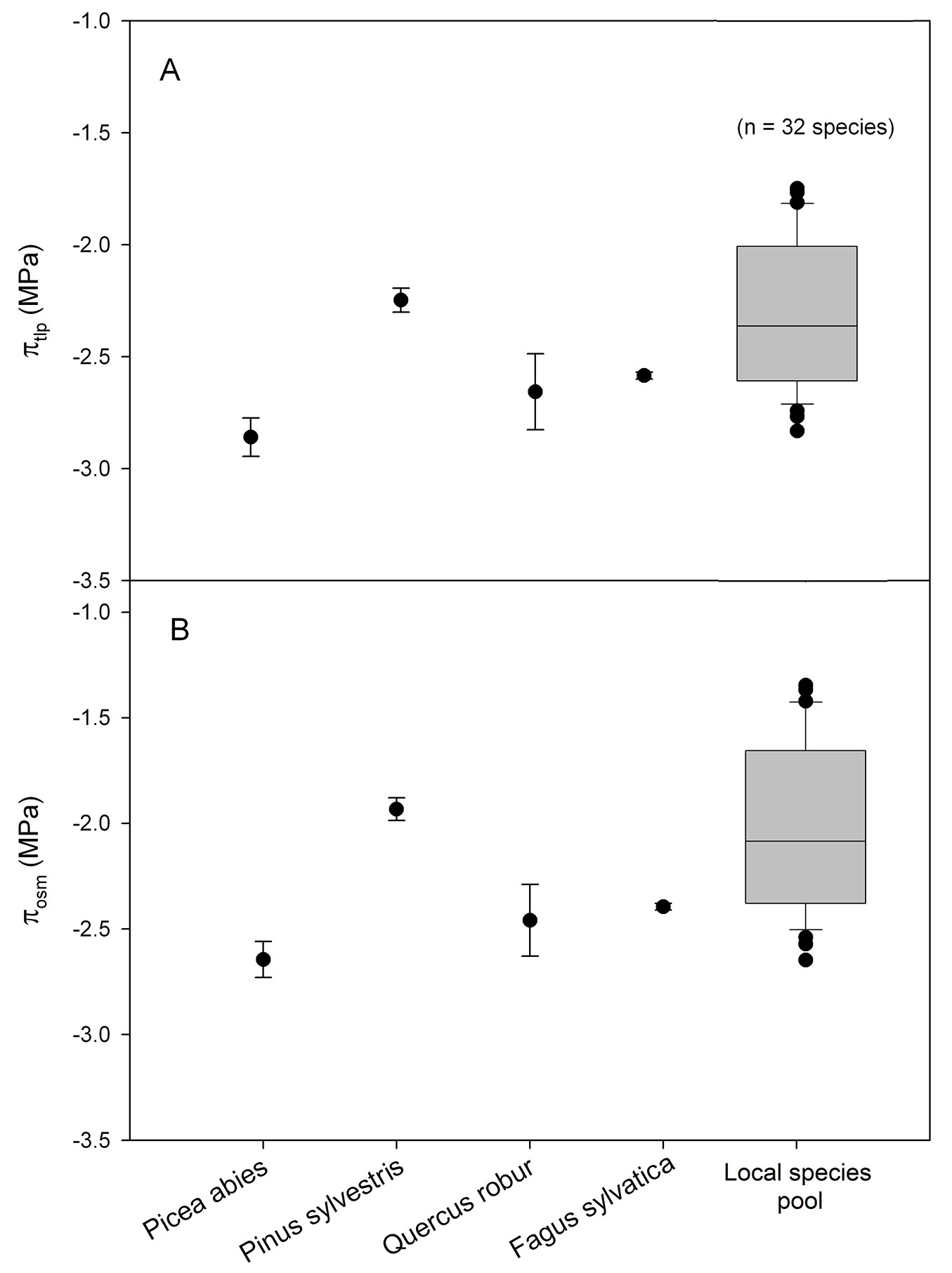
The main results of this study are summarized in Fig. 1.
Fig. 1 - Mean values and standard deviation of the (A) water potential at turgor loss (πtlp); and (B) osmotic leaf water potential (πosm) of the target species and the most economically important broadleaved species in Central Europe. The box plot presents πtlp and πosm of 32 woody species collected in the study area. Three individual (n = 3) for each species were sampled to measure πtlp and πosm. Values for the box plot are based on the mean values of the 32 species. The upper and lower limits of the box plots represent estimates of the 75th and 25th percentiles.
The 75th and 25th percentile of the species pool were -2.01 and -2.60 MPa for πtlp and -1.66 and -2.37 MPa for πosm. Mean π tlp lies at -2.33 ± 0.33 MPa and mean πosm at -2.04 ± 0.40 MPa. The two target species had a πtlp of -2.83 ± 0.085 MPa and -2.24 ± 0.054 MPa (Picea abies and Pinus sylvestris, respectively). Their πosm was at -2.64 ± 0.10 MPa and -1.93 ± 0.065 MPa. Common broadleaved species like Quercus robur and Fagus sylvatica had a πtlp of -2.68 ± 0.17 MPa and -2.62 ± 0.02 MPa, respectively. πosm was at -2.46 ± 0.20 MPa and -2.39 ± 0.02 MPa.
Discussion
πtlp measurements revealed that Norway spruce loses its turgor at a more negative leaf water potential. In contrary, Scots pine loses its turgor earlier with less negative leaf water potential. This means that Scots pine reaches its permanent wilting point earlier under water limitation than Norway spruce ([2]). This approach disregards possible differences in rooting depth of the two species and water accessibility during drought; however, various studies have shown that both species have most of their fine roots in the same soil layer and do not access layers deeper than 50 cm with a significantly different amount of fine roots ([12], [8], [10]). Hence, it can be assumed that both species are accessing water resources at the same soil layer and that from leaf hydraulic point of view Norway spruce is much more drought tolerant than Scots pine. Despite possible differences in rooting and soil water uptake pattern, Norway spruce has even lower πosm and πtlp than the 25th percentile of the species in the area. This means that Norway spruce might be potentially more drought tolerant than most other species, even more, tolerant to drought than oak and beech trees (Fig. 1). I conclude that the main underlying mechanism of accelerating Norway spruce mortality does not lie in water stress but rather in heat stress caused by heatwaves. In the last years a significantly increasing number of heatwaves, defined as at least three consecutive days with a maximum daily air temperature of >30°C, has been observed in Central Europe ([18]). Norway spruce has been planted in many lowland areas near or at the edge of its thermal tolerance. Those temperature regimes during the heat waves are most likely reaching the thermal tolerance threshold ([6],[5]) of the species leading to lethal heating of leaves ([16]). As higher altitudes are affected by heatwaves too, the thermal tolerance threshold could explain mortality in higher and cooler environments. To my knowledge there is no study on the thermal tolerance threshold of Norway spruce to support my speculation; most studies focus rather on the freezing tolerance of these species (e.g., combination of freezing and drought tolerance - [3]). Similar mechanisms of tree mortality concerning heat might apply to the currently observed wilting of beech trees all over central Europe. From the leaf hydraulic perspective, European beech and oak have similar πtlp; however, species distributional range of European oak reaches much further south with historically higher maximum temperatures (Southern Turkey and Northern Iraq, an area with scorching high temperatures to quite high altitudes). The principle mechanism of mortality in Scots pine might be defined by drought stress followed by pathogen outbreaks such as the Diplodia tip blight ([14]), often combined with the cauliflower fungus (Sparassis crispa), a cellulose digesting species significantly reducing water uptake in the trunk ([15]).
Conclusion
Mortality in the two most economically important conifer species in Central Europe might be explained by two different mechanisms. Norway spruce might be more affected by being pushed to its thermal tolerance threshold, whereas Scots pine suffers from water limitation. However, both drought and heat are threatening forest ecosystems with progressing climate change and will be the most pressing task to be tackled by forest managers in the coming years. Therefore, the search for suitable species must account for the thermal tolerance threshold and a species’ drought tolerance.
Acknowledgements
I would like to thank Jürgen Bauhus to assure funding for the consumables used in this study. I also thank Alida Mercado Cardenas for language editing. Finally, I am deeply indebted to two anonymous reviewers who helped to improve essentially the manuscript.
References
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Conservation Ecology Center, Smithsonian Conservation Biology Institute, Front Royal, VA (USA)
Center for Tropical Forest Science - Forest Global Earth Observatory, Smithsonian Tropical Research Institute, Panama (Republic of Panama)
Corresponding author
Paper Info
Citation
Kunert N (2020). Preliminary indications for diverging heat and drought sensitivities in Norway spruce and Scots pine in Central Europe. iForest 13: 89-91. - doi: 10.3832/ifor3216-012
Academic Editor
Tamir Klein
Paper history
Received: Aug 18, 2019
Accepted: Dec 11, 2019
First online: Mar 01, 2020
Publication Date: Apr 30, 2020
Publication Time: 2.70 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 43237
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 36518
Abstract Page Views: 3421
PDF Downloads: 2535
Citation/Reference Downloads: 16
XML Downloads: 747
Web Metrics
Days since publication: 2165
Overall contacts: 43237
Avg. contacts per week: 139.80
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 26
Average cites per year: 4.33
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effect of drought stress on some growth, morphological, physiological, and biochemical parameters of two different populations of Quercus brantii
vol. 11, pp. 212-220 (online: 01 March 2018)
Research Articles
Use of δ13C as water stress indicator and potential silvicultural decision support tool in Pinus radiata stand management in South Africa
vol. 12, pp. 51-60 (online: 24 January 2019)
Research Articles
Extreme climatic events, biotic interactions and species-specific responses drive tree crown defoliation and mortality in Italian forests
vol. 17, pp. 300-308 (online: 30 September 2024)
Research Articles
A physiological approach for pre-selection of Eucalyptus clones resistant to drought
vol. 13, pp. 16-23 (online: 15 January 2020)
Research Articles
Response of juvenile progeny of seven forest tree species and their populations to simulated climate change-related stressors, heat, elevated humidity and drought
vol. 11, pp. 374-388 (online: 15 May 2018)
Research Articles
Variations in the performance of hybrid poplars subjected to the inoculation of a microbial consortium and water restriction
vol. 16, pp. 352-360 (online: 13 December 2023)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Nutrient uptake, allocation and biochemical changes in two Chinese fir cuttings under heterogeneous phosphorus supply
vol. 11, pp. 411-417 (online: 05 June 2018)
Research Articles
Oak sprouts grow better than seedlings under drought stress
vol. 9, pp. 529-535 (online: 17 March 2016)
Research Articles
Sodium and potassium allocation under drought stress in Atlas mastic tree (Pistacia atlantica subsp. mutica)
vol. 6, pp. 90-94 (online: 07 February 2013)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword