Seedling emergence capacity and morphological traits are under strong genetic control in the resin tree Pinus oocarpa
iForest - Biogeosciences and Forestry, Volume 17, Issue 4, Pages 245-251 (2024)
doi: https://doi.org/10.3832/ifor4397-017
Published: Aug 16, 2024 - Copyright © 2024 SISEF
Research Articles
Abstract
Pinus oocarpa is a widely distributed species essential for resin production in Mexico, where demand surpasses supply. This study aimed to identify differences and variation levels of seedling emergence capacity and morphological traits in resin-producing high-yield P. oocarpa trees and estimate their genetic control. Seeds from 72 open-pollinated families were planted using a randomized complete block experimental design. Differences between families were determined and pooled using cluster analysis. We recorded wide differences in seed emergence capacity and morphological traits (cotyledon number and length, and hypocotyl length), allowing to establish three family groups. We also calculated the contribution of the variation sources to the total variance and genetic parameters involved. Our data evidenced high genetic control for all tested variables. We found a moderate and positive genetic correlation between cotyledon length, cotyledon number, and hypocotyl length. We also found a high negative genetic correlation between emergence capacity and hypocotyl length. At the phenotypic level, we found a high and significant correlation between cotyledon length and number. Grouping P. oocarpa into families should aid decision-making for sexual propagation since a high propagation capacity of the high-yield trees is essential for genetic improvement programs. Moreover, we demonstrate that the heritability of the emergence capacity and other morphological traits is high; these traits can be useful for the early selection of high-yield families.
Keywords
Egg-cone pine, Cotyledons, Genetic Control, Genetic Correlation, Genetic Variation, Heritability
Introduction
Pinus oocarpa Schiede ex Schltdl. (egg-cone pine) is naturally distributed along 3.100 km from Sonora, Mexico, through Belize, Guatemala, El Salvador, Honduras, and Nicaragua, between 29° to 12° N latitude ([27]). Outside its natural distribution, this species is grown in Brazil, Colombia, Honduras, Mexico, Venezuela, Asia, Africa, the Caribbean, and Oceania, especially Australia ([18], [10]). P. oocarpa is widely used in the forest industry in Mexico; its wood is used for manufacturing posts, beams, packaging, sheets, plywood, toys, doors, sawmills, toothpicks, pulp for paper, and firewood. The tree also has medicinal and ornamental uses. In Mexico, P. oocarpa is the main resin-producing species, from which turpentine (a liquid substance) and tar (a solid substance) are obtained through an industrial process. These two raw materials are used to manufacture adhesives, tires, paint, rubber, soap, varnish, perfume, and pharmaceutical products ([6]).
Resin is the second most important non-timber forest product in Mexico, representing 39%-44% of national annual non-timber forest profits ([34]). The demand for pine resin in Mexico has increased in recent years, exceeding the national production, while its production has decreased in the last two decades. The demand has thus been mainly supplied by Venezuela, Honduras, China, and Cuba ([6]). The deficit in resin production in Mexico is partly due to the lack of research focused on generating methods and integrative technological strategies to increase its production. A genetic improvement program should be implemented to address supply problems and increase resin production, starting with selecting pine species with the highest resin production.
In Mexico, P. oocarpa has been studied for the magnitude of its wood density variation ([19]) and the genetic control of seedling growth ([37]). In Michoacán, incipient initiatives for genetically improving P. oocarpa to increase its wood and resin production are ongoing ([13], [14]). In 2019, such initiatives were included in a broader research program ([31]). Evaluating the reproductive potential (seed production and germination) of the selected resin trees and estimating the genetic variation, heritability, and genetic correlation between the traits of their progenies is essential for such purpose ([31]).
In Mexico, the forest genetic improvement program begins with the phenotypic selection of superior trees in natural stands. These trees are evaluated through genetic testing, and seed orchards are established. However, no attention has been paid to the reproductive capacity of the selected superior trees, a crucial aspect for forest genetic improvement programs ([40], [39]).
Information on the physical and physiological qualities of seeds from Pinus trees subjected to intensive resin production is insufficient. In P. pseudostrobus Lindl. resin trees, although the germination rate is high, the germination speed indicates wide variation among trees ([26]). In contrast, in the Burseraceae family, resin and exudate extraction negatively affects the species’ reproductive success and particularly decreases the seeds’ viability and germination rate ([12], [1]). Therefore, since the selected P. oocarpa resinous trees ([31]) constitute the base population for the forest genetic improvement program and the seed resources to cover urgent needs in reforestation programs and forest plantations ([40], [39]), it is essential to know the differences between families in seedling emergence. This information will allow decisions to be made to continue the cycle of genetic improvement; that is, the study of the emergence capacity of P. oocarpa resin trees will allow the selection of families without reproductive problems to establish seed orchards in the future. Likewise, gaining insight into the variation level and genetic control of the emergence and morphological characteristics of P. oocarpa seedlings is crucial; if a high level of genetic control is found in these characteristics, the selection of phenotypes with high reproductive capacity will be facilitated ([40], [39]). In turn, this will facilitate the study of the genetic relationships between emergence and morphological characteristics ([15], [11]).
The objectives of this study are to: (i) determine the differences between P. oocarpa families in seedling emergence capacity and morphological characteristics; (ii) determine the level of contribution of families to the total variance of selector characteristics; and (iii) estimate the genetic control of the emergence capacity and morphological characteristics of seedlings through heritability and genetic/phenotypic correlations. Our hypotheses are: (a) the differences between families are large and allow the integration of the high-yield trees into several groups; (b) the contribution of P. oocarpa families to the total variance will be low due to the reduced geographical interval where the high-yield trees were selected; and (c) the characteristics that we evaluated have high genetic control and present a high genetic correlation between them since they depend on the embryo’s viability.
Materials and methods
Tree selection and cone collection
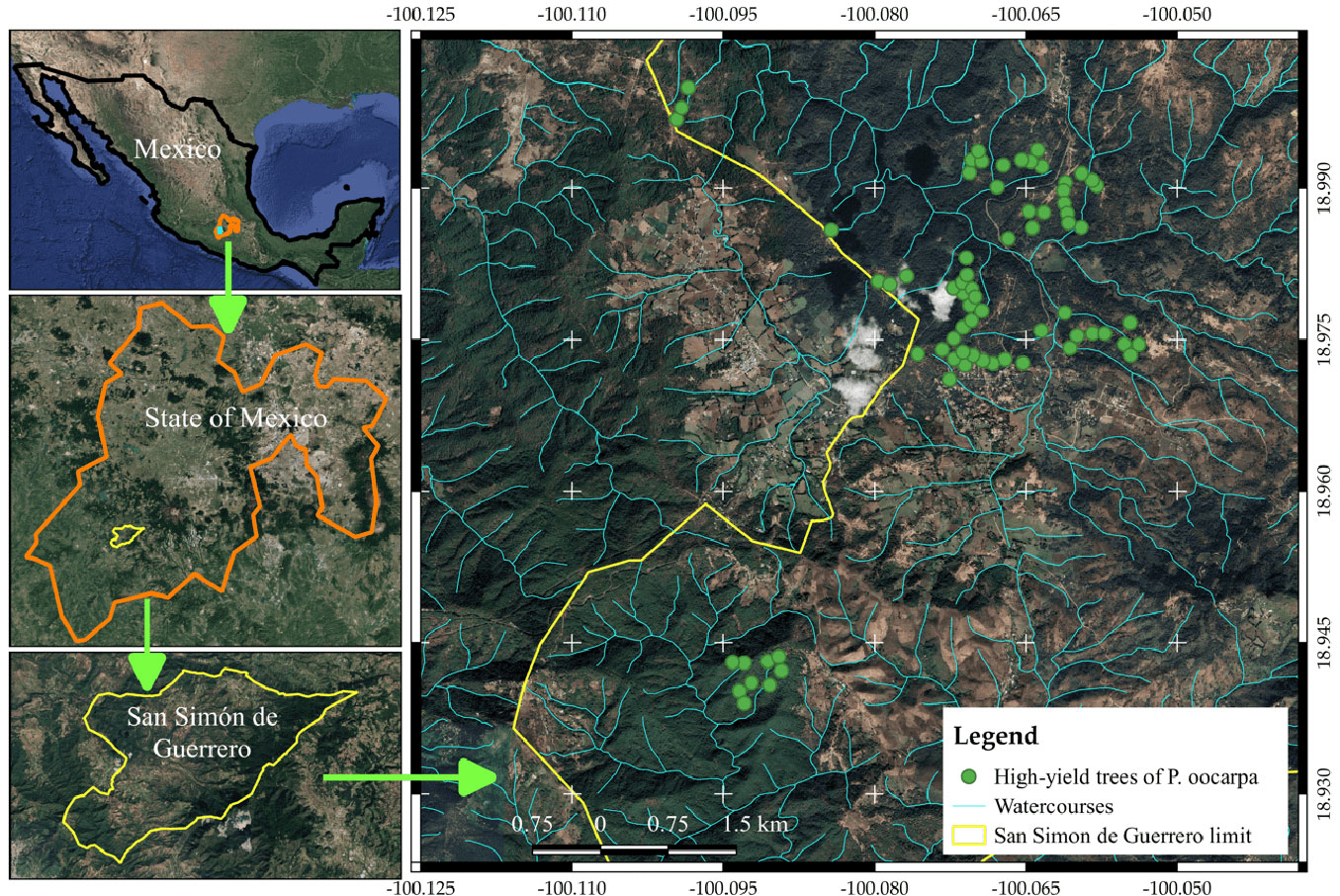
High-yield P. oocarpa trees were selected from the natural forest properties of “Bienes Comunales de San Gabriel Cuentla” in San Simón de Guerrero, Mexico (Fig. 1). The 100 trees with the highest resin production were chosen. Cones from these trees were collected in November-December 2019, and the seeds were cleaned, preserving the trees’ identity. Due to seed availability, only 72 trees were selected for seed emergence trials (Fig. 1). The percentage of vain seeds from these trees was below 18%. The selected trees are distributed at elevations of 1661-1825 m a.s.l. The mean annual temperature in the collecting site varies from 19.2 to 21.5 °C and mean annual precipitations from 1.055 to 1.093 mm ([9]). The soil types were luvisol, cambisol, and regosol ([20]).
Seed planting
To separate seeds with reserves (full seeds) from vain seeds by flotation, a sample of 300 seeds per tree was soaked in distilled water for 24 h. Sixty full seeds were taken from each tree and sown in pairs in 280 ml tubes. Each tube was labelled with a key number (tree number selected in the field and consecutive seed number) that was kept until the end of the trial. The substrate was a mixture of peatmoss®, perlita®, and vermiculita® (60:20:20). Additionally, 5 g·l-1 of Multicote® brand-controlled release fertilizer (06-18-12 + Em) was added. During the first month, samples were irrigated three times a week. Captan WP (1 g·l-1) was applied once a week to avoid fungal attack. All these experiments were conducted in a greenhouse in La Protectora de Bosques (PROBOSQUE), Mexico. The average temperature in the greenhouse was 19.5 °C, with minimum and maximum temperatures of 10 and 35 °C, respectively.
Experimental design and evaluated variables
The experimental design was randomized complete blocks with six repetitions (blocks) per family. The experimental unit comprised ten seeds. All germinated seeds from the same tree were considered as a half-sibs family ([40], [16]).
To determine the emergence capacity (EC), the number of seeds that emerged 30 days after sowing was recorded, using the following equation (eqn. 1):
where Sem is the number of emerging seeds and Stot is the total number of seed tested. Forty-five days after sowing, the number of cotyledons was counted, and hypocotyl length and cotyledon length were measured with a ruler.
Statistical analysis
Differences and grouping of families
Compliance with the assumption of normality and homogeneity of variances of all tested variables (emergence capacity, cotyledon number, hypocotyl length, and cotyledon length) was verified using the Kolmogorov-Smirnov and Levene tests, respectively. None of the variables met any of the assumptions (p < 0.01); therefore, to identify differences between P. oocarpa families, non-parametric RT4 variance analyses and comparisons of range means were performed ([7]). To establish groups of P. oocarpa families, cluster analysis was performed based on Euclidean distances using Ward’s grouping ([25]).
Estimation of variance components
The variance components associated with each variation source were determined with the VARCOMP procedure using the Restricted Maximum Likelihood method of the statistical package SAS® ver. 9.4 ([33]), using the following statistical model (eqn. 2):
where Yijk is the observed value, μ is the overall mean, Bi is the effect of the i-th block, Fj is the effect of the j-th family, Bi×Fj is the effect of the interaction of the i-th block with the j-th family and εijk is the experimental error.
Genetic parameters and correlations
To calculate the genetic parameters and establish correlations, the variance and covariance components were obtained with the same statistical model as above; the block was considered as a fixed effect, the family was a random effect, and interaction block by family was not considered ([11], [29]). The individual heritability (hi2) and family means (hf2) were determined with the following equations ([15] - eqn. 3, eqn. 4):
where σf2 is the family variance, σe2 is the error variance, b is the harmonic mean of the number of seedlings per family.
To avoid overestimating heritability, the additive variance (σA = 3σf2) was calculated with the coefficient of genetic determination 3, since P. oocarpa families come from open pollination and are composed of a mixture of half-sibs and full-sibs ([11], [29]). The standard error of individual heritability (EE(hi2)) and the coefficient of genetic variation (CVg) were calculated using the following equations ([15] - eqn. 5, eqn. 6):
where na is the number of seedlings per family, nf is the number of families, σA2 is the additive variance, bar{X} is the general mean.
The phenotypic correlation coefficient between pairs of variables was obtained with the Pearson’s correlation coefficient and the genetic correlation coefficients were estimated using the Falconer ([15]) equation (eqn. 7):
where σf2(X) and σf2(Y) are the variances of families X and Y, respectively; COVf(X,Y) is the family covariance of those variables, obtained using the following formula ([38] - eqn. 8):
where σf2(X+Y) is the covariance of families of the variable X + Y. The standard error of the genetic correlation was obtained with the following equation ([16] - eqn. 9):
Results
Differences between morphological traits and grouping into families
The general average emergence capacity was 80%, the average cotyledon number was 5.97, the average cotyledon length was 3.29 cm, and the average hypocotyl length was 0.77 cm. All the variables presented significant differences between P. oocarpa families (p < 0.0001). The mean emergence capacity per family ranged from 25% (family 21) to 98% (families 7, 43, and 87). The mean cotyledon number per family ranged from 4.97 cm (family 85) to 6.93 cm (family 34). The mean cotyledon length per family ranged from 2.09 cm (family 21) to 4.17 cm (family 65), and the mean hypocotyl length per family ranged from 0.55 cm (family 84) to 1.08 cm (family 56 - Tab. 1).
Tab. 1 - Overall mean, minimum, and maximum averages of traits evaluated by family groups of resin-producing high-yield trees of P. oocarpa.
| Variables | Overall mean |
Family averages | |||||
|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | |||||
| Min | Max | Min | Max | Min | Max | ||
| Emergence capacity (%) | 80.0 | 66.7 | 98.3 | 25.0 | 85.0 | 68.3 | 98.3 |
| Number of cotyledons | 5.97 | 5.75 | 6.93 | 4.97 | 6.48 | 5.40 | 6.28 |
| Cotyledon length (cm) | 3.29 | 2.94 | 4.17 | 2.09 | 3.40 | 2.70 | 3.60 |
| Hypocotyl length (cm) | 0.77 | 0.64 | 1.08 | 0.55 | 0.88 | 0.65 | 0.88 |
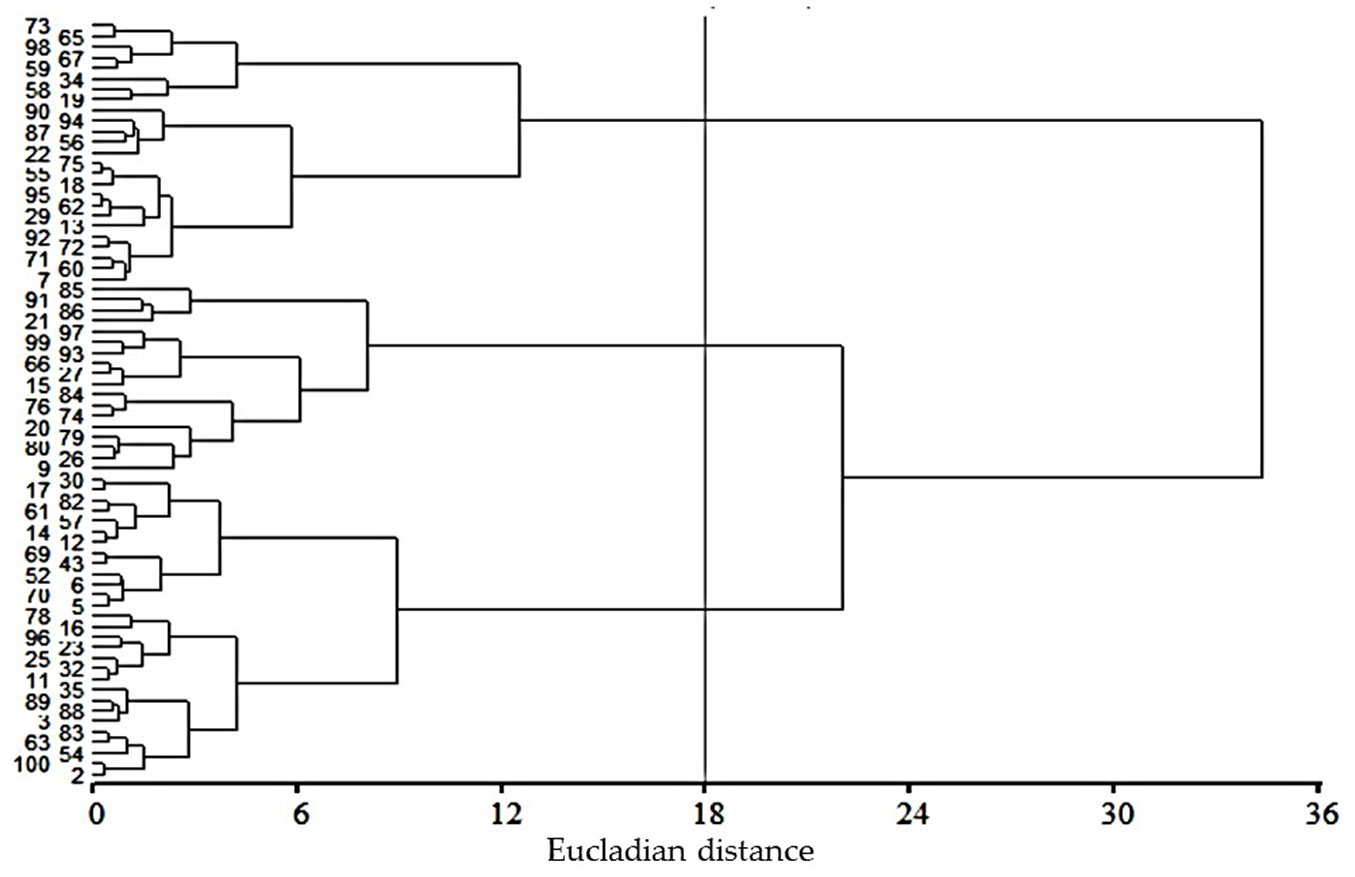
Our multivariate analysis divided P. oocarpa families into three groups: the first comprises 25 families, the second includes 18 families, and the third group has 29 families (Fig. 2). Generally, for all the variables, P. oocarpa families of groups 1 and 2 presented high and low mean values, respectively. Group 3 families presented intermediate mean values (Tab. 1).
Variation
On average, the contribution of P. oocarpa families to the total variance was 15.8%. The average contribution of the block, block interaction per family, and error were 7.6%, 12.3%, and 64.4%, respectively. For all the variables, the contribution of the error to the total variance was higher than the contribution of the family, block, and block by family (Tab. 2). The highest contribution of P. oocarpa families to the total variance corresponded to the cotyledon number, while the lowest contribution was that of hypocotyl length (Tab. 2).
Tab. 2 - Contribution of variance of seedlings morphological variables and emergence of families of resin-producing high-yield trees of Pinus oocarpa.
| Variable | Variance components (%) | Total Variance |
|||
|---|---|---|---|---|---|
| Block | Family | Block× Family |
Error | ||
| Emergence capacity | 0.7 | 15.64 | 11.48 | 72.18 | 0.16 |
| Number of cotyledons | 0.01 | 19.12 | 1.21 | 79.66 | 0.66 |
| Cotyledon lenght | 11.43 | 17.9 | 21.27 | 49.4 | 0.79 |
| Hypocotyl lenght | 18.28 | 10.38 | 15.16 | 56.19 | 0.1 |
| Mean | 7.6 | 15.76 | 12.28 | 64.36 | - |
Genetic control
The individual heritability values (hi2) of the evaluated traits ranged from 0.46 (hypocotyl length) to 0.70 (cotyledon length), while the heritability value of the family means (hf2) varied from 0.67 (hypocotyl length) to 0.70 (cotyledon length). The additive genetic variation coefficient ranged from 10.3% (number of cotyledons) to 36.5% (emergence capacity - Tab. 3).
Tab. 3 - Coefficient of additive genetic variation (CVga), individual heritability (hi2), standard error (EE(hi2)) and heritability of family means (hf2) of morphological variables of seedling and emergence of families of resin-producing high-yield trees of Pinus oocarpa.
| Variables | CVga (%) | h i 2 | EE(hi2) | h f 2 |
|---|---|---|---|---|
| Emergence capacity | 36.45 | 0.53 | 0.05 | 0.7 |
| Number of cotyledons | 10.32 | 0.58 | 0.07 | 0.69 |
| Cotyledon lenght | 20.52 | 0.7 | 0.06 | 0.7 |
| Hypocotyl lenght | 24.06 | 0.46 | 0.07 | 0.67 |
Genetic correlations between the number of cotyledons, hypocotyl length, and cotyledon length were positive, while the genetic correlation between these variables and the emergence capacity was negative. Half of the genetic correlations between variables were low; cotyledon length moderately correlated with hypocotyl length and cotyledon number. Only cotyledon length had a high genetic correlation with emergence capacity, but it was negative (Tab. 4). On the other hand, only the phenotypic correlation between cotyledon length and number was high and significant; the rest of the phenotypic correlation values between variables were low (Tab. 4).
Tab. 4 - Genetic correlations (with standard error, right of diagonal) and phenotypic correlations (with p-values, left of diagonal) between the characteristics evaluated in Pinus oocarpa.
| Variables | Number of cotyledons | Cotyledon length | Hypocotyl length | Emergence capacity |
|---|---|---|---|---|
| Number of cotyledons | - | 0.414 ± 0.046 | 0.169 ± 0.108 | -0.235 ± 0.120 |
| Cotyledon length | 0.539 ± 0.001 | - | 0.544 ± 0.046 | -0.211 ± 0.091 |
| Hypocotyl length | -0.045 ± 0.794 | -0.134 ± 0.435 | - | -0.774 ± 0.221 |
| Emergence capacity | -0.254 ± 0.135 | 0.091 ± 0.599 | -0.06 ± 0.73 | - |
Discussion
Differences and grouping of families
Our first objective was to determine the differences between P. oocarpa families’ seedling emergence capacity and morphological characteristics. The wide differences observed in seed emergence capacity and seedling morphological traits of P. oocarpa trees with the highest resin yields allowed us to establish three groups. This grouping provides important guidelines for the sexual propagation of these trees. A high propagation capacity is essential for the selection of high-yield trees. According to our data, P. oocarpa families of groups 1 and 3 are better than families of group 2 in terms of emergence.
In our study, the average seed emergence capacity of high-yield resin-bearing P. oocarpa trees was higher than that previously reported for the same species ([30]) and P. pseudostrobus Lindl. (78% germination - [2]). However, in our study the emergence capacity was similar to other species, such as P. leiophylla Schiede ex Schltdl. & Cham. and P. ayacahuite var. veitchii (Roezl) Shaw ([17], [23]). Likewise, the overall average emergence capacity was lower than the germination rate of P. pseudostrobus resin trees in Michoacán, Mexico ([26]).
Notably, the average emergence capacity of group 2 P. oocarpa families was lower than in other pine species ([17], [23], [2]), which may result from deficient pollination and a possible inbreeding effect ([4], [3]). Intensive resination might also decrease the physiological quality and, consequently, the viability of these families’ seeds ([12], [1]), warranting further investigations.
A previous study reported that the differences in the number and length of P. oocarpa cotyledons are under strong genetic control ([37]). This information is evidenced by comparing our data with reports on other species. For instance, in our study, the average cotyledon number was the same as that reported for P. oocarpa species in Michoacán ([37]). Also, the general and group average number of P. oocarpa cotyledons was lower than the average number of P. sylvestris L. cotyledons ([36]). The cotyledon number was lower in groups 2 and 3 than in group 1, which had more cotyledons than the mean value reported for P. oocarpa trees ([37]). Additionally, the average (general and by groups) cotyledon length was lower than that of P. oocarpa trees in Michoacán ([37]). Regarding hypocotyl length, group 2 families presented lower values than groups 1 and 3.
The differentiation between families by emergence capacity and seedling morphological traits (e.g., hypocotyl and cotyledon length) has implications for plant production due to a positive relationship between these variables and survival and initial growth in the nursery phase ([21]). Moreover, hypocotyls are considered an early selection trait because they represent the initial growth vigor of the seedlings and an indicator of morphological variation ([21], [36]). Thus, our grouping of P. oocarpa trees into families differentiated by emergence capacity, hypocotyl, and cotyledon length may represent a methodological advantage for future studies.
Variation
The family’s contribution to the total variance indicates a high genetic control for the traits studied here; therefore, selecting between and within families to obtain genetic gains should be feasible. In our study, the variance of cotyledon number and length was similar to that of another population of P. oocarpa ([37]). On the other hand, the family’s contribution to the total variance of emergence capacity was lower than that of P. greggii Engelm. ([24]) and P. leiophylla Schiede ex Schltdl. & Cham. ([17]). The high contribution of the error to the total variance indicated greater variation between plants within the same family, which agrees with reports on P. oocarpa and P. leiophylla ([37]. [17]). The contribution of the block to the total variance indicated a moderate environmental effect for cotyledon and hypocotyl length. However, the block had a very low effect on emergence capacity and cotyledon number since these traits depend on the embryo’s viability and are independent of the environmental effect of the experiment ([32], [36]). Notably, the cotyledon number is not affected by the age of the parent trees ([36]).
Genetic control and correlation between variables
In previous reports ([35], [8]), the heritability values of emergence capacity, number of cotyledons, and cotyledon length have been regarded as “high”; for instance, these variables have heritability values higher than those of growth traits, shape, wood quality, and morphology in forest species ([8], [11], [13], [29]). High heritability values indicate a strong genetic control on the emergence and seedling characteristics of P. oocarpa. These characteristics can thus be useful for the early selection of families ([24]).
In our study, the individual heritability values of cotyledon number and length were similar to those of other P. oocarpa populations (0.89 and 0.84 - [37]). In contrast, the heritability of the mean cotyledon number and length of families with the same characteristics was slightly lower than the values reported for another P. oocarpa population (0.90 and 0.84 - [37]). Similarly, we found that the heritability of cotyledon number and length was lower than the reported heritability in the broad sense in P. sylvestris (0.983 and 0. 956 - [36]). In contrast, the heritability values of the cotyledon number in P. oocarpa were higher than the broad sense heritability (0.503) in Pinus wallichiana A. B. Jack ([28]). Additionally, we found that the individual and family mean heritability values of emergence capacity in P. oocarpa were lower than the broad sense heritability mean of the germinative capacity of P. wallichiana (0.80 - [22]) and Picea sitchensis (Bong.) Car. (0.78 - [5]). However, the final germination rate of P. wallichiana had a lower broad sense heritability (0.665 - [28]) than the heritability value of family means obtained for emergence capacity in the present study.
In our study, the additive genetic variability of emergence capacity and hypocotyl and cotyledon length was higher than the mean additive genetic variability reported for other forest trees (14.7% - [8]). Our high values of the coefficient of additive genetic variation indicate a high genetic variability between families of P. oocarpa trees relative to the population mean, reflecting a high genetic variability of these traits. We found a moderate positive correlation between cotyledon and hypocotyl length and cotyledon length and number. Moreover, the genetic correlation between cotyledon number and length reported here is very similar to that obtained (0.44) for other P. oocarpa populations from Michoacán ([37]).
We also found a high negative correlation between hypocotyl length and emergence capacity, possibly due to common genes affecting these traits and the linkage effect between nearby genes ([15]). From the point of view of plant selection, this correlation is unfavorable; although it is desirable to select families with high emergence capacity, a negative genetic correlation with hypocotyl length could negatively influence plant growth in the nursery phase. This observation suggests that the genetic correlation between seedling morphological traits and growth in the nursery phase should be further investigated in P. oocarpa families selected for high resin production. For instance, an important genetic correlation has been reported between cotyledon length, growth, and height in the rearing phase ([37]). In our study, the significant phenotypic correlation between cotyledon number and length was consistent with their genotypic correlation. The value of the phenotypic correlation between cotyledon number and length was higher than the value reported for another population of the same species (0.036 - [37]).
We did not find a phenotypic correlation between cotyledons number and hypocotyl length, consistent with previous reports for P. oocarpa ([37]) and P. sylvestris ([36]). The low and non-significant phenotypic correlation between cotyledon length and emergence capacity is possible because the genetic component, environmental effect, and interaction between these factors do not favor the genetic response in these variables. However, even without a strong correlation between all the variables, some seedling traits could have an important phenotypic correlation with height and diameter growth in the nursery phase. For instance, in P. oocarpa and P. sylvestris, a significant phenotypic correlation between cotyledon length and number on one hand and seedling height and diameter on the other hand was observed in the nursery stage ([37], [36]). These observations illustrate the importance of studying the phenotypic relationship between seedling traits, height and diameter growth, and other variables of high-yield resin-producing P. oocarpa trees. Grouping P. oocarpa into families, as illustrated here, should aid decision-making for sexual propagation because a high propagation capacity of high-yield trees is essential to any genetic improvement program. In addition, we demonstrate here that the heritability of emergence and other seedling traits is high; therefore, these traits could be useful for selecting superior trees with high sexual reproductive capacity.
Conclusions
Seed emergence capacity and seedling characteristics of P. oocarpa differ widely, allowing the grouping of families into three groups, one of which presents emergence problems. The family’s contribution to the total variance of the evaluated characteristics was moderate and lower than the contribution of the error; therefore, there is a larger variation between seedlings and seeds within families than between families. However, the variation levels indicated the possibility of selecting high-yield P. oocarpa trees between and within families.
A strong genetic control was detected for the emergence and other seedling traits. There was a high and negative genetic correlation between hypocotyl length and emergence capacity that is unfavorable because hypocotyl length influences early growth, and families with high germination capacity and growth are required.
The differences, the grouping, the variation levels between families, and the heritability will allow identifying high-yield individuals in seedling emergence capacity and morphological traits, key aspects for forest genetic improvement programs.
Author Contributions
Conceptualization, M.V.V.-G. and L.M.-G; methodology, M.V.V.-G. and L.M.-G; formal analysis, M.V.V.-G.; investigation, M.V.V.-G. and L.M.-G; resources, M.V.V.-G.; data curation, L.M.-G and G.M.-C.; writing - original draft preparation, M.V.V.-G. and L.M.-G; writing - review and editing, L.M.-G and G.M.-C.; project administration, M.V.V.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute on Forestry, Agriculture and Livestock Research through Fiscal Project No. 1218634780 “Phenotypic selection and establishment of sexual seed orchards of Pinus oocarpa for resin production”.
Data Availability Statement
The data present in this study are available upon request to the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
We thank the authority of Bienes Comunales and resineros of San Gabriel Cuentla for the field support and facilities granted. We also thank Diego Antonio Becerril and Ricardo Conde Ávila of the Protector of Forests of the State of Mexico for their support in the field for data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
CrossRef | Gscholar
Gscholar
Gscholar
Online | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Liliana Muñoz-Gutiérrez 0000-0001-5207-7665
National Institute on Forestry, Agriculture and Livestock Research, Progreso 5, Barrio de Santa Catarina, Coyoacán, 04010 Mexico City (Mexico)
Protector of Forests of the State of Mexico, Rancho Guadalupe Manzana 009, 52148 Llano Grande, State of Mexico (Mexico)
Corresponding author
Paper Info
Citation
Velasco-García Mario V, Muñoz-Gutiérrez L, Martínez-Cantera G (2024). Seedling emergence capacity and morphological traits are under strong genetic control in the resin tree Pinus oocarpa. iForest 17: 245-251. - doi: 10.3832/ifor4397-017
Academic Editor
Claudia Cocozza
Paper history
Received: Jun 01, 2023
Accepted: Mar 27, 2024
First online: Aug 16, 2024
Publication Date: Aug 31, 2024
Publication Time: 4.73 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2024
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 9825
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 5940
Abstract Page Views: 1777
PDF Downloads: 1776
Citation/Reference Downloads: 1
XML Downloads: 331
Web Metrics
Days since publication: 531
Overall contacts: 9825
Avg. contacts per week: 129.52
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2024): 1
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Patterns of genetic variation in bud flushing of Abies alba populations
vol. 11, pp. 284-290 (online: 13 April 2018)
Research Articles
Genetic variation and heritability estimates of Ulmus minor and Ulmus pumila hybrids for budburst, growth and tolerance to Ophiostoma novo-ulmi
vol. 8, pp. 422-430 (online: 15 December 2014)
Research Articles
Comparison of genetic parameters between optimal and marginal populations of oriental sweet gum on adaptive traits
vol. 11, pp. 510-516 (online: 18 July 2018)
Research Articles
Age trends in genetic parameters for growth and quality traits in Abies alba
vol. 9, pp. 954-959 (online: 07 July 2016)
Research Articles
Genetic variation of Fraxinus excelsior half-sib families in response to ash dieback disease following simulated spring frost and summer drought treatments
vol. 9, pp. 12-22 (online: 08 September 2015)
Research Articles
Genetic diversity of core vs. peripheral Norway spruce native populations at a local scale in Slovenia
vol. 11, pp. 104-110 (online: 31 January 2018)
Research Articles
Comparison of range-wide chloroplast microsatellite and needle trait variation patterns in Pinus mugo Turra (dwarf mountain pine)
vol. 10, pp. 250-258 (online: 11 February 2017)
Review Papers
Genetic diversity and forest reproductive material - from seed source selection to planting
vol. 9, pp. 801-812 (online: 13 June 2016)
Research Articles
Genetic control of intra-annual height growth in 6-year-old Norway spruce progenies in Latvia
vol. 12, pp. 214-219 (online: 25 April 2019)
Commentaries & Perspectives
The genetic consequences of habitat fragmentation: the case of forests
vol. 2, pp. 75-76 (online: 10 June 2009)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword