Sodium and potassium allocation under drought stress in Atlas mastic tree (Pistacia atlantica subsp. mutica)
iForest - Biogeosciences and Forestry, Volume 6, Issue 2, Pages 90-94 (2013)
doi: https://doi.org/10.3832/ifor0856-006
Published: Feb 07, 2013 - Copyright © 2013 SISEF
Research Articles
Abstract
Sodium and potassium ions have crucial roles in drought tolerant mechanisms of plants. In a pot experiment, seedlings of Pistacia atlantica subsp. mutica were cultivated in a silty clay soil (Kex=21 mg/l, Naex=143 mg/l) and exposed to different levels of soil water content (90, 65 and 45% FC). P. atlantica maintained leaf transpiration rate, and relative water content and cation (Na+ and K+) content of different organs under moderate water deficit (above permanent wilting point). At higher water shortage, the amount of transpiration rate and the relative water content of tissues were reduced, but the amount of Na+ and K+ remained unaffected even under severe drought stress (below permanent wilting point). Generally, the amount of K+ content in plant organism was higher than that of Na+. The amount of root to shoot Na+ was higher than that of K+. Potassium contents of leaves and stems were positively related with transpiration rate, whereas Na+ contents of roots and stems were positively related with root relative water content. Roots and stem of Atlas mastic tree seedlings exhibit high drought tolerance index for Na+ and K+.
Keywords
Pistacia atlantica Desf., Univalent Cations, Physiological Traits, Ion Translocation, Water Deficit
Introduction
Among the many physiological features by which plants cope with water shortage, maintaining cation uptake and mobility in different tissues plays many crucial roles to deal with water deficit. Monitoring of cation changes under soil water shortage is so important that it was recently used as a drought tolerance index for selecting cultivars ([1], [2]). Nutrients uptake and consequent cation translocation highly depend on transpiration stream ([15]). Reduced root lateral growth can also restrict nutrient uptake. Potassium is the most abundant cation in higher plants and is involved primarily in osmotic-mediated cell expansion ([12]), protein synthesis, glycolytic enzyme activity, membrane permeability, photosynthesis, stomatal movement, drought resistance ([17]) and tropism ([29]). In contrast, sodium at elevated concentrations - usually more than 100 mM - has toxic effects on plants. Sodium has lower replacing power than K+ as revealed from Hofmeister’s cation sequence, higher affinity with anionic groups as the carboxyl groups of proteins ([37]) and generally impairs the positive roles of K+ in living cells. Because of high frequency, similar valence electron in outer shell, similar hydrated ion radius and deficiency of high performance discriminating antiporters ([24]), Na+ interferes in K+ uptake and translocation. It can also enhance plant performance, being involved in osmotic regulation in vacuoles ([27], [25]). This role of Na+ is even more relevant under water deficit where total nutrient uptake is affected by decreasing soil water content and Na+ toxicity is not an issue. Most of studies emphasize the necessity of reducing Na+ under salt stress to maintain high K+/Na+ ratio in the intracellular space ([24]). Although changing K+/Na+ ratio is not the main concern under drought stress, increasing of this ratio can facilitate plant survival and development under this condition. Exposing six cultivars of sorghum seedlings to progressive water deficit reduced the content of these univalent cations both in root and shoot, whereas the amount of sodium increased and potassium remained constant in the roots of a drought tolerance cultivar ([1]). Plants that are able to take more K+ and maintain higher K+/Na+ ratio in the intracellular space, are able to ameliorate the negative effects of drought on water relations and organic and inorganic solute accumulation in different organs ([28]).
The genus Pistacia (Anacardiaceae), commonly known for the edible nuts of P. vera, has at least 11 species worldwide. P. atlantica Desf. is a wild pistachio species which produces mastic (an edible resin) and small edible nuts with high content of valuable essential oils ([3], [35], [14]). As a xerophyte species, P. atlantica is widely distributed from south-west Asia to north-west Africa ([38]) and has wide range of pharmacological ([5]), cosmetic and food consumptions at local and industrial scale. At least three subspecies - cabulica (Stocks) Rech. F., kurdica (Zohary) Rech. F., and mutica (Fish and May) Rech. F. - have been identified so far ([19]). Pistacia atlantica subsp. mutica is a predominant tree species in Irano-Turanian region and mostly distributed in altitude of 900-2800 m a.s.l. Contrary to importance, the physiological mechanisms to deal with water shortage, one of the main growth limiting factors in P. atlantica habitats, are poorly investigated. Benhassaini et al. ([6]) noted that RWC of the plantlet leaves of P. atlantica subsp. atlantica remained unaffected up to 200 meq.l-1 NaCl+CaCl2 and decreased at 400 meq.l-1. Some studies on P. terebinthus and P. lentiscus, two similar wild pistachio species, describe these species as a drought-avoider, water-spender plants ([23]) that sustain high stomatal conductance and leaf RWC under water shortage and typically cannot stand long under harsh conditions ([33], [36]). However by increasing water deficit, drought avoidance strategy of wild pistachio turns towards water saving mechanisms by decreasing stomatal conductance and sap flow ([9], [36]). Despite many progress, our current knowledge about drought tolerance strategies, especially in woody plants, is not comprehensive. In this paper, the behavior of Atlas mastic tree (P. atlantica) seedlings under water deficit condition is investigated, with emphasis on altering univalent cations content and translocation in different organs. Also the relationship with leaf transpiration rate and relative water content of different organs is discussed. Finally drought tolerance indexes for Na+ and K+ accumulation in different tissues of P. atlantica are compared and discussed.
Materials and methods
Plant preparation and treatments
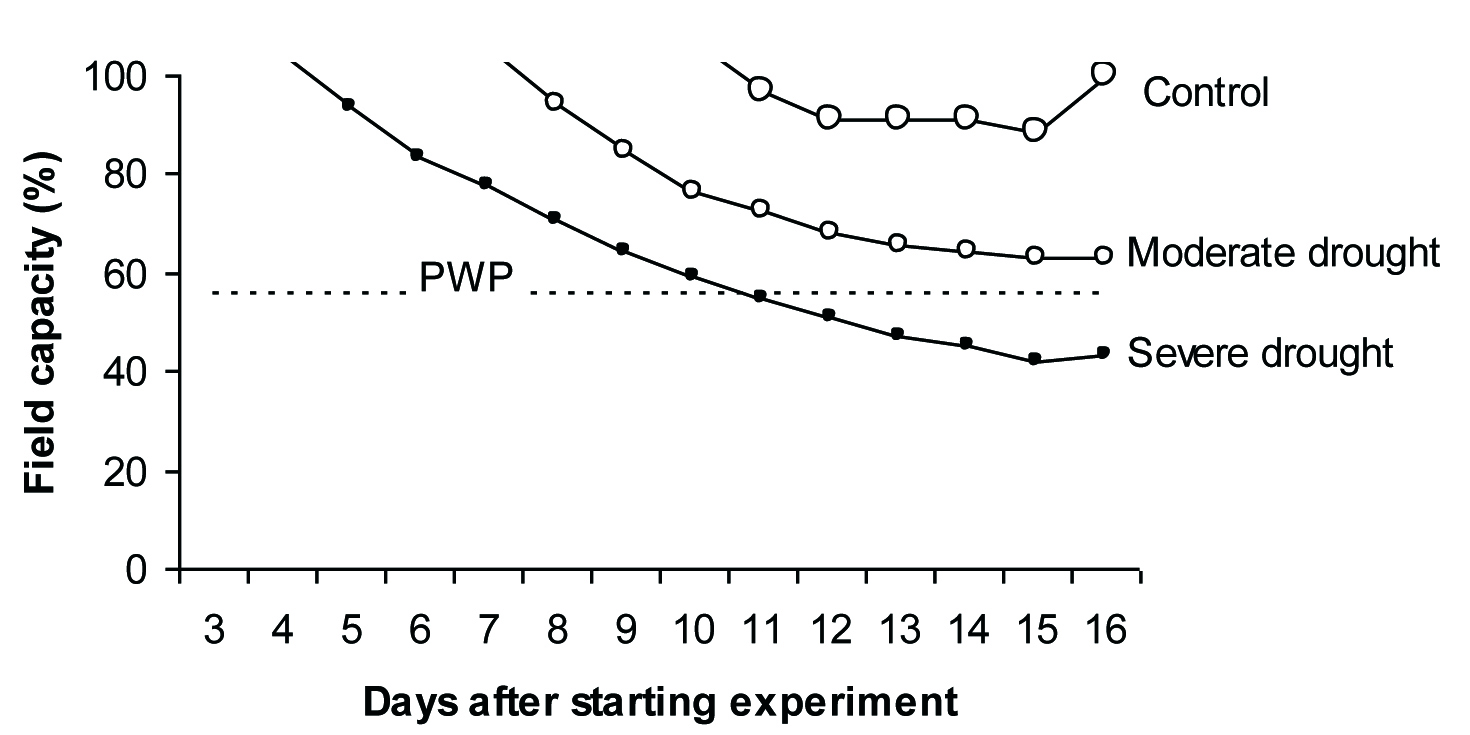
The experiment was conducted using 36, 3-year-old saplings of P. atlantica Desf. subsp. mutica (Fisch. & C. A. Mey.) Rech. f. (about 30 cm height and 4 mm collar diameter) under a plastic greenhouse in uncontrolled climatic condition in Yasouj (also Romanized as Yasuj and Yasooj), the center of “Kohgiluyeh and Boyer-Ahmad” province in Iran (30°40’06” N, 51°35’17” E), during summer 2010. In February 2010, plants were obtained from Yasouj nursery and transferred into 3-liter pots (15 cm diameter) containing 80% field soil and 20% humus (sand 11%, silt 45%, clay 44%, pH 7.9, Kex 21 mg/l, Naex 143 mg/l, CEC 2.5%, OC 7.8%) and divided into four groups with similar amount of leaves per group. Whole plants were irrigated 2-3 times per week until the beginning of experiment. Prior to experiment onset, soil moisture characteristics including field capacity and permanent wilting point were determined by pressure-plate apparatus ([31]). Soil water content at 0.33 atm. (FC) and 15 atm. (PWP) was 28.75% and 15.95% w/w, respectively (PWP 55% of FC - Fig. 1).
Fig. 1 - Kinetics of soil water content, expressed as percent of water content at field capacity, under different irrigating regimes. A dash line shows the permanent wilting point (PWP). Each point represents the mean of 12 observation ± SE.
The experiment started on July 20th with controlled irrigation to reach the desired percentage of soil field capacity i.e., 90, 65 and 45%. The average value of meteorological parameters together with 95% confidence interval during the experiment were as follow: PAR (9:00 am - 3:00 pm) = 1270 ± 39 μmol m-2 s-1; hours of sunshine = 11.2 ± 0.5 h; Tmax = 39 ± 0.5 °C; Tmin = 18 ± 0.5 °C; pan evaporation rate = 11.6 ± 0.9 mm/day; relative humidity at 09:00 am = 24.6±5.2%. The desired soil water content level was achieved after 12 days of water holding and plants were kept in these conditions for 4 further days.
Transpiration rate and relative water content
One day before harvest, transpiration rate of the youngest fully developed leaf of the plants was measured using a portable IRGA instrument (ADC LCA4 Bioscientific, United Kingdom) from 9:30 to 11:30 am on a full-sun day (1270 μmol m-2 s-1 PAR, 42° C chamber temperature). Seven leaves per treatment were measured within 2 hours. After determining real leaf area in chamber using INAHFJ software ([30]), area correction coefficient was applied to calculate corrected leaf transpiration rate. To determine relative water content (RWC), nearly 0.1-0.2 g of different tissues (leaf, stem and root) were weighed to accuracy of 0.001 g (FW) by a digital balance (GF-400, A&D, Japan) and floated in distilled water for 24 h at room temperature in a dark place. Then samples were blotted by a tissue paper to measure the turgid weight (TW) and oven dried for 48 h at 80 °C to determining the dry weight (DW). Finally, RWC was calculated as follows (eqn. 1 - [4]):
Element analysis
The concentration of Na+ and K+ in different organs (leaf, stem and root) was determined by dry ashing followed by photometric analysis ([32]). A pool of three plant tissues was replicated 4 times per each water treatment. About 0.1 g of dried tissues was ashed in an oven at 500 °C for 7 h. Samples were then dissolved in 1 ml 2N HCl and diluted up to 10 ml using distilled water. After filtration, the concentrations of Na+ and K+ were determined using flame photometer (Jenway PFP7, UK).
Drought tolerance index
Drought tolerance index (DTI) for cation accumulation (Na+ and K+ separately and simultaneously) in different organs were calculated on the basis of Achakzai ([1], [2]) with some modifications as follows (eqn. 2):
where Ind(CAdrougth) is the individual cation accumulation under severe drought (%) and Avg(CAcontrol) is the average of cation accumulation under control conditions (%). In order to achieve within group variations, DTI was calculated by dividing the cation content of each pool of plants standing under severe drought condition, by an average value of cation accumulation under control condition.
Statistical analysis
Determination of transpiration rate and relative water content were obtained from 7 plants for each treatment and a one-way analysis of variance was applied to evaluate the effect of water shortage treatments on these parameters. In the case of element analysis, four pools from each water regimes and different organs (leaf, stem and root) were measured and a two-way analysis of variance was performed to assess the main and interaction effects of water regime and organ type. A bivariate correlation analysis was performed to illustrate the relationship between desired traits. Also, a partial correlation analysis was conducted to evaluate the changes in the root and stem univalent cations (Na+ and K+) by controlling either the leaf Na+ or K+ content. Drought tolerance indexes for cations accumulation were measured in four pools of plants underling severe water deficit level from each organ and a one-way analysis of variance was conducted to determine the effect of organ type on DTI for different cation accumulation. The putative outliers were found by drawing box-and-whisker plots, and values more than 3 folds of standard deviation from the mean were discarded. The analysis of variance was performed by applying a general linear model (GLM) and different levels of water deficit were considered as a fixed effect variable in the model. Tests of normality and equality of variances of the residuals were conducted by Kolmogorov-Smirnov (with Lilliefors significance correction) and Levene’s test. A natural logarithm transformation was applied for sodium concentration data. Differences of means were tested by Duncan’s multiple- range test. Differences were considered statistically significant based on an alpha level of 0.05. All statistical analyses were performed using IBM SPSS Statistics 19 software (SPSS Inc., Chicago, IL, USA).
Results
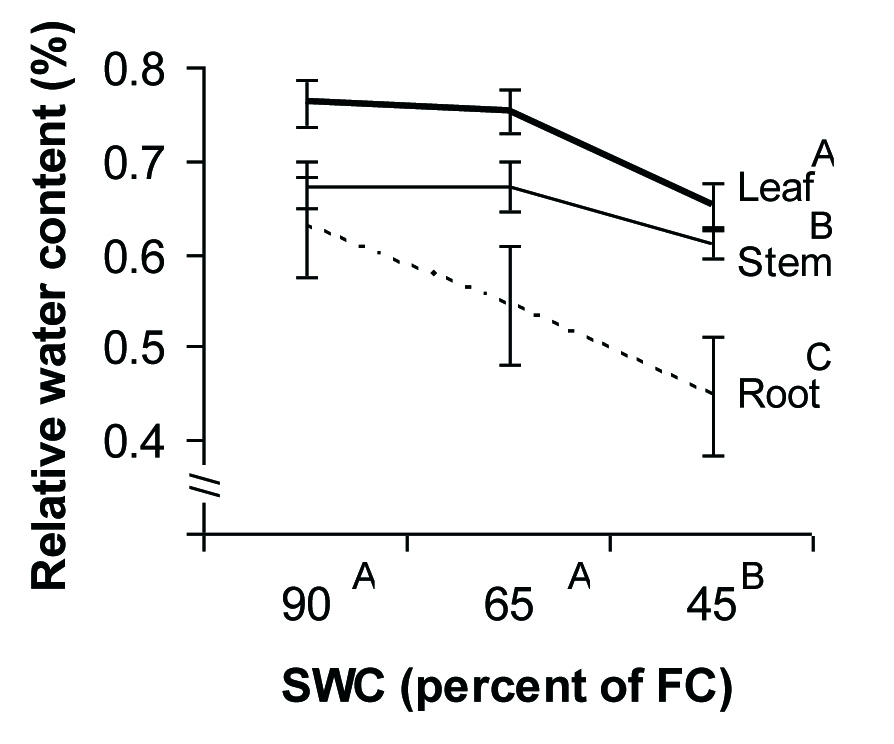
Decreasing soil water content from 90 to 45% of FC gradually diminished transpiration rate of the seedlings of P. atlantica from 5 to 2 mmol H2O m-2 s-1, but the amount of transpiration rate under 65% of FC did not change significantly (Fig. 2). Organ type and SWC affected RWC, but no significant interaction was observed between these factors. In all water regimes, the amount of RWC in P. atlantica increased from roots to leaves (Fig. 3). Regardless of the organ type, RWC of seedlings remained constant up to 65 % of FC and decreased under severe drought condition (45% of FC).
Fig. 2 - Leaf transpiration rate in P. atlantica seedlings as a function of soil water shortage. Each point represents the mean of 7 observations ± SE. Different letters indicate significant differences between means after Duncan’s test (α = 0.05).
Fig. 3 - Relative water content of different tissues in P. atlantica seedlings as a function of soil water shortage. Each point represents the mean of 7 observations ± SE. Different letters indicate significant differences between means after Duncan’s test (α = 0.05).
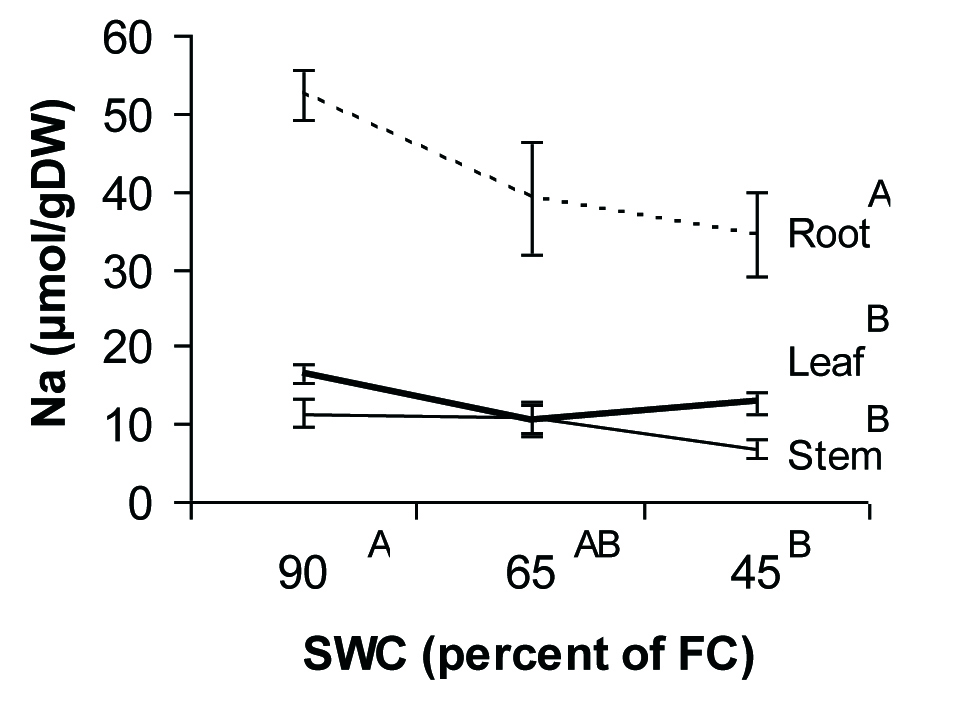
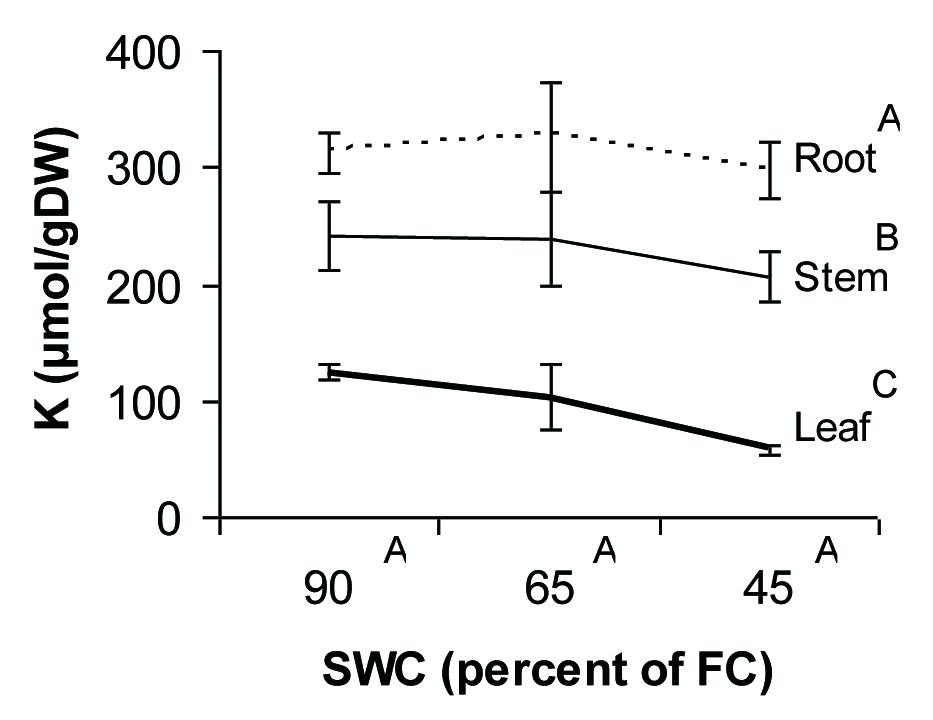
The univalent cations, Na+ and K+, showed different patterns of allocation and response to drought in seedlings of P. atlantica. Na+ ions mostly accumulated in roots and their concentration in both leaves and stems were at the lowest level (Fig. 4). K+ ions had more free move than Na+ ions from root to the aerial parts of the plant (Fig. 5). Also, K+ content remained constant during water deficit conditions, while concentration of Na+ decreased under severe water deficit.
Fig. 4 - Sodium concentration in different tissues of P. atlantica seedlings as a function of soil water shortage. Each point represents the mean of 4 observations ± SE. Different letters indicate significant differences between means after Duncan’s test (α = 0.05).
Fig. 5 - Potassium concentration in different tissues of P. atlantica seedlings as a function of soil water shortage. Each point represents the mean of 4 observations ± SE. Different letters indicate significant differences between means after Duncan’s test (α = 0.05).
Simple correlation analysis between soil water content, transpiration rate, relative water content and sodium and potassium content of different organs illustrated that transpiration increased with increasing leaf and stem K+ content (Tab. 1). Also a positive correlation was observed between root RWC and Na+ content of root and stem. Transpiration was not affected by the RWC of different organs (Tab. 1). The results of partial correlation analysis revealed that the part of root and stem K+ content, that is not related with leaf K+ content, was not related with RWC and transpiration. In contrast, the part of root Na+ content that is independent from leaf Na+ content was still highly and positively correlated with root relative water content (r = 0.791, p = 0.006).
Tab. 1 - Pearson’s correlation coefficients and significance levels obtained comparing leaf transpiration rate (E), relative water content (RWC) and ion contents of different organs, and soil water content (SWC - n=12). (*): p<0.05; (**): p<0.01.
| Parameters | Relative water content |
K+ content (μmol/gDW) |
Na+ content (μmol/gDW) |
SWC (%FC) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Root | Leaf | Stem | Root | Leaf | Stem | Root | ||
| E (mmol H2O m-2 s-1) | 0.24 | 0.41 | -0.19 | 0.63* | 0.71* | 0.21 | 0.33 | -0.12 | 0.02 | 0.52 |
| Leaf RWC | - | 0.75** | 0.39 | 0.34 | 0.41 | 0.02 | -0.28 | 0.45 | 0.25 | 0.67* |
| Stem RWC | - | - | 0.19 | 0.44 | 0.70* | 0.00 | -0.22 | -0.03 | -0.01 | 0.42 |
| Root RWC | - | - | - | -0.20 | -0.02 | -0.10 | 0.11 | 0.72* | 0.75** | 0.53 |
DTI for potassium accumulation was lower in the leaves than in the stem and roots, but DTI for sodium accumulation in different organs did not vary (Tab. 2). Considering both Na+ and K+ simultaneously resulted in a response similar to that obtained by considering K+ only. Performing multiple comparisons revealed that leaves of P. atlantica seedlings had lower DTI for K+ and conjugated univalent cations (Na+ and K+) than roots and stems (Tab. 2).
Tab. 2 - Tolerance index (DTI) as percentage of drought-induced reduction in cation content in different organs of P. atlantica seedlings. Each group represents the mean of 4 observation ± SE. Different letters in each column indicate significant differences with 95 % confidence intervals after Duncan’s multiple mean comparison.
| Organs | Drought Tolerance Index (%, Mean ± SE) | ||
|---|---|---|---|
| Na+ | K+ | Na+ and K+ | |
| Leaf | 77.26 ± 5.5 a | 46.44 ± 3.0 b | 50.05 ± 3.0 b |
| Stem | 57.46 ± 10.1 a | 82.05 ± 8.8 a | 80.94 ± 8.4 a |
| Root | 66.80 ± 10.8 a | 96.72 ± 8.0 a | 92.45 ± 7.5 a |
Discussion
In this study we demonstrated that seedlings of P. atlantica maintain a high level of transpiration and relative water content of different organs under moderate water deficit conditions (65% FC) and decrease transpiration and RWC under severe stress (45% FC). These results confirm the two-step drought- avoider mechanisms of water spending and saving in P. atlantica as reported for P. terebinthus and P. lentiscus ([9], [36]). Despite of relatively low soil exchangeable potassium availability for seedlings in this experiment (Kex= 21 mg/l), K+ content remained constant during soil water depletion. K+ levels in root, stem and leaf were about 15, 10 and 5 times higher than that of the soil medium, respectively. The ability of P. atlantica to transport considerable amount of K+ to the shoots and enhancing K+ content of soil surface has been previously reported by Hosseini et al. ([18]). Constant K+ levels in different organs even under both K+ deficit conditions and dropping of transpiration stream suggests the presence of high efficient K+ uptake and transport mechanisms in P. atlantica. P. atlantica might benefit from some highly specific K+ channels such as Shaker-type K+ channel ([16]) and SKOR (Stelar K+ Outward Rectifier - [20]). Also, some proteins like CIPKs (Calcineurin B-like proteins interacting protein kinases) that regulate stomatal closure and K+ uptake under drought conditions could be involved ([8]). In P. atlantica K+ content was 5 to 10 times higher than that of Na+, indicating highly distinct absorption pathway with different efficiency for K+ and Na+ as reported for other plants ([22]). Also the stem to root K+ content was 0.7, whereas this ratio for Na+ was lower (about 0.2). This result supports the conclusion that the rate of Na+ translocation from root to shoot was more limited than that of K+. Restricting Na+ transport, especially in stems, maintains a negative charge and facilitates K+ entrance.
Regulation of K+ transport in leaves affects cell turgidity, stomatal conductance, photosynthesis, cell expansion and oxidative metabolism ([26]). K+ channels of guard cells have an important role in drought-induced stomatal closure ([21], [34]). K+ content of leaves and stems of P. atlantica were positively correlated with transpiration rate, but no correlation was observed between K+ content of root and transpiration. Some studies suggested that K+ contents in roots and shoots are independent, so that shoot K+ content is correlated with transpiration, while variations in root K+ content is not in relation with leaf transpiration rate ([7]). Plants containing high amount of K+ are able to absorb more water under drought stress ([11]). Generally, in the early stages of drought stress the amount of K+ uptake increases and finally declines at higher drought stress ([10], [13]). Comparing DTI for Na+ and K+ accumulation (separately and simultaneously) suggested higher sensitivity of the leaves than of the stems and roots of Atlas mastic tree to water deficit, mostly due to restriction of K+ transport into the leaves. The DTI for K+ accumulation in leaves of P. atlantica (about 46%) is in agreement with cultivars of sorghum (about 50%) and maize (about 25%) as reported by Achakzai ([1], [2]). DTI for K+ in P. atlantica roots (96%) was higher than that in cultivars of sorghum (about 50%) and maize (about 65% - [1], [2]). In conclusion, P. atlantica is able to maintain high K+ and Na+ affinity even under low soil water and K+ content. This behavior of P. atlantica is so marked that in natural forests, concentration of K+ and Na+ in soil surface decreases with distance from the tree base ([18]).
P. atlantica showed both water-spending and water-saving strategies to deal with soil water depletion. Sodium and potassium accumulation and translocation were highly efficient and segregated. Transpiration and water content of shoots were positively correlated with K+ content of shoots, whereas root RWC was affected by Na+ content of root and stem. To increase drought tolerance of P. atlantica plantations, it is recommended to select varieties able to uptake more Na+ and K+. It can be also concluded that application of K+ fertilizer can reduce drought damage to P. atlantica seedlings located in plantation and natural forests.
Acknowledgments
PF supervised the experimental design, performed the statistical analysis and drafted the manuscript, EE carried out the element analysis, NJ conducted the experiment, RZ contributed as advisor. We thank general director of Natural Resources and Watershed Management of Kohgiluyeh-and-Boyer-Ahmad Province for providing seedlings of P. atlantica. We wish also to express our thanks to Drs. H.R. Owliaie and M. Sedghi (Department of soil sciences, Yasouj University, Iran) for their help in measuring field capacity and physico-chemical characteristics of soil. This work is a part of a master student project that was financially supported by Yasouj University (Iran).
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
E Etemadi
N Julaiee-Manesh
R Zolfaghari
Institut of Natural Resources and Environment, University of Yasouj, 75918-74831 Yasouj (Iran)
Corresponding author
Paper Info
Citation
Fayyaz P, Etemadi E, Julaiee-Manesh N, Zolfaghari R (2013). Sodium and potassium allocation under drought stress in Atlas mastic tree (Pistacia atlantica subsp. mutica). iForest 6: 90-94. - doi: 10.3832/ifor0856-006
Academic Editor
Giustino Tonon
Paper history
Received: Nov 07, 2012
Accepted: Nov 14, 2012
First online: Feb 07, 2013
Publication Date: Apr 02, 2013
Publication Time: 2.83 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2013
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 57789
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 47602
Abstract Page Views: 3566
PDF Downloads: 5108
Citation/Reference Downloads: 21
XML Downloads: 1492
Web Metrics
Days since publication: 4750
Overall contacts: 57789
Avg. contacts per week: 85.16
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2013): 8
Average cites per year: 0.62
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Variations in the performance of hybrid poplars subjected to the inoculation of a microbial consortium and water restriction
vol. 16, pp. 352-360 (online: 13 December 2023)
Research Articles
A physiological approach for pre-selection of Eucalyptus clones resistant to drought
vol. 13, pp. 16-23 (online: 15 January 2020)
Technical Reports
Detecting tree water deficit by very low altitude remote sensing
vol. 10, pp. 215-219 (online: 11 February 2017)
Research Articles
Adaptive variation in physiological traits of beech provenances in Central Europe
vol. 11, pp. 24-31 (online: 09 January 2018)
Short Communications
Evidence of Alectoris chukar (Aves, Galliformes) as seed dispersal and germinating agent for Pistacia khinjuk in Balochistan, Pakistan
vol. 14, pp. 378-382 (online: 22 August 2021)
Research Articles
Diurnal dynamics of water transport, storage and hydraulic conductivity in pine trees under seasonal drought
vol. 9, pp. 710-719 (online: 21 August 2016)
Research Articles
Addressing post-transplant summer water stress in Pinus pinea and Quercus ilex seedlings
vol. 8, pp. 348-358 (online: 16 September 2014)
Research Articles
Evergreen species response to Mediterranean climate stress factors
vol. 9, pp. 946-953 (online: 07 July 2016)
Research Articles
Assessment of cadmium tolerance and phytoextraction ability in young Populus deltoides L. and Populus × euramericana plants through morpho-anatomical and physiological responses to growth in cadmium enriched soil
vol. 10, pp. 635-644 (online: 01 June 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword