Effect of drought stress on some growth, morphological, physiological, and biochemical parameters of two different populations of Quercus brantii
iForest - Biogeosciences and Forestry, Volume 11, Issue 2, Pages 212-220 (2018)
doi: https://doi.org/10.3832/ifor2496-010
Published: Mar 01, 2018 - Copyright © 2018 SISEF
Research Articles
Abstract
In recent years, drought-induced tree mortality has occurred in the oak forests of the Zagros Mountains (western Iran). The impacts of climate change induced by drought stress have been most acutely experienced by two populations of Persian oaks (Quercus brantii Lindl) grown in the western provinces (Ilam and Lorestan) of the Zagros region. We surveyed growth, physiological, and biochemical responses of one-year-old Persian oak seedlings from Melasyah (Ilam) and Chegeni (Lorestan) provenances, which were subjected to three watering regimes (100%, 40%, and 20% of field capacity) in a greenhouse. The severe drought stress decreased the diameter and height growth, total biomass, net photosynthesis, gas exchange, xylem water potential, maximum Rubisco activity (Vcmax) as well as the maximum PSII photochemical efficiency of the oak seedlings in both populations, but the rate of decrease was greater in Chegeni seedlings as compared to Melasyah seedlings. Although proline and soluble sugar contents significantly increased in response to drought in both populations under stress, the rate of increase was higher in Melasyah seedlings as compared to Chegeni seedlings. In addition, the activities of peroxidase, superoxide dismutase, catalase, and ascorbic peroxidase as well as that of phenylalanine ammonia lyase were promoted in both populations under drought stress. However, the incremental rate was higher in the Melasyah population than in the Chegeni population. Under severe drought stress, the MDA content, electrolyte leakage, the content of hydrogen peroxide, and superoxide radical significantly increased in both the populations. The rate of increase, however, was higher in the Chegeni population. Under drought stress, the total phenol and flavonoid contents of Melasyah seedlings were higher than those of Chegeni seedlings. The results showed that Chegeni seedlings are more sensitive than Melasyah seedlings when exposed to a water limitation stress. Our findings suggest that the climate conditions of the Persian oak stands should be considered by nursery managers while creating establishment and restoration programs.
Keywords
Drought Stress, Persian Oak, Zagros Mountain, Provenance, Drought Resistance
Introduction
In recent years, the negative impacts of climate change on forest trees has intensified ([40]), thus exposing forests to increasingly stressful conditions, such as heat and drought ([23]). The most alarming outcome of climate change is the increased number of trees dying off because of drought. During recent years, many trees have been lost in the forests of the Zagros Mountains, located in the western provinces of Iran (Ilam, Lorestan, and Kermanshah), as a result of decreased precipitation and increased temperature in the region. The Zagros forests cover an area of 5 million hectares (about 40% of all Iranian forests), and represent the widest forest region of the country ([1]). Persian oak (Quercus brantii Lindl.) is the dominant tree species in these forests and is extensively distributed throughout the mountains. Drought has also increased the vulnerability of trees to charcoal disease ([15]).
The survival and growth of Persian oaks depend on their tolerance to drought stress ([15]). Therefore, conservation activities aimed at preserving Persian oaks in the damaged Zagros forests might greatly benefit from the selection of individuals or provenances more tolerant to drought.
Drought is one of the most important limitation factors for growth and plant production. Under drought stress, some disorder generally occurs in most physiological processes, such as decreases in photosynthesis rate and growth ([20]), stomatal conductance ([19]), as well as cell dehydration ([26]) and chlorophyll degradation ([13]). Besides, biochemical reactions are part of the plant’s response to environmental stress ([33]). Plants respond to drought stress using a combination of biochemical processes ([2]). Proline and soluble sugars are overproduced in plants in response to drought stress. Proline and carbohydrates act as osmolytes to maintain water in the cytoplasm ([2]). Furthermore, they prevent protein denaturation and cell membrane damage, and induce stability in the structure of enzymatic proteins, thereby preserving their activity ([17]).
The production of reactive oxygen species (ROS), including superoxide radicals and hydrogen peroxide, is an important biochemical response to oxidative stress ([11]). High ROS concentrations can disrupt normal plant metabolism damaging lipids, proteins, chlorophyll, and nucleic acids ([42]). ROS directly damage cell membrane phospholipids and increases lipid peroxidation, which can be determined by the content of malondialdehyde (MDA) by-products ([48]). On the other hand, oxidative damage to cell membranes results in electrolyte leakage (EL) and, ultimately, in cell death ([37]).
Plants use different enzymatic and non-enzymatic systems to control ROS production induced by drought stress. The most important antioxidant enzymes include superoxide dismutase (SOD), catalase and peroxidases (POXs), glutathione reductase, and ascorbate peroxidase (APX) to prevent cell damage ([25]). In addition, phenolic components - especially flavonoids and phenylalanine ammonia lyase (PAL) - form another class of plant defense molecules that respond to drought stress ([1]). In fact, PAL is the primer of the phenylpropanoid pathway that finally leads to phenolic components biosynthesis.
Although previous studies focused on growth and physiological responses of oak species to drought stress ([26], [9]), there is limited data concerning the effect of drought stress on morphological, physiological, and biochemical responses of the Persian oak. In this study, Persian oak seedlings were selected from two provinces in the Zagros region with different precipitation regimes, and subjected to different levels of drought stress. The growth, physiological, and biochemical responses of the seedlings were measured to compare the level of drought tolerance between and within the individuals of the two populations. Here, we focus more on biochemical reactions of the species in response to drought stress since there was no comprehensive information about the subject. The aim was to evaluate the diversity of Persian oak populations in the response to water limitation and elucidate whether climatic conditions occurring at Persian oak stands are related with the resistance to drought stress of their seedlings.
Material and methods
Plant material and experimental design
Different precipitation regimes occur on the Zagros mountains, as the mean annual rainfall declines from north to south. Southern Zagros region has a longer dry season period and higher mean annual temperature compared to the northern area. We selected Ilam and Lorestan provinces from the southern and central Zagros Mountains, respectively. Ilam province has a lower average annual precipitation, higher mean temperature, and longer dry season, compared with Lorestan province. The general climatic characteristics of both provinces are presented in Tab. 1.
Tab. 1 - Climatic characteristics of the provenances selected in this study. (‡): 10-year-annual means according to data of meteorological stations of each province.
| Province | Provenance | Altitude (m.a.s.l.) |
Coordinates | Temp (‡) (°C) |
Rainfall (‡) (mm) |
|---|---|---|---|---|---|
| Ilam | Melasyah | 1490 | 33° 43′ 04″ N 46° 13′ 67″ E |
19.8 | 235 |
| Lorestan | Chegeni | 1975 | 33° 27′ 20″ N 48° 11′ 28″ E |
16.4 | 409 |
Ten healthy Persian oak individuals were randomly selected from Chegeni (Lorestan province) and Melasyah (Ilam province) provenances, and their healthy and mature seeds were collected ([48]). The sampled seeds were surface-sterilized with 3.5% Na-hypochlorite for 10 minutes and then rinsed three times with sterile distilled water. The sterilized seeds were planted in polyethylene pots (10 × 15 cm) containing a mixture of compost and perlite (3:1 v/v). The seeds were germinated under controlled condition including a light period of 13 hours, mean temperature of 25 ± 2 °C, and optical photosynthetic radiation of 1200 µmol m-2 S-1 for a one-year period with daily irrigation. The grown seedlings were replanted into plastic pots (5 L) containing sandy loam soil (51% sand, 34% silt, 15% clay, 1.25% organic matter, 0.34% N, 12.52 mg kg-1 available phosphorus, 174 mg kg-1 available potassium, pH 7.21).
In late May of 2015, 240 uniformly sized seedlings underwent different drought stress treatments. At the beginning of the experiment, the average of diameter and height of the seedlings were 0.8 ± 0.19 and 25 ± 1.92 cm respectively. The experiment was carried out in a completely randomized design with two factors (two provenances and three watering regimes). Each treatment was conducted in four replications and 10 seedlings were used in each replication. Seedlings of each provenance were imposed to three watering regimes including 100% (W: control), 40% (M: moderate), and 20% (S: severe) of soil field capacity. The stress treatments continued for three months.
Growth parameters
At the end of the experiment, the stem diameter and height of each seedling were measured. Then, the seedlings were removed from their pots and the soil around the roots was washed away. Each plantlet was divided into roots, stems, and leaves. Biomass samples were dried at 70 °C for 48 hours and weighted ([48]). The root/shoot ratio was then calculated.
Physiological parameters
The maximum photochemical efficiency of photosystem II, xylem water potential, and gas exchanges were measured three times (once every month). Net photosynthesis (A), transpiration (E), stomatal conductance (gs), and intercellular CO2 concentration (Ci) were determined using a portable photosynthesis measuring device LI-6400XT® (LI-COR, Lincoln, USA). Fully-developed leaves from the lower part of the stem (the same in all seedlings) were selected for gas exchange measurements ([31]). The maximum Rubisco activity, Vcmax, was estimated through the analysis of A/Ci curves, according to Sharkey et al. ([35]).
Leaf water potential (Ψ) was measured using a Scholander-type pressure chamber (Skye, SKPM 1400, UK). From each seedling, five fully-developed leaves were collected. Gas exchange parameters, water potential in stable conditions of CO2 (350 ppm), relative humidity (60% to 80%), and constant leaf temperature (25 °C to 30 °C) were measured at midday and under full-light conditions ([47]).
The maximum PSII photochemical efficiency in leaves adapted to darkness (for 20 minutes) was measured using a fluorometer PAM-2000® (H Walz GmbH, Effeltrich, Germany) connected with a leaf-clip holder and with a trifurcated fiber-optic (2010-F, Walz). The maximum PSII photochemical efficiency was calculated as (eqn. 1):
where Fv was the variable fluorescence, Fm was the maximum fluorescence of the leaves adapted to darkness and F0 was the minimum fluorescence yield in leaves adapted to darkness.
Pigment content
At the end of the experiment, fully developed leaves were collected from each seedling and their chlorophyll and carotenoid contents were extracted using acetone (80%, v/v). The amounts of total chlorophyll (a + b) and carotenoid contents were calculated using the Arnon’s method ([3]).
Proline and soluble sugar content
Free proline content in leaves was quantified in accordance with Bates et al. ([5]). To measure the content of soluble sugars, 200 µL alcoholic extract (ethanol 80%, v/v) was mixed with 3 mL anthrone. The soluble obtained was placed in a boiling water bath for 10 minutes and then cooled in an ice bath. The content of soluble sugars was determined using a spectrophotometer (FCC Compliance, Epoch, Biotek Instrument, USA) by reading the absorbance at 625 nm.
Lipid peroxidation, electrolyte leakage, and ROS determination
Lipid peroxidation was measured by the concentration of MDA byproducts. First, 0.5 g of fresh leaf was mixed with 0.5% (w/v) thiobarbituric acid solution containing 20% (w/v) trichloroacetic acid. The mixture was heated at 95°C for 25 minutes and the reaction was stopped by quickly placing it in an ice-bath. The absorbance of the supernatant was read by spectrophotometer at 532 nm ([43]).
To determine the electrolyte leakage (EL), 100 mg fresh leaf samples were cut into 5 mm length and placed in test tubes containing 10 mL distilled deionized water. The tubes were placed in a water bath maintained at a constant 32 °C. After two hours, the initial electrical conductivity of the medium (EC1) was measured using an electrical conductivity meter. Then, the samples were put in an oven at 120 °C for 120 minutes. Then, samples were cooled to 25 °C and the final electrical conductivity (EC2) was measured. The EL was calculated by the following equation ([30] - eqn. 2):
Hydrogen peroxide (H2O2) content was measured as described by Velikova et al. ([44]). Leaf samples (0.2 g) were homogenized in an ice bath with 5 mL of 0.1% TCA and centrifuged at 12.000×g for 15 minutes; then, 1 mL of the obtained supernatant was added to 1 mL of 10 mM potassium phosphate buffer (pH 7.0) and 2 mL of 1 M potassium iodide, and put in a dark room for one hour. The absorbance of the supernatant was read using a spectrophotometer at 390 nm.
Superoxide radical (O2•-) content of tissue was determined in accordance with Bai et al. ([4]). Briefly, 1 g of fresh leaf was homogenized in 4 mL of 65 mM phosphate buffer (pH 7.8) and centrifuged at 5.000×g for 10 minutes. Then, 1 mL of the obtained supernatant was mixed with 0.1 mL of 10 mM hydroxylamine chloride and 0.9 mL of 65 mM phosphate buffer (pH 7.8), and held for 20 minutes at 25 °C in a water bath. Subsequently, 1 mL of this mixture was mixed with 1 mL of 17 mM sulfanilic acid and 1 mL of 7 mM α-naphthylamine, and held for 20 minutes at 25 °C. The absorbance of supernatant was read at 530 nm. Nitrogen dioxide radical was used as a standard.
Quantitative analysis of activities of antioxidant enzymes
Fresh leaves were collected from each seedling subjected to three watering regimes and immediately put in liquid nitrogen and kept at -80 °C for enzymes assay. Leaf tissue (1 g) was homogenized with 3 mL 0.05 mM sodium phosphate buffer (pH 7.8) containing 1 mM ethylenediaminetetraacetic acid (EDTA) and 2% polyvinylpolypyrrolidone (PVPP) and centrifuged at 15.000×g for 40 minutes. The supernatant was used to determine enzyme activities.
The activity of SOD and POXs was measured in accordance with Beauchamp & Fridovich ([6]). CAT activity was determined as reported by Bergmeyer ([7]). APX assay was performed in accordance with Nakano & Asada ([29]).
Determination of PAL activity, total phenol and flavonoid contents
PAL activity was determined by trans-cinnamic acid production rate in accordance with Wang et al. ([46]). Total leaf phenol content was determined by the Folin-Ciocalteu method ([38]) and leaf flavonoid content was determined in accordance with the method suggested by Zhishen et al. ([49]).
Statistical analysis
The experiment was performed in a factorial-based completely random design (CRD) with a 2 × 3 factorial arrangement. The data was analyzed by analysis of variance (ANOVA) using the statistical package SPSS® ver. 16.0 (IBM, Armonk, NY, USA); the mean values were compared using Duncan’s multiple range test (α = 0.05).
Results
Growth parameters
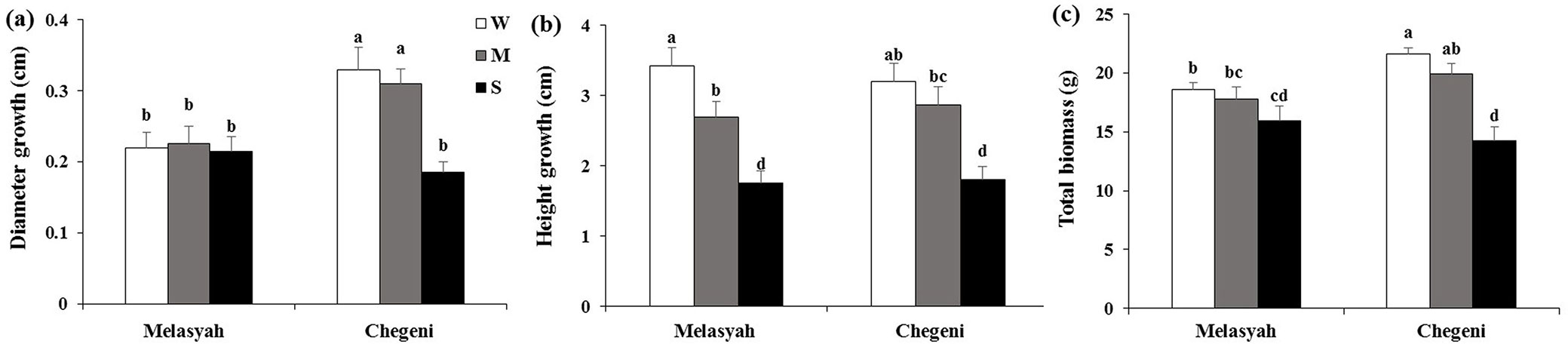
Typically, Chegeni seedlings had larger diameter compared with Melasyah seedlings. No significant difference were found between stem diameter of seedlings under control (W) and moderate (M) drought conditions (Fig. 1a). However, severe (S) drought significantly reduced the diameter of Chegeni seedlings, but not that of Melasyah seedlings.
Fig. 1 - Mean values (± standard error) of (a) diameter growth, (b) height growth and (c) total biomass of Persian oak seedlings from Melasyah and Chegeni provenances under different drought stress treatments. (W): irrigation to 100% field capacity; (M): irrigation to 50% field capacity; (S): irrigation to 20% field capacity. Different letters on the top of bars indicate significant difference between treatments after Duncan’s test (p<0.05, n=4).
Drought stress significantly reduced the height of seedlings in both populations (Fig. 1b). Severe stress (S) significantly reduced the height of seedlings when compared to control (W) and moderate (M) stress.
The total biomass of Chegeni seedlings was significantly influenced by drought stress, but Melasyah population was not (Fig. 1c). A significant interaction effect between population and drought stress was also detected for total biomass. As a result, the total seedling biomass for the Chegeni provenance was higher than that for Melasyah under control condition. In addition, severe stress (S) decreased the total biomass of Chegeni seedlings compared to moderate stress (M).
Physiological parameters
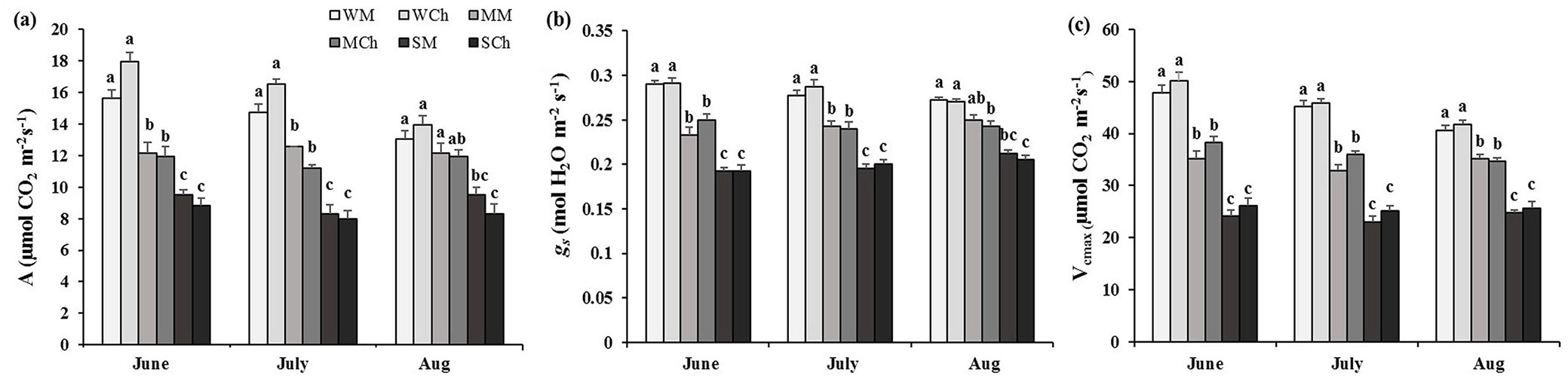
In June, one month after the drought stress was applied, the net photosynthesis rate, stomatal conductance, and Vcmax in moderate and severe drought-stressed seedlings from both provenances declined, in comparison with unstressed seedlings, and there was no significant difference between the two provenances (Fig. 2a, Fig. 2b). After two months of drought stress, in July, the gas exchange parameters were similar to those observed in June. In August (after three month of drought stress), the net photosynthesis rate declined considerably in seedlings subjected to severe drought stress (SM and SCh); however, we did not find any significant difference between moderate stress (MM and MCh) and control (WM and WCh) seedlings. Furthermore, we did not observe a significant difference of net photosynthesis after three months of drought among the two provenances. In August, stomatal conductance extensively decreased in the seedlings from Melasyah only under severe drought stress (SM). On the other hand, moderate and severe drought stress negatively affected stomatal conductance of Chegeni seedlings (MCh and SCh, respectivey). The results of Vcmax were the same for all three months (Fig. 2c).
Fig. 2 - Mean values (± standard error) of (a) net photosynthesis rate (A), (b) stomatal conductance (gs) and (c) Vcmax in Persian oak seedlings from Melasyah (M) and Chegeni (Ch) provenances under different drought stress treatments. (W): irrigation to 100% field capacity; (M): irrigation to 50% field capacity; (S): irrigation to 20% field capacity. Different letters on the top of bars indicate significant difference between treatments after Duncan’s test (p<0.05, n=4).
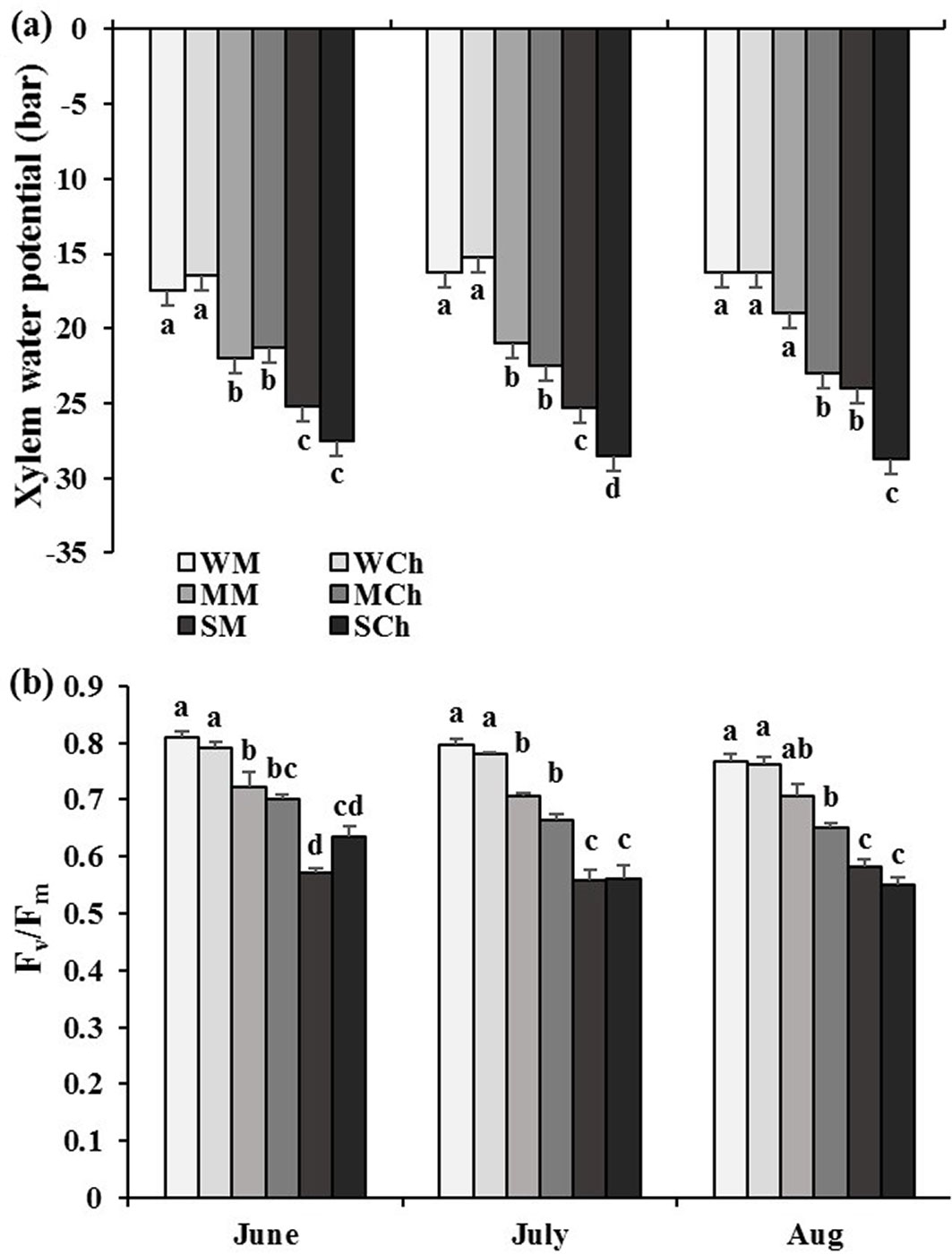
In June and July, the xylem water potential of seedlings from both provenances declined in response to moderate and severe drought stress. In July, Chegeni seedlings under severe drought stress showed the lowest mean value of xylem water potential and the decreasing rate was greater in this provenance in comparison with the Melasyah provenance. On the other hand, moderate drought stress only affected the xylem water potential of Chegeni provenance seedlings (Fig. 3a). The maximum PSII photochemical efficiency in both provenances significantly decreased in response to moderate and severe drought stress when they were measured in June and July. In August, PSII in Melasyah seedlings was affected only by severe drought stress, while both moderate and severe drought stress led to a decrease in the parameter values of Chegeni seedlings at the same time (Fig. 3b).
Fig. 3 - Mean values (± standard error) of (a) xylem water potential (ψ) and (b) the maximum PSII photochemical efficiency in leaves of Persian oak seedlings from Melasyah (M) and Chegeni (Ch) provenances under different drought stress treatments. (W): irrigation to 100% field capacity; (M): irrigation to 50% field capacity ; (S): irrigation to 20% field capacity. Different letters on the top of bars indicate significant differences between treatments after Duncan’s test (p<0.05, n=4).
Pigment content, proline and soluble sugar concentration
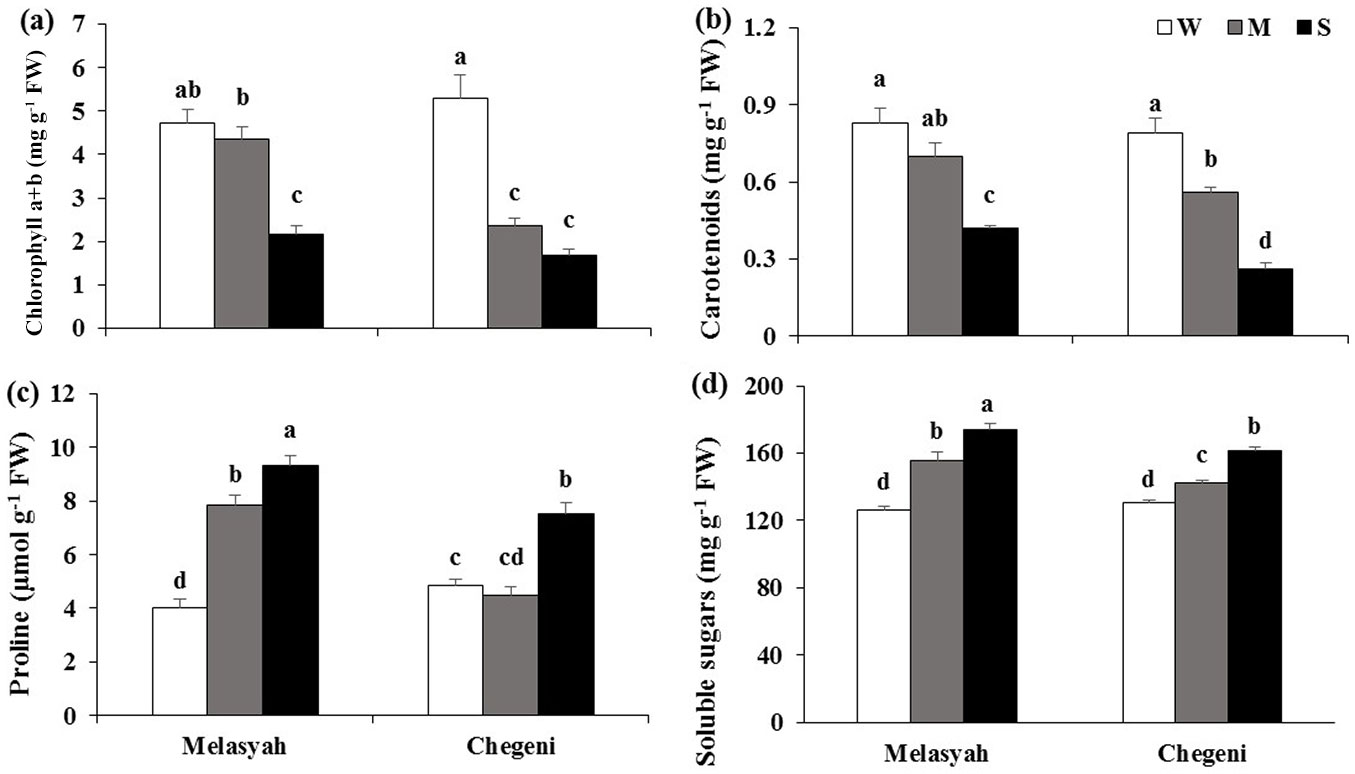
Chlorophyll and carotenoid content of the seedlings decreased in response to drought stress (Fig. 4a, Fig. 4b). Moderate or severe stress drastically decreased the chlorophyll contents of Chegeni’s seedlings. However, the chlorophyll contents of Melasyah seedlings were not affected by moderate stress compared to control conditions. Furthermore, carotenoid content of Chegeni seedlings decreased more than Melasyah seedlings under stress.
Fig. 4 - Mean values (± standard error) of (a) chlorophyll a+b content, (b) carotenoid, (c) proline and (d) soluble sugar concentrations in leaves of Persian oak seedlings from Melasyah and Chegeni provenances under different drought stress treatments. (W): irrigation to 100% field capacity; (M): irrigation to 50% field capacity ; (S): irrigation to 20% field capacity. Different letters on the top of bars indicate significant differences between treatments according to Duncan’s test (p<0.05, n=4).
Proline concentration increased in seedlings of both populations under moderate and severe drought stress (Fig. 4c). It increased in Melasyah provenance during moderate and severe stresses, while proline content of Chegeni seedlings increased just under severe drought stress.
The concentration of soluble sugars increased in seedlings of both provenances in response to drought stress, and the highest concentration of soluble sugars was recorded in Melasyah seedlings under severe stress.
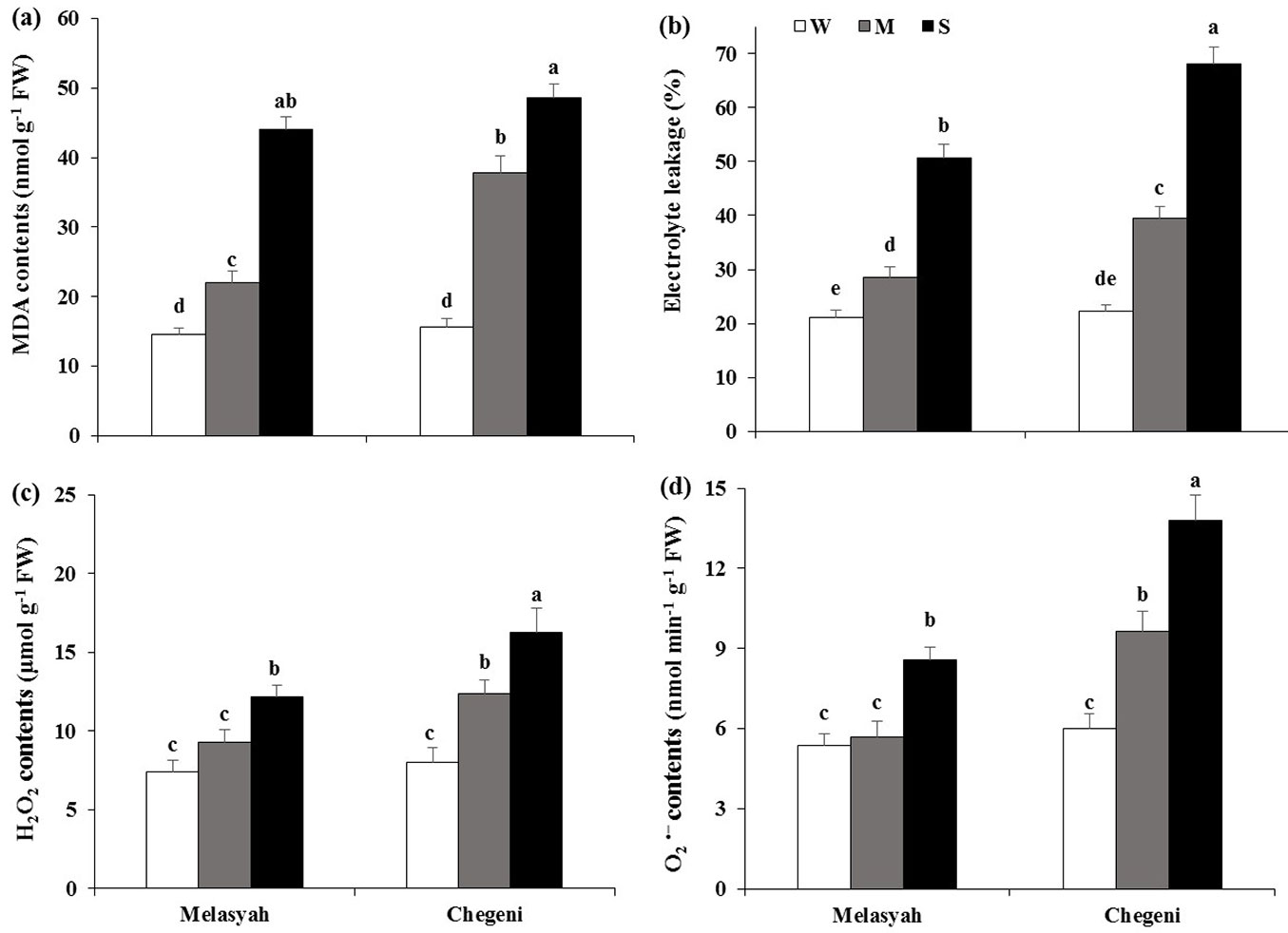
Cell damage and ROS concentrations
Under drought stress, MDA content increased in seedlings of both provenances. MDA increased more in seedlings from Chegeni compared to the Melasyah provenance (Fig. 5a). Drought stress also increased EL in seedlings of both populations (Fig. 5b). The highest value of EL was detected for seedlings from Chegeni under drought stress.
Fig. 5 - Mean values (± standard error) of MDA (a), electrolyte leakage (b), hydrogen peroxide (c) and superoxide radical (d) in leaves of Persian oak seedlings from Melasyah and Chegeni provenances under different drought stress treatments. (W): irrigation to 100% field capacity; (M): irrigation to 50% field capacity; (S): irrigation to 20% field capacity). Different letters on the top of bars indicate significant differences between treatments according to Duncan’s test (p<0.05, n=4).
Hydrogen peroxide and superoxide radical increased in response to water limitation when the seedlings of both provenances were exposed to drought stress (Fig. 5c, Fig. 5d). The production of hydrogen peroxide and superoxide radical in response to moderate drought stress increased in seedlings from Chegeni. Under both drought treatments, ROS production was higher in Chegeni seedlings as compared to Melasyah seedlings.
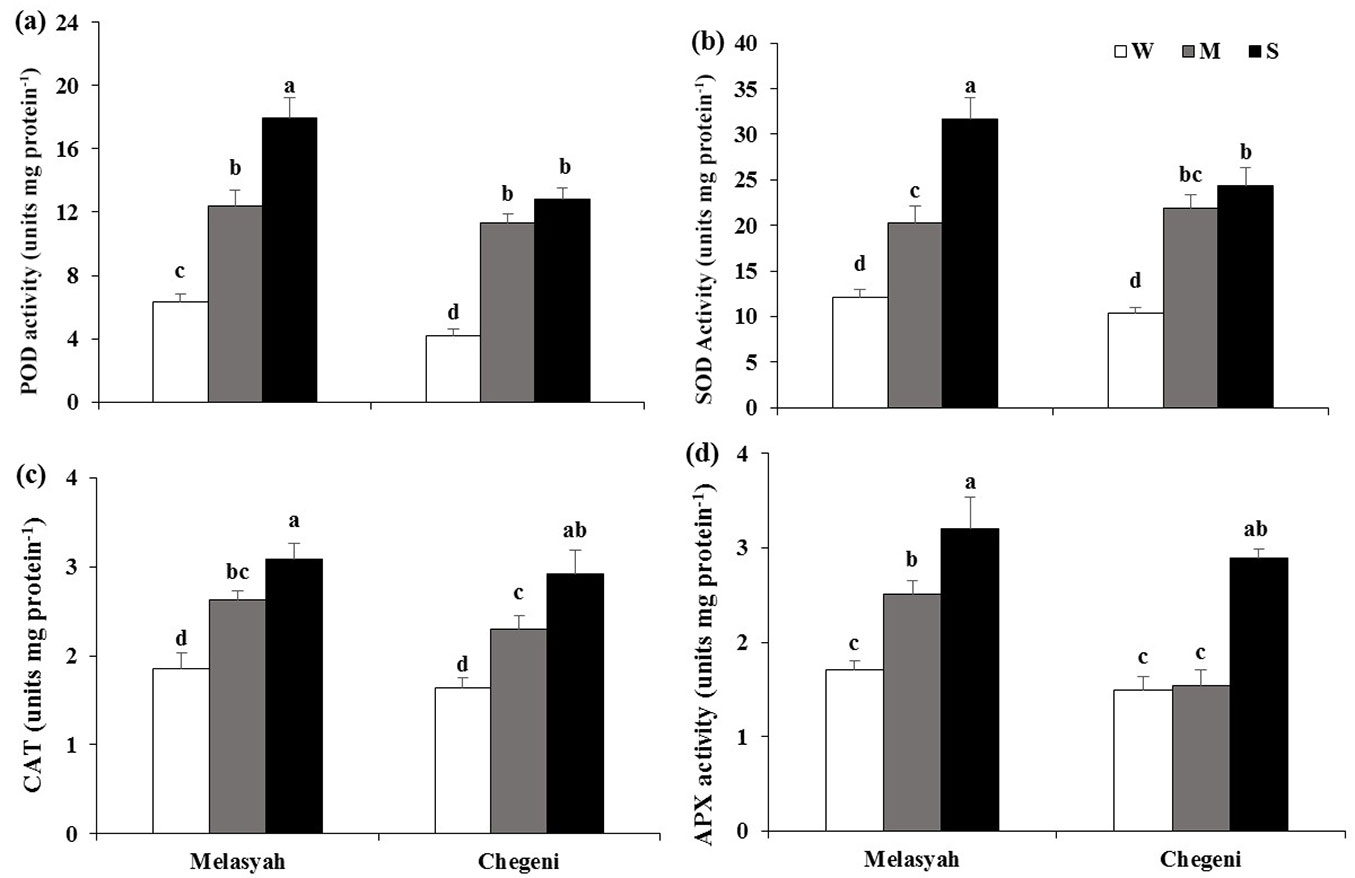
Antioxidant enzymes
The activities of POD and SOD were higher when seedlings were exposed to moderate and severe water stress compared to well-watered seedlings, and the highest enzyme activities were recorded in Melasyah seedlings subjected to severe drought stress (Fig. 6a, Fig. 6b). The Melasyah seedlings subjected to both drought treatments showed higher activity of CAT than well-irrigated seedlings, while the enzyme activity in Chegeni seedlings just increased under severe water limitations (Fig. 6c). In the Chegeni population, there was no difference in APX activity between moderately and non-stressed seedlings, but the enzyme activity was highest in the severe drought treatment (Fig. 6d).
Fig. 6 - Mean values (± standard error) of antioxidant enzyme activities (a: POD; b: SOD; c: CAT; d: APX) in leaves of Persian oak seedlings from Melasyah and Chegeni provenances under different drought stress treatments. (W): irrigation to 100% field capacity; (M): irrigation to 50% field capacity; (S): irrigation to 20% field capacity). Different letters on the top of bars indicate significant differences between treatments according to Duncan’s test (p<0.05, n=4).
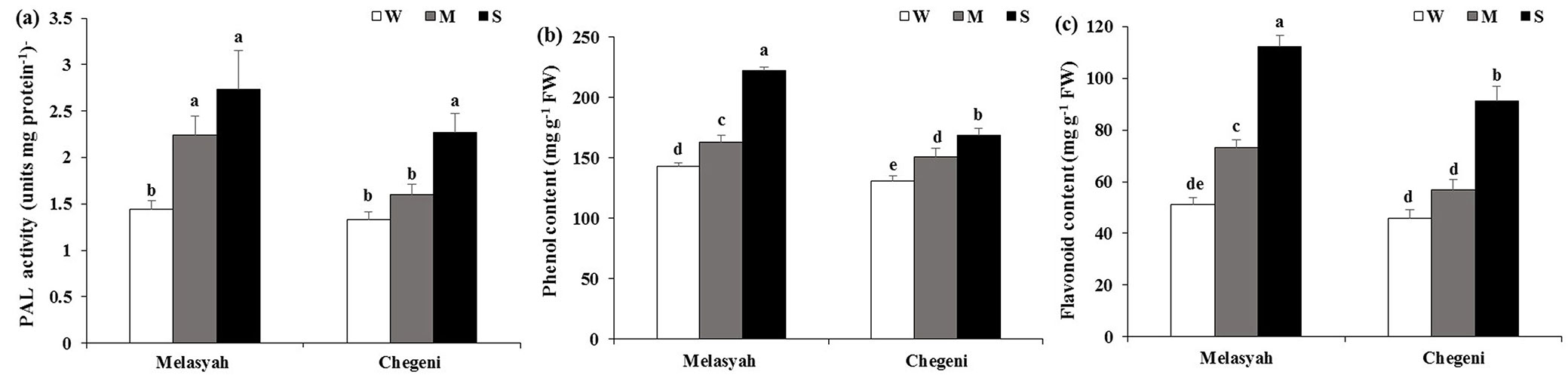
PAL activity, total phenol and flavonoid content
PAL activity increased after drought stress in seedlings of both populations. Although Melasyah seedlings had higher levels of PAL activity under moderate and severe drought stress in comparison with control seedlings, no difference was found in well-watered and moderate drought-stressed seedlings from Chegeni provenance and the enzyme activity just increased in response to severe stress (Fig. 7a).
Fig. 7 - Mean values (± standard error) of (a) PAL enzyme activity, (b) total phenol and (c) flavonoid content in leaves of Persian oak seedlings from Melasyah and Chegeni provenances under different drought stress treatments. (W): irrigation to 100% field capacity; (M): irrigation to 50% field capacity; (S): irrigation to 20% field capacity). Different letters on the top of bars indicate significant differences between treatments according to Duncan’s test (p<0.05, n=4).
In both populations, total phenol content in stressed seedlings was higher than in control seedlings (Fig. 7b). The highest phenol content was found in Melasyah seedlings subjected to severe stress.
Both levels of drought stress led to a significant increase in flavonoid content of Melasyah seedlings in comparison with control seedlings, whereas flavonoid content in Chegeni seedlings significantly increased only in response to severe drought stress (Fig. 7c). Under both levels of water limitation, the foliar flavonoid content of Melasyah seedlings was higher than of Chegeni seedlings.
Discussion
Populations from high precipitation regions are reported to be less resistant to drought than those growing under low precipitation regimes ([48]). In this study, we compared two Persian oak provenances from different precipitation regimes, observing significant differences in terms of growth, physiological, and biochemical differences; hence, a local adaptation to drier conditions may be hypothesized. Under water limitations, the Melasyah provenance significantly differed from the Chegeni provenance, probably as a result of its origin from a lower precipitation region. Our findings are supported by the results of Wanjiku & Bohne ([45]), who worked on growth and drought responses of three Prunus spinosa L. ecotypes from Germany and Italy. They reported that P. spinosa tends to vary in susceptibility to drought because of local adaptation.
As expected, photosynthetic performance and aboveground growth of oak seedlings from both provenances significantly declined when exposed to severe drought stress in comparison with control seedlings. Diameter growth significantly decreased by 52.52% in Chegeni seedlings under severe drought stress compared with non-stressed seedlings, while the height growth of seedlings from both provenances declined under moderate and severe water limitations (by 18% and 51% at Melasyah and 18% and 39% at Chegeni provenance under moderate and severe drought stress, respectively). Moreover, the total seedling biomass for Melasyah and Chegeni provenances declined by 14% and 34% respectively, when subjected to severe drought stress. The reduction in plant growth is a common response to water shortage as a result of reduced photosynthesis ([15]), which is positively correlated with plant growth ([26]). In this study, the results of morphological analysis showed that the Chegeni population was more sensitive to drought stress compared to the Melasyah population.
Drought is one of the most important abiotic stresses in distribution range of oak species and leads to a decrease in gas exchange parameters (including photosynthesis rate, stomatal conductance, and transpiration rate - [9], [15]). However, the negative effects can vary depending on drought severity and genotype. In this study, the rate of photosynthesis and the stomatal conductance of oak seedlings declined in response to drought stress, especially when exposed to severe drought. Furthermore, the decreasing rate of the photosynthetic parameters was more prominent in seedlings from Chegeni than from Melasyah provenance. Stomatal conductance is a primary driver of hydrological changes that control plant response to environmental stress, and is highly correlated with the photosynthesis rate as well ([14]). The reduced rate of photosynthesis can be associated with the decrease in stomatal conductance, transpiration, and mesophilic changes, such as changes in Ci and the reduced apparent Vcmax ([9]), finally leading to a decrease in photochemical efficiency of PS II.
It is well known that the decrease in water potential and maintenance of water relations is one of the plant strategies in response to water limitation, which finally helps develop resistance to drought stress. Our findings revealed that Persian oak seedlings reduced xylem water potential when they were exposed to severe drought stress. Moreover, xylem water potential of Chegeni seedlings under drought stress was more negative compared with those of Melasyah seedlings. The reduced water potential of plants under drought is a common physiological response to perform osmoregulation and maintain plant water so as to enhance drought resistance, as reported in different studies on oak species ([9]). We surveyed the stability of PSII in seedlings by means of Fv/Fm measurement because the photochemical efficiency of photosystem II is among the most important components of the photosynthetic apparatus. We clearly observed a decrease of Fv/Fm in seedlings from both provenances under water limitation treatments. Hence, it can be hypothesized that a reduction in Fv/Fm could represent a deep disorder in PSII. Moreover, Boussadia et al. ([8]) stated that the reduction in Fv/Fm is related to down-regulation of PSII, reflecting a protective or regulative mechanism to preclude energy loss and damage to photosynthetic apparatus. Our results support the last findings about changes in PSII when plants were subjected to drought stress ([10]).
Pigment content is an important indicator of the plant status, and can be used to survey the capability and activity of plant photosynthesis ([12]). The reduction in chlorophyll content under drought stress is a common response following biotic or abiotic stresses because of photo-oxidation, chlorophyll degradation, or impaired chlorophyll biosynthesis ([39]). In out study, chlorophyll a+b and carotenoid contents of Persian oak seedlings from both populations declined under drought stress treatments, though the decreasing rate was greater in the Chegeni population. In addition, a decline in pigment content under moderate drought stress was recorded only for the Chegeni provenance. The reduction in chlorophyll and carotenoid content in response to water shortage have been also reported by previous studies ([2]).
An increase in proline content under water limitation seems related to drought resistance in plants ([25]). Based on out results, Melasyah seedlings had higher proline content compared to the Chegeni provenance. The increase of proline under water limitation was repeatedly observed in other plants ([9]), where it acts as an osmotic molecule and protects the plant cell from ROS ([41]).
We observed that drought stress stimulated an increase in soluble sugar in Persian oak leaves as compared with unstressed seedlings. Furthermore, soluble sugar content was higher in Melasyah than in Chegeni seedlings. Typically, drought stress accelerates soluble sugar content in the leaf and subsequently leads to induced osmotic adjustment ([18]). The accumulation of soluble sugar promotes water penetration and finally maintains cell volume to avoid wilting ([18]). Not only are soluble sugars metabolic sources and compounds of cell structure, they also act as substrates for cellular respiration ([26]).
Electrolyte leakage is an indicator of cell integrity and cellular membrane stability which reflects the degree of damage to the plant by stress agents ([22]). Generally, the content of MDA byproducts is a measure of the degree of lipid peroxidation caused by oxidative stress ([24]). In the current study, the increase in MDA content in response to drought stress occurred in stressed seedlings of both populations, and was concomitant with the increase in electrolyte leakage. The result indicates that lipid peroxidation increased membrane permeability. In fact, lipid peroxidation determines changes in cellular membrane causing an increase in electrolyte leakage and osmotic imbalance ([34]). Previous studies have reported a similar increase in MDA content and electrolyte leakage in plants subjected to water limitation stress ([9], [25]).
ROS (including superoxide radicals and hydrogen peroxide) are the first messengers in plants subjected to drought stress ([42]), and their increase in response to drought stress has been proved ([36]). In our study, the enhancement of superoxide radicals and hydrogen peroxide content in drought treatments was expected, indicating that oak seedlings were subjected to oxidative stress. ROS causes lipid peroxidation by stimulating decomposition of unsaturated lipids and finally has negative effects on the structure, fluidity, and integrity of plant cells ([21]). Like MDA and electrolyte leakage, superoxide radicals and hydrogen peroxide content only increased in the Chegeni provenance under moderate water limitation, while Melasyah seedlings showed more resistance under the same condition.
Antioxidant enzymes (peroxidase, superoxide dismutase, catalase, etc.) are part of the most important metabolic pathways for the removal of ROS and limitation of lipid peroxidation ([25]). In this study, antioxidant enzyme activities, including peroxidase, superoxide dismutase, catalase, and ascorbate peroxidase, increased when the oak seedlings were exposed to the water limitation.
The increase in antioxidant enzymes in Persian oak seedlings under drought condition could be an adaption mechanism to face the high production of ROS. Among all the antioxidant enzymes, superoxide dismutase converts superoxide radicals to hydrogen peroxide, which subsequently should be scavenged to water and molecular oxygen by the others like POD, CAT, and APX ([32]). Catalase is mainly located in peroxisome, where its main function is to remove hydrogen peroxide (H2O2-detoxifying), while peroxidase converts hydrogen peroxide to water by means of some available reductions in the cell ([28]). Similarly to our results, other studied have reported an increase in antioxidant enzyme activities in plants in response to water limitation stress ([16]). Our results showed that Melasyah seedlings had higher antioxidant activity than Chegeni seedlings.
In the current study, the increase in PAL activity and accumulation of phenolic components in leaves of Persian oak seedlings under severe drought conditions reflects changes in the phenylpropanoid pathway. Under drought stress, total phenol and flavonoid contents of Melasyah seedlings were higher than those of Chegeni seedlings. In fact, the increase in PAL activity determines the activation of the phenylpropanoid pathway, and thus causes accumulation of phenolic and flavonoid compounds. Phenolic compounds are secondary metabolites with antioxidant effects in plants exposed to oxidative stress ([27]). In line with our findings, an increase in phenolic compounds associated with increasing PAL in drought-stressed plants have been reported ([1]).
Conclusions
We evaluated morpho-physiological and biochemical responses to drought of two provenances of Persian Oak to test for their local adaption to contrasting climatic conditions. Overall, our results show that there is a systematic relationship between responses to drought stress and provenance. Growth parameters, gas exchange, and pigment content of seedlings from Melasyah provenance (drier climate) were less affected by drought stress when were compared with those from Chegeni provenance (wetter climate). Our findings revealed a significant relationship between the mean annual precipitations of Persian oak stands and their drought tolerance. It can be concluded that the climatic condition of Persian oak stands is an important factor to be be considered by forest managers who are responsible for establishment and restoration programs.
Acknowledgements
The authors acknowledge the help of Dr. Ehsan Ghanbary, Mr. Jebreil, and Dr. Mohammad Khezry in providing plant materials and soil preparation, greenhouse maintenance, and watering.
References
Gscholar
CrossRef | Gscholar
Online | Gscholar
Authors’ Info
Authors’ Affiliation
Moslem Akbarinia
Department of Forestry, Faculty of Natural Resources, Tarbiat Modares University, P. O. Box 14115-111 (Iran)
Department of Agriculture, Iranian Research Organization for Science and Technology (IROST), P. O. Box 3353-5111, Tehran (Iran)
Department of Agronomy, Faculty of Agriculture, Tarbiat Modares University, P. O. Box 14115-336, Tehran (Iran)
Department of Horticultural Sciences, Faculty of Agriculture and Natural Resources, University of Tehran, P. O. Box 41111, Tehran (Iran)
Corresponding author
Paper Info
Citation
Jafarnia S, Akbarinia M, Hosseinpour B, Modarres Sanavi SAM, Salami SA (2018). Effect of drought stress on some growth, morphological, physiological, and biochemical parameters of two different populations of Quercus brantii. iForest 11: 212-220. - doi: 10.3832/ifor2496-010
Academic Editor
Claudia Cocozza
Paper history
Received: May 19, 2017
Accepted: Nov 22, 2017
First online: Mar 01, 2018
Publication Date: Apr 30, 2018
Publication Time: 3.30 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 54245
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 43013
Abstract Page Views: 5011
PDF Downloads: 4929
Citation/Reference Downloads: 32
XML Downloads: 1260
Web Metrics
Days since publication: 2902
Overall contacts: 54245
Avg. contacts per week: 130.85
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 45
Average cites per year: 5.63
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Oak sprouts grow better than seedlings under drought stress
vol. 9, pp. 529-535 (online: 17 March 2016)
Review Papers
Indicators of drought effects in Pinus sylvestris: genetic analyses to corroborate the results of empirical methods
vol. 3, pp. 89-91 (online: 15 July 2010)
Research Articles
Drought tolerance in cork oak is associated with low leaf stomatal and hydraulic conductances
vol. 11, pp. 728-733 (online: 06 November 2018)
Research Articles
Effect of salt and drought on growth, physiological and biochemical responses of two Tamarix species
vol. 8, pp. 772-779 (online: 25 March 2015)
Research Articles
Effects of mild drought on the morphology of sun and shade needles in 20-year-old Norway spruce trees
vol. 12, pp. 27-34 (online: 10 January 2019)
Research Articles
Tracing the acclimation of European beech (Fagus sylvatica L.) populations to climatic stress by analyzing the antioxidant system
vol. 14, pp. 95-103 (online: 01 March 2021)
Review Papers
Drought-induced mortality of Scots pines at the southern limits of its distribution in Europe: causes and consequences
vol. 3, pp. 95-97 (online: 15 July 2010)
Research Articles
Magnolia grandiflora L. shows better responses to drought than Magnolia × soulangeana in urban environment
vol. 13, pp. 575-583 (online: 07 December 2020)
Short Communications
Preliminary indications for diverging heat and drought sensitivities in Norway spruce and Scots pine in Central Europe
vol. 13, pp. 89-91 (online: 01 March 2020)
Research Articles
Spatial and temporal variation of drought impact on black locust (Robinia pseudoacacia L.) water status and growth
vol. 8, pp. 743-747 (online: 18 June 2015)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword