Comparison of assimilation parameters of coppiced and non-coppiced sessile oaks
iForest - Biogeosciences and Forestry, Volume 9, Issue 4, Pages 553-559 (2016)
doi: https://doi.org/10.3832/ifor1824-009
Published: Mar 25, 2016 - Copyright © 2016 SISEF
Research Articles
Collection/Special Issue: IUFRO division 8.02 - Mendel University Brno (Czech Republic) 2015
Coppice forests: past, present and future
Guest Editors: Tomas Vrska, Renzo Motta, Alex Mosseler
Abstract
Coppice forest is an alternative to high forest mainly aimed at the production of firewood with a short rotation period. A new interest in this silvicultural system has arisen with the demand for renewable energy resources. Exploiting the existing root system of the stump, sprouts are advantaged over plants of seed origin, and this advantage could induce changes at the level of a photosynthetic apparatus, especially in young plants. This paper presents a comparison of the photosynthetic ability of young sprouts, young seedlings and mature trees of sessile oak (Quercus petraea (Matt.) Liebl.) growing in a forest stand managed as a coppice-with-standards in the southeast of the Czech Republic. The basic photosynthetic characteristics and transpiration rate at the leaf level were determined using gas-exchange measurement techniques. Data taken in non-limiting conditions were compared with those obtained under limiting soil moisture. The results revealed no differences between the measured parameters of sprouts, seedlings and old trees in non-limiting conditions. Contrastingly, sprouts had the highest photosynthetic capacity and transpiration during drought due to their ability to maintain a higher stomatal conductance as compared with seedlings and old trees. This suggests a better drought tolerance of sprouts compared to seedlings.
Keywords
Introduction
The production of sprouts after cutting, damage or dramatic changes in environmental conditions is a common attribute of temperate angiosperm trees ([6]). Coppicing, the oldest type of forest management, induces the sprouting of basal shoots and the fast production of wood without any subsequent silvicultural treatment ([17]). Due to the exploitation of the stump root system, sprouts rapidly grow in their first years of life ([7]). The height growth rate varies from 0.5 to 1 m per year, depending on many factors, such as the tree species, site conditions, stump age or the timing of cutting ([6]). The sprouting ability of tree species of Central Europe was investigated by Matula et al. ([22]) and Splíchalová et al. ([29]). They reported that the stump sprouting ability of Quercus petraea (Matt.) Liebl. decreased with increasing stump diameter and age. Contrariwise, Carpinus betulus (L.) and Tilia cordata (Mill.) resprouted well from stumps of all diameters. A faster growth rate of sprouts, compared to trees of seed origin, leads to an earlier growth culmination and a shorter rotation period that can range from 5 to 40 years, depending on the purpose. For example, a 5-year rotation period is usually taken for the production of wicker from willow, 15 years for oaks to obtain tan barks and 40 years for fuel production ([5]). Coppice management provides sustainable production of thin stems of lower quality (mainly used as firewood) as compared with those from high forests, with a lower financial profit ([13]); however, in terms of costs for forest cultivation, coppice is cheaper than high forest ([18]).
Interest in coppice management has recently arisen after the claim of the European Union to increase the proportion of energy obtained from renewable resources and to reduce the consumption of fossil fuels ([19]). Short-rotation coppices are often established for afforestation of unused agricultural soils and restoration of landfills ([23]). The most frequently claimed advantage of coppice is an increase in the overall biodiversity of the site, especially in terms of heliophile forest species ([27], [30], [1]). Due to its low managing costs, coppice seems a valuable alternative for small- and medium-sized-forest owners, as well as for the regeneration of stands where seedling establishment is difficult. For example, fast-growing sprouts can be used for soil stabilization on steep slopes ([24]). On the other hand, coppice has several disadvantages compared with high forests, such as the lower quality of timber production, the reduced genetic diversity of sprouts as compared to seedlings, that may lead to a decreased plasticity of the stand in terms of response to environmental constraints ([21]). Moreover, natural regeneration from seedlings can be hampered by sprouts in stands of fast-growing broadleaved species ([31]). Finally, the more frequent removal of the standing biomass in coppice as compared with high forests may lead to an impoverishment of soil nutrients ([17]).
This paper is focused on the comparison under different soil moisture conditions over two growing seasons of the photosynthetic ability and assimilation characteristics of young sprouts, young seedlings and mature trees of sessile oak (Quercus petraea (Matt.) Liebl.) growing in a coppice-with-standards forest located in the southeastern Czech Republic. Our starting hypothesis was that young oak sprouts have higher photosynthetic rate due to the possibility of using resources from the existing stump root system ([26], [32], [21], [7]).
Material and methods
Site description
The stydy site of Sobešice is located in the southeast of the Czech Republic (49° 14′ 42″ N, 16° 35′ 59″ E, 355 m a.s.l.) in the Training Forest Enterprise KrÂtiny of the Mendel University in Brno. This site is characterized by a mean annual temperature of 7.5 °C and a mean annual precipitation from 550 to 650 mm. A study area of 200 × 200 m was established in 2009/2010 in a 73-year-old oak forest, where 95% of the total number of trees was sessile oak of predominantly coppiced origin. The stand was characterized by the presence of seedlings naturally regenerated from seeds of left standards and sprouts grown as a multi-stem coppice from stumps, with an average of 12 sprouts per stump. The area was divided into 16 square-shaped plots with an area of 2500 m2 differing in felling intensity, i.e., from clearcut plots (100% of trees harvested) to medium-harvested plots (76% of trees harvested). The aim of the treatment was to convert the existing forest into coppice and coppice-with-standards with different stand density. More detailed description of the conversion procedures is in Kadavý et al. ([17]). The different stand density of the plots did not affect assimilation parameters at the leaf level and therefore it was not reflected in this study. In 2014, ten sprouts and ten seedlings were harvested to compare their biometric characteristics. The diameter at breast height (DBH) and tree height of ten old trees were measured for each plot (Tab. 1).
Tab. 1 - Age and mean values ± standard deviations of basic characteristics of three studied groups of sessile oak measured in 2014 (n = 10). (H): plant height; (D0.1): sprouts and seedlings stem diameter 10 cm above the ground/stump base; (DBH): the old trees stem diameter at breast height; (LA): plant leaf area.
| Group | Age (years) |
H (m) |
D0.1 or DBH (mm) |
LA (m 2 plant -1) |
|---|---|---|---|---|
| sprouts | 5 | 2.6 ± 0.4 | 32.0 ± 6.5 | 3.2 ± 0.9 |
| seedlings | 5 | 1.7 ± 0.3 | 15.2 ± 1.8 | 0.7 ± 0.2 |
| branches | 77 | 17.1 ± 1.2 | 256.7 ± 36.3 | - |
In situ measurement
The in situ physiological measurements were carried out during three days of the growing season 2013 (20th June, 1st August, 5th September) and two days of 2014 (17th June, 8th August). Days with different level of drought stress were chosen based on measurements along the growing seasons of the following variables: air temperature (°C) and relative humidity (%), measured using a Minikin RTHi (EMS, Brno, CZ), and precipitation (mm) using a MetOne 380 (EMS, Brno, CZ). Soil temperature at a depth of 1.5 cm and the soil volumetric water content in the range 0-6 cm below the ground surface were monitored during the measuring days using the TPD32 penetrate thermometer (°C - Omega, CZ) and the ThetaProbe ML2x soil moisture sensor (m3 m-3 - Delta-T Devices, UK). Four plants from each group (young sprouts, young seedlings and lower branches of old trees) were randomly selected within the plot. On every plant, a sun-exposed and fully developed leaf without sign of damage was measured at 9:00-12:0 a.m. The measurement was repeated on the same leaf in the next measuring day of the growing season. Assimilation parameters, such as the light-saturated CO2 assimilation rate (Amax), the stomatal conductance (gs) and the transpiration rate (E) were measured using a gas-exchange system (LI-6400, LI-COR, USA). The leaves were exposed to the same air flow rate (500 μmol s-1), the ambient CO2 concentration (400 μmol CO2 mol-1) and the saturated photosynthetic photon flux density (PPFD, 1400 μmol photons m-2 s-1 - LI-6400-2 LED light source, LI-COR, USA) in the measuring chamber during all the sampling days. The saturated level of PPFD was chosen high enough so as the CO2 assimilation rate (A) did not increase further above this level, and the measured leaves were commonly exposed to this level during sunny days. Temperature and air humidity in measuring chamber followed the actual environmental conditions. The relationship between A and the intercellular concentration of CO2 (Ci, A-Ci response curve) at a saturated PPFD (1400 μmol photons m-2 s-1) was recorded. A-Ci response curves were not measured only in the first sampling day (20th June), The A-Ci response curve started at ambient CO2 concentration (400 µmol CO2mol-1), decreased stepwise to 200, 100, 50 µmol CO2mol-1 and then increased to 700, 1500 µmol CO2mol-1. The values of the A-Ci response curves were used for the estimation of the light and CO2-saturated CO2 assimilation rate (Asat), the rates of in vivo Rubisco carboxylation (VCmax), electron transport (Jmax) and triose phosphate utilisation (TPU). The equations of Farquhar et al. ([10]) with modification of the Michaelis-Menten constants of Rubisco by Harley et al. ([14]) were used for A-Ci response curve analyses.
Statistical analysis
The obtained data were tested for normality and homogeneity of variances. Two-way analysis of variance (ANOVA) was performed to evaluate the differences in the mean values among different groups (sprouts, seedlings and branches), among different date of measurement and their dependence. Differences among means were tested using the Tukey’s post-hoc test (α = 0.05). All statistical tests were performed using the software package SigmaPlot® version 11.0 (Systat Software, Inc., USA).
Results
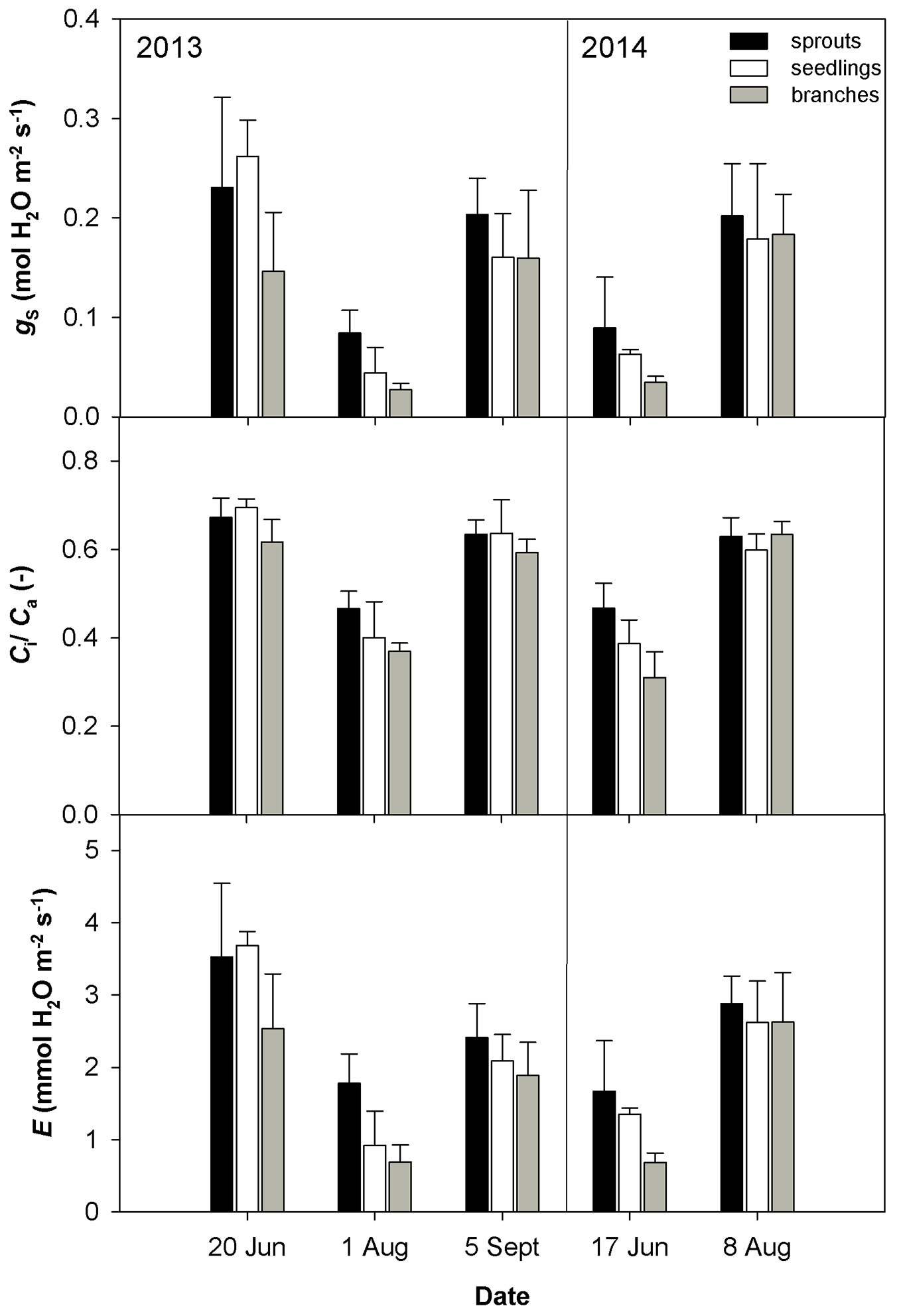
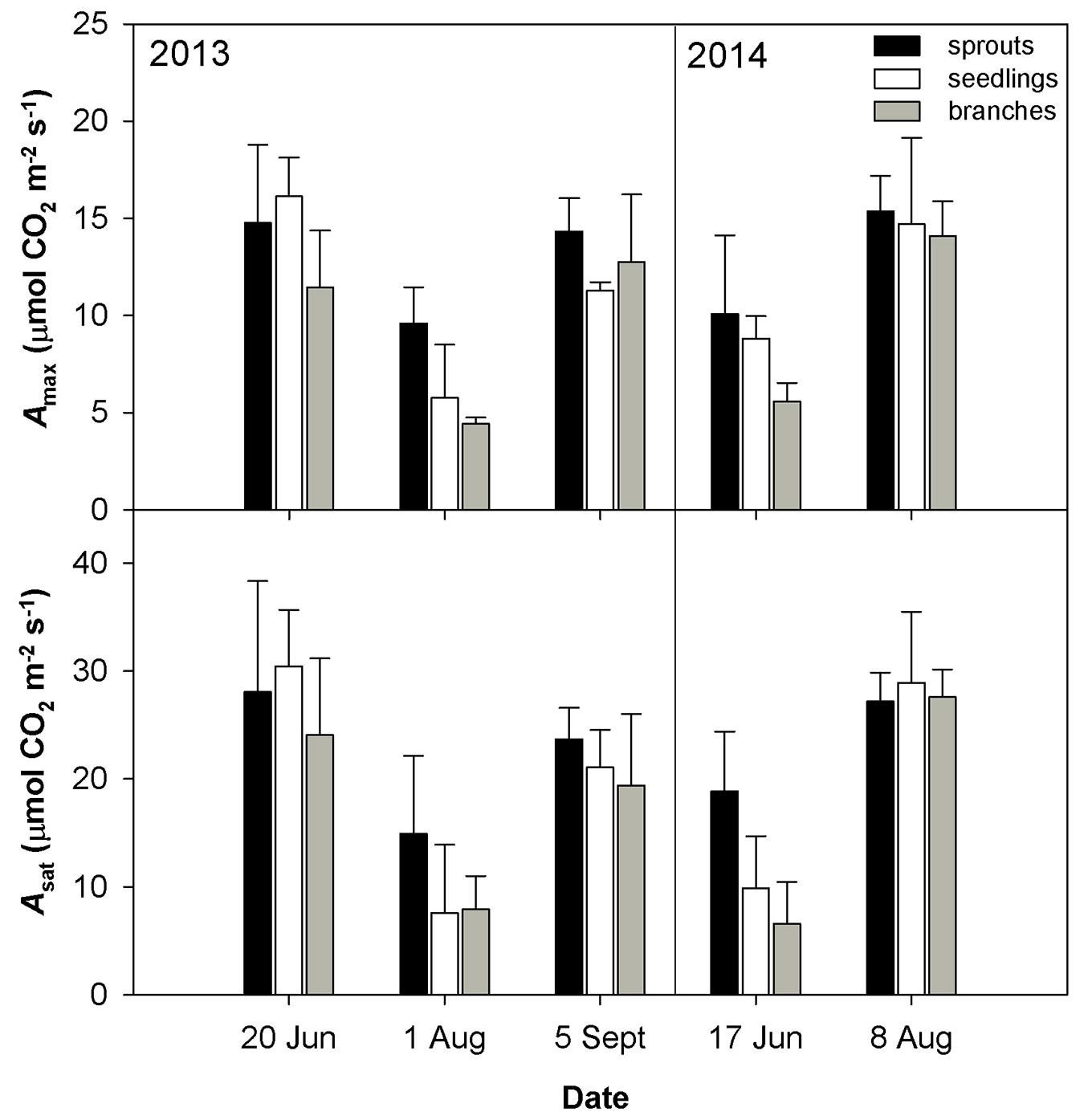
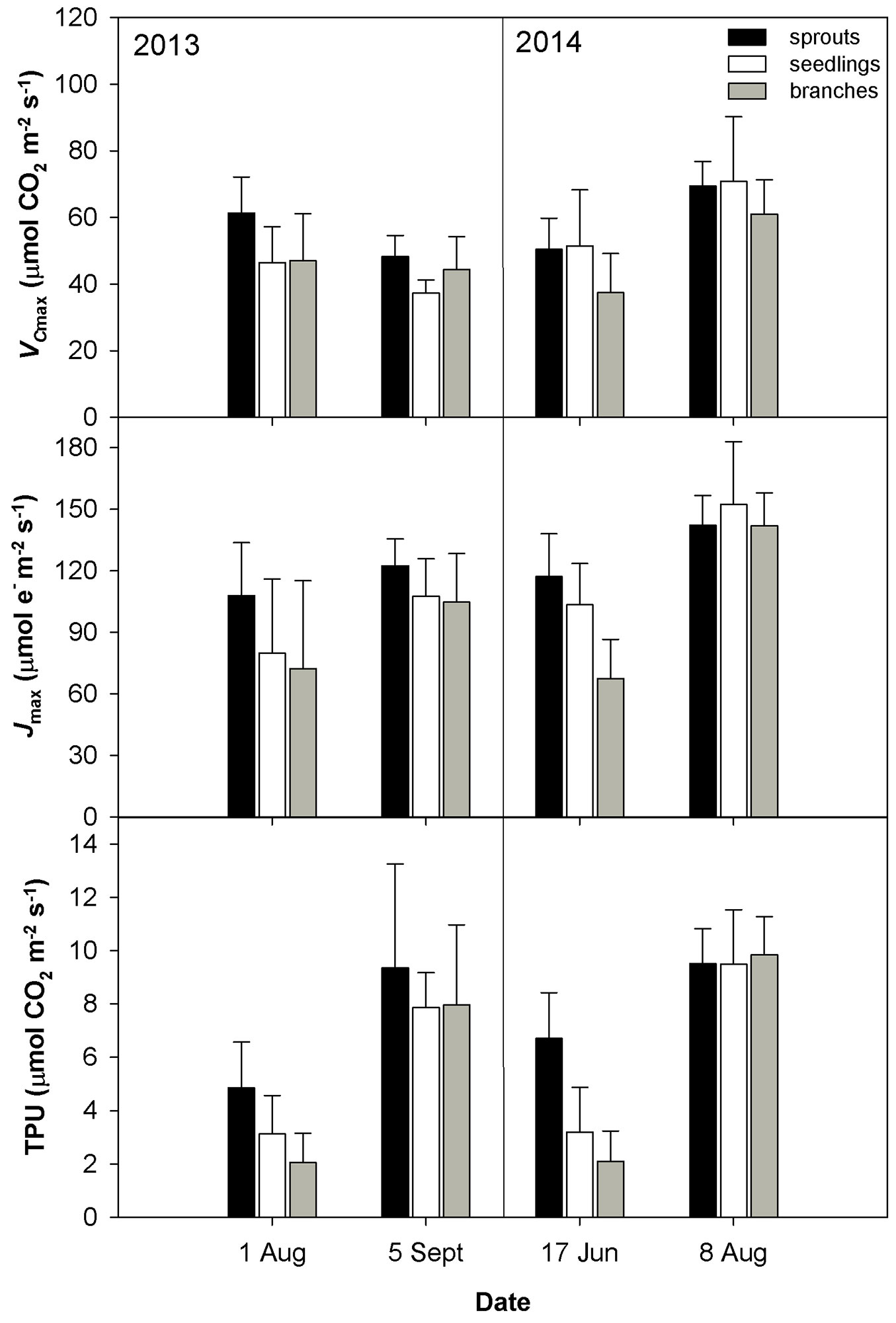
The drought conditions occurred in August 2013 and June 2014 were characterized by low soil volumetric water content (less than 10 m3 m-3) and a high vapor pressure deficit (VPD). The drought in August 2013 was accompanied by higher temperature as compared with June 2014 (Tab. 2). The dry weather induced a significant reduction in gs (Fig. 1) and an increase in the limitation of CO2 diffusion into the leaf, as revealed by the decreasing ratio between the intercellular and air CO2 concentrations (Ci/Ca - Fig. 1). The low gs also caused a decline in E (Fig. 1). The drought conditions brought about a reduction in the CO2 assimilation rate (Amax, Asat - Fig. 2). Amax and Asat were lower in the hot and dry August 2013 than in the dry June 2014. The VCmax seemed not to respond to drought in 2013. In 2014, VCmax was approximately 23-39% lower in June than in August (Fig. 3). Jmax was reduced by drought in both years; however, its reduction was significant only in 2014 (Fig. 3). The rate of utilization of the created triose phosphates (TPU) decreased during drought more than Jmax and VCmax. The reduction of TPU was approximately 29-79 %, the lowest being in sprouts and the highest in branches (Fig. 3).
Tab. 2 - Mean values ± standard deviations of soil volumetric water content (SWC, n = 60), soil temperature (TS, n = 60), vapor pressure deficit based on leaf temperature (VPD, n = 12) and leaf temperature (TL, n = 12) measured in 2013 and 2014.
| Date | SWC (m 3 m -3) |
TS (°C) |
VPD (kPa) |
TL (°C) |
|---|---|---|---|---|
| 20 Jun 2013 | 21.4 ± 6.2 | 27.1 ± 2.4 | 1.6 ± 0.2 | 34.7 ± 0.8 |
| 1 Aug 2013 | 8.0 ± 1.7 | 26.6 ± 3.7 | 2.2 ± 0.3 | 30.5 ± 0.7 |
| 5 Sept 2013 | 14.7 ± 3.2 | 20.7 ± 1.9 | 1.3 ± 0.1 | 25.3 ± 0.8 |
| 17 Jun 2014 | 7.2 ± 1.4 | 18.4 ± 1.5 | 2.0 ± 0.2 | 24.9 ± 0.7 |
| 8 Sept 2014 | 17.3 ± 3.8 | 20.9 ± 1.9 | 1.5 ± 0.2 | 28.4 ± 0.7 |
Fig. 1 - Mean values ± standard deviations (n = 4) of stomatal conductance (gs) measured under ambient CO2 concentration 400 μmol CO2 mol-1, ratio between intercellular and air CO2 concentration (Ci/Ca) and transpiration rate based on gas exchange measurement (E) in 2013 and 2014 conducted on three studied groups (sprouts, seedlings and lower branches of old trees) of sessile oak.
Fig. 2 - Mean values ± standard deviations (n = 4) of light-saturated CO2 assimilation rate (Amax) and light- and CO2-saturated CO2 assimilation rate (Asat) during two years of measurements conducted on three studied groups (sprouts, seedlings and lower branches of old trees) of sessile oak.
Fig. 3 - Mean values ± standard deviations (n = 4) of in vivo Rubisco carboxylation rate (VCmax), electron transport rate (Jmax) and rate of triose phosphate utilisation (TPU) during two years of measurements conducted on three studied groups (sprouts, seedlings and lower branches of old trees) of sessile oak.
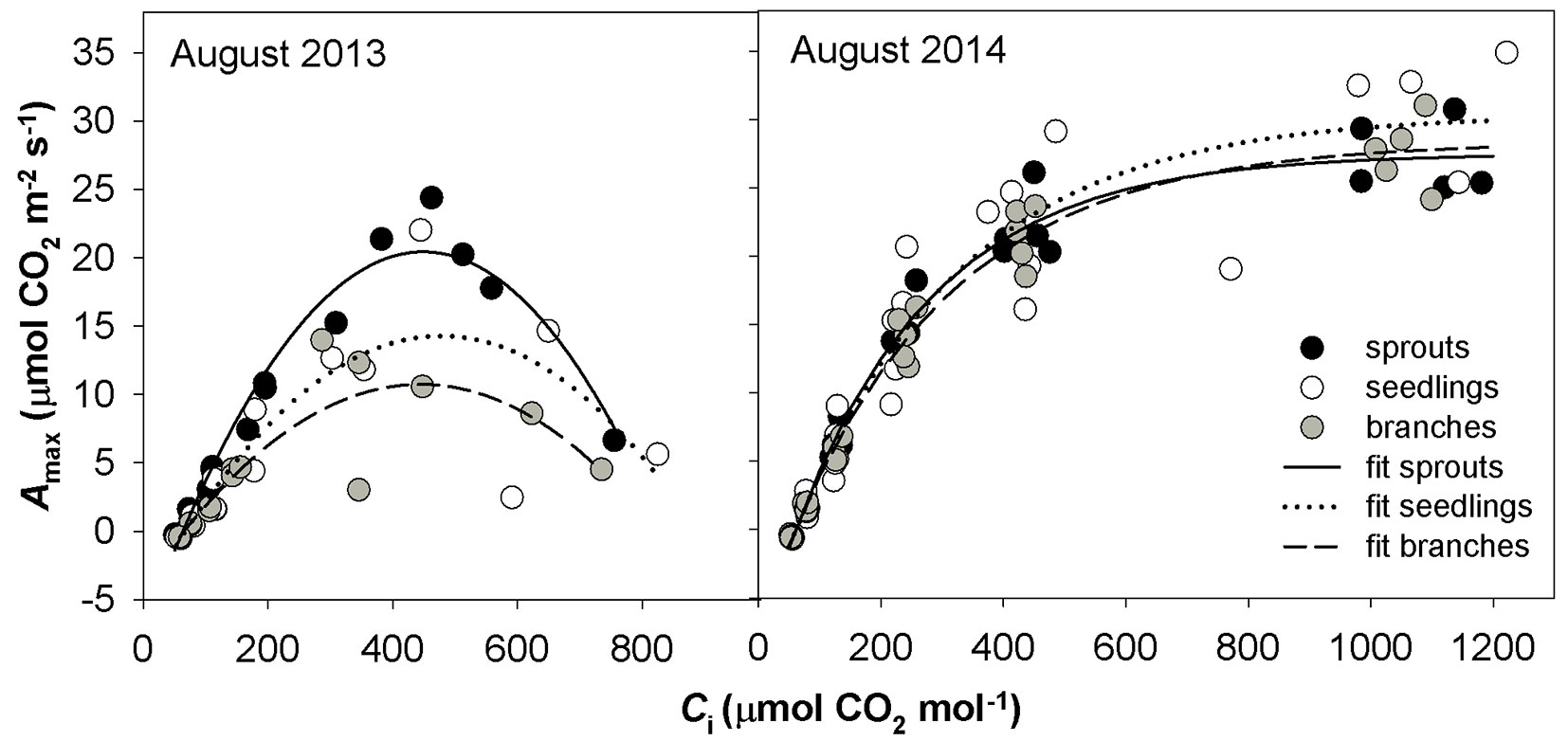
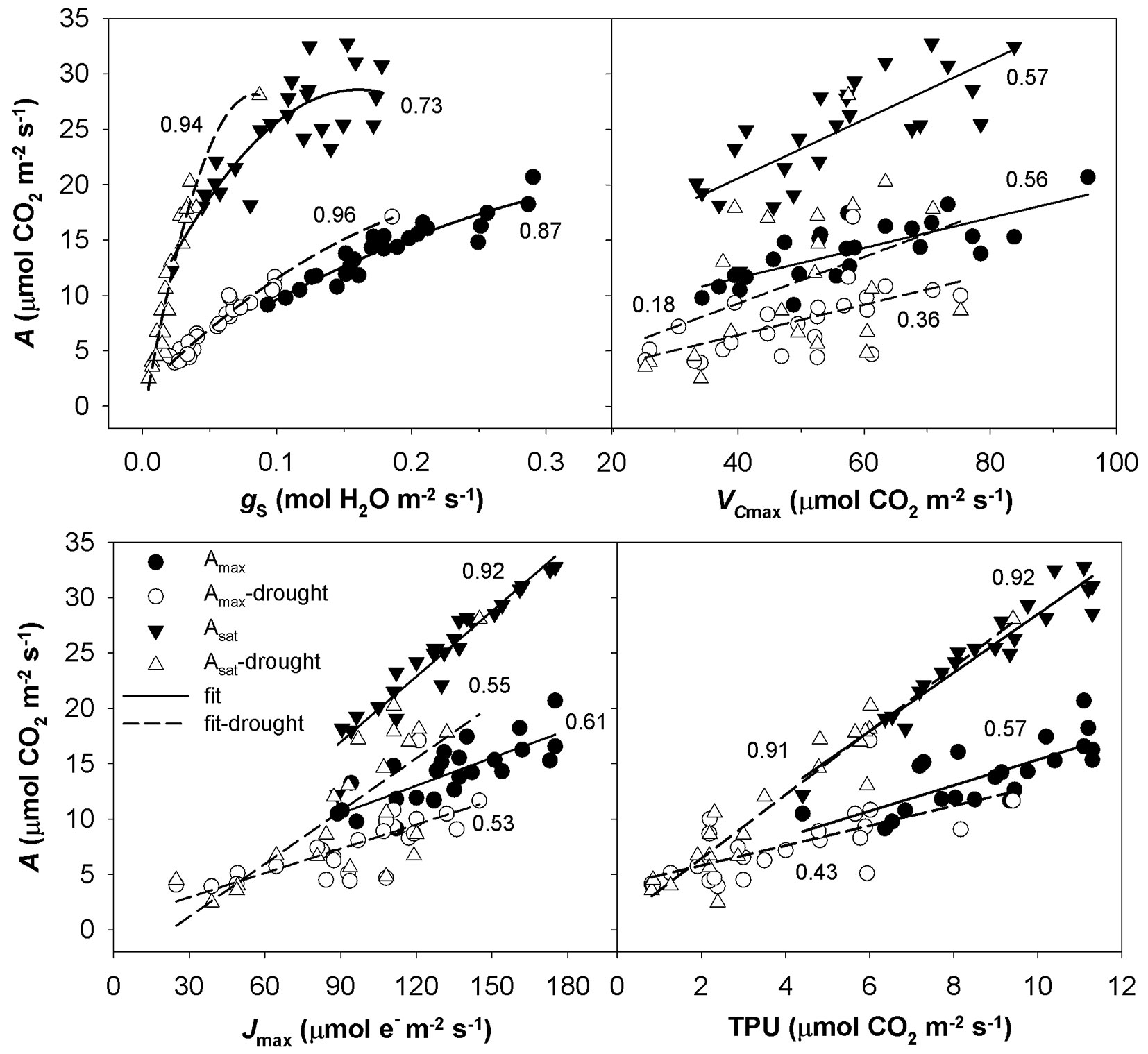
The A-Ci response curves in the hot and dry days of August 2013 showed a reversed sensitivity to Ci, i.e., the rate of photosynthesis did not increase with increasing Ci to a saturated value as for August 2014, but reached a maximum value and started to decrease (Fig. 4). The A-Ci response curves measured in September 2013 were similar to those taken in August 2014. Not all A-Ci response curves observed for June 2014 showed a reversed sensitivity to Ci as in the case of August 2013. To understand which process controls the photosynthesis, a pairwise correlation analysis using the parameters Amax, Asat and gs, Jmax, VCmax and TPU was conducted (Fig. 5). Amax resulted tightly correlated with gs, while Asat was correlated with gs only in the case of drought-affected values. Amax and Asat showed moderate dependence on VCmax only in drought-unaffected months. A high correlation was found between drought-unaffected Asat and Jmax. Both drought-affected and drought-unaffected Asat highly depended on TPU.
Fig. 4 - The relationship between light-saturated CO2 assimilation rate (Amax) and intercellular CO2 concentration (Ci) under drought in August 2013 and in moisture-normal August 2014 in three studied groups of sessile oaks. The graphs indicate individual measured values and their fits.
Fig. 5 - The relationship between CO2 assimilation rate (A) and stomatal conductance (gs), in vivo Rubisco carboxylation rate (VCmax), electron transport rate (Jmax) and rate of triose phosphate utilisation (TPU). The individual measured values are divided in A measured under light-saturated and drought-unaffected condition (Amax) and drought affected condition (Amax-drought), A measured under light- and CO2-saturated and in drought-unaffected condition (Asat) and drought affected condition (Asat-drought). Quadratic and linear function were used to fit data; the coefficients of determination from regression analysis are shown near to the corresponding curve.
Differences in the measured parameters between sprouts, seedlings and lower branches of mature trees were low in the non-dry parts of the growing season (June and September 2013, August 2014). However, such differences increased under drought (August 2013 and June 2014) regardless of the time along the growing season. Under drought conditions, sprouts usually showed the highest values of the studied parameters, and the branches showed the lowest. The greatest differences between the studied groups were found for Asat, TPU, gs and the lowest for VCmax (Fig. 1, Fig. 2, Fig. 3).
Discussion
Our starting hypothesis that young oak sprouts have higher photosynthetic activity as compared to other groups was not confirmed; however, sprouts showed some indication of better coping with drought stress.
Compared with seedlings, sprouts are characterized by a faster above-ground growth during early development ([16], [20], [9]), although this does not entails a higher photosynthetic ability of sprouts. Indeed, seedlings allocate more assimilated carbon to below-ground biomass and root development than plants from coppicing ([8]). Therefore, seedlings can have the same or even a higher photosynthetic rate than sprouts, though their above-ground growth can be smaller ([8]).
In this study, significant differences in the measured assimilation parameters among sprouts, seedlings and branches of mature trees were observed only during periods of drought. Decreased CO2 diffusion from the atmosphere to the site of carboxylation due to stomatal closure is generally considered the main cause for decreased photosynthesis under mild to moderate water deficit ([3], [12]). Different gs under drought between sprouts and seedlings can be caused by different root absorptive area and root depth. Under drought conditions, sprouts benefit from the developed root system, that allows a higher water uptake than that of seedlings ([21]). A lower water limitation and a higher water potential of sprouts compared to seedlings was also confirmed in studies describing the effect of drought after wildfire on seedlings and sprouts survival ([33], [4]). A higher gs allowed the sprouts to maintain a higher rate of use of the assimilates, which led to a lower down-regulation of photosynthesis compared to that of seedlings and branches of old trees. Lower branches of old trees had the lowest gs during drought. It is known that gs usually declines with tree height and age, due to longer hydraulic pathways and an increase in the leaf-area-to-sapwood-area ratio ([28]). A greater reduction in the stomatal conductance in leaves of large trees can be observed during drought in comparison with young plants. Moreover, many sprouts on a single stump (as in our study) may create a more favorable microclimate by self-shading sprouts, thus overcoming hot and dry weather. Drake et al. ([8]) tested the hypothesis that the disproportionate amount of below-ground biomass in coppices can change the leaf-to-air vapor pressure difference or intrinsic water-use efficiency when compared to those of seedlings, but no differences were observed.
Water deficit can diminish the photosynthesis close to zero without a decline in photosynthetic capacity (Asat). Down-regulation of Asat occurs when drought stress is too high or accompanied by another stress, such as high PPFD or heat stress ([2]). In the present study, drought occurred with high PPFD and high temperature, especially in August 2013, when Asat was mostly down-regulated. In such cases the products of photochemical reaction overcomes the capacity for their utilization and insufficient inorganic phosphate (Pi) regeneration limits ATP production that affects electron transport and RuBP regeneration ([15]). Regression analysis showed that Asat was dependent on Jmax and TPU under drought-unaffected condition. Drought led to the disappearance of Asat dependence on Jmax, but dependence on TPU remained and dependence on gs was developed (Fig. 5), although this relationship is unusual during measurements in high Ci. These conditions led to atypical course of A-Ci response curves, especially in August 2013 (Fig. 4). Harley & Sharkey ([15]) explained the reversed sensitivity of photosynthesis to Ci by suppressed photorespiration in high Ci that does not allow Pi regeneration in chloroplasts, which would lead to alleviation of Pi-limitation. Response of plant to drought is very complex process and stomatal closure is not the only regulatory mechanism of photosynthesis. A water deficit reduces the flow rate in the phloem and also reduces the movement of assimilates from mesophyll ([25]). The recent assimilates are accumulated in source leaves, the rate of triose phosphates utilization is reduced and can be followed by down-regulation of the assimilation capacity. Stomata can respond to changes in mesophyll metabolism, so their dependency on assimilation capacity can be observed ([11]).
Conclusion
Although sprouts had a greater above-ground biomass than seedlings of the same age, their photosynthetic ability at the leaf level was not higher than that in seedlings or old trees in water non-limiting conditions. On the other hand, a larger root system of sprouts, as compared to seedlings, likely enabled them to maintain a higher stomatal conductance during drought and led to a lower down-regulation of CO2 assimilation. This study showed better drought tolerance of sprouts compared to that of seedlings.
Acknowledgements
This work was supported by the Ministry of Education, Youth and Sports of CR within the National Sustainability Program I (NPU I), grant number LO1415 and by the project “Coppice as a production and biological alternative for the future” (Reg. No. CZ.1.07/2.3.00/20.0267), funded by the EU and the government of the Czech Republic.
References
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Justyna Pietras
Eva Darenová
Katerina Novosadová
Radek Pokorný
Global Change Research Centre, Academy of Sciences of the Czech Republic, Belidla 986/4a, CZ-603 00 Brno (Czech Republic)
Corresponding author
Paper Info
Citation
Holišová P, Pietras J, Darenová E, Novosadová K, Pokorný R (2016). Comparison of assimilation parameters of coppiced and non-coppiced sessile oaks. iForest 9: 553-559. - doi: 10.3832/ifor1824-009
Academic Editor
Tomas Vrska
Paper history
Received: Aug 26, 2015
Accepted: Feb 11, 2016
First online: Mar 25, 2016
Publication Date: Aug 09, 2016
Publication Time: 1.43 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2016
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 48748
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 40976
Abstract Page Views: 2548
PDF Downloads: 3887
Citation/Reference Downloads: 35
XML Downloads: 1302
Web Metrics
Days since publication: 3615
Overall contacts: 48748
Avg. contacts per week: 94.39
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 5
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Oak sprouts grow better than seedlings under drought stress
vol. 9, pp. 529-535 (online: 17 March 2016)
Research Articles
Effect of drought stress on some growth, morphological, physiological, and biochemical parameters of two different populations of Quercus brantii
vol. 11, pp. 212-220 (online: 01 March 2018)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Photosynthesis of three evergreen broad-leaved tree species, Castanopsis sieboldii, Quercus glauca, and Q. myrsinaefolia, under elevated ozone
vol. 11, pp. 360-366 (online: 04 May 2018)
Research Articles
Drought tolerance in cork oak is associated with low leaf stomatal and hydraulic conductances
vol. 11, pp. 728-733 (online: 06 November 2018)
Research Articles
Magnolia grandiflora L. shows better responses to drought than Magnolia × soulangeana in urban environment
vol. 13, pp. 575-583 (online: 07 December 2020)
Research Articles
Adjustment of photosynthetic carbon assimilation to higher growth irradiance in three-year-old seedlings of two Tunisian provenances of Cork Oak (Quercus suber L.)
vol. 10, pp. 618-624 (online: 17 May 2017)
Research Articles
Evergreen species response to Mediterranean climate stress factors
vol. 9, pp. 946-953 (online: 07 July 2016)
Research Articles
The use of branch enclosures to assess direct and indirect effects of elevated CO2 on photosynthesis, respiration and isoprene emission of Populus alba leaves
vol. 1, pp. 49-54 (online: 28 February 2008)
Research Articles
Multi-temporal influence of vegetation on soil respiration in a drought-affected forest
vol. 11, pp. 189-198 (online: 01 March 2018)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword