Light acclimation of leaf gas exchange in two Tunisian cork oak populations from contrasting environmental conditions
iForest - Biogeosciences and Forestry, Volume 8, Issue 5, Pages 700-706 (2015)
doi: https://doi.org/10.3832/ifor1306-007
Published: Jan 08, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
Due to diverse environmental conditions, Mediterranean plant populations are exposed to a range of selective pressures that may lead to phenotypic plasticity and local adaptation. We examined the effect of light acclimation on photosynthetic capacity in two Quercus suber (L.) populations that are native to different ecological conditions. Low-light adapted seedlings from both populations were exposed to three light treatments: full sunlight (HL), medium light (ML, 43% sunlight) and low light (LL, 15% sunlight) for one month. Photosynthetic performance was monitored by measuring leaf gas exchange and chlorophyll fluorescence parameters. The light environment influences light-saturated carbon assimilation (Amax) in the leaves of the population inhabiting the hot and dry region (from Gaafour). In contrast, there was no significant difference in Amax between leaves grown in high light and low light from Feija (the population native to a cold and humid climate), which suggests an inability of the Feija population to adjust its photosynthesis to respond to higher irradiance. The inability of the Feija population to adjust its photosynthesis did not result from a light acclimation failure in terms of chlorophyll content and ratio compared with the Gaafour population. Instead, it seems to be the consequence of lower stomatal conductance in the Feija population at HL compared to Gaafour.
Keywords
Quercus suber L., Photosynthesis, Stomatal Conductance, Light, Acclimation
Introduction
Light is one of the most heterogeneous factors affecting plant growth and development, though plants are well-adapted to seasonal and diurnal light fluctuations ([12]). High light can be considered to be a stress factor in Mediterranean ecosystems.
Forest tree species are distributed across a wide range of environmental conditions and subjected to different selective pressures ([29]). Long-term selection can lead to the development of morphological and physiological adaptations to the local environment, giving rise to ecotypic differentiation ([4], [19], [20]). Plant species can cope with this environmental variability by enhancing phenotypic plasticity and adapting to local climates ([37]).
Cork oak (Quercus suber L.) is a sclerophyllous, evergreen Mediterranean tree species adapted to long dry summer conditions with little or no precipitation, maximum temperatures reaching 35-40°C, and irradiance exceeding 2000 µmol m-2 s-1 (PAR) at midday. Such high irradiance can inhibit net carbon uptake, especially in sun-exposed leaves ([8]). Sun leaves are thicker, and their chloroplasts are smaller with lower concentrations of total chlorophyll in comparison with shade leaves. These differences between sun and shade leaves can be readily observed in many species or when a single species is grown under different light intensities ([5]). Walters ([42]) observed that acclimation to high light intensity increases maximum photosynthetic rate, while Price et al. ([28]) suggested that the marked changes in the cytochrome b/f complex can be linked to changes in photosynthetic capacity. Since the 1980’s, it has been thought that the acclimation of plants to their environment is generally dictated by strategies aimed at maximizing the photosynthetic rate ([26]). The factors influencing the photosynthetic rate in both sun and shade plants have been reported by Boardman ([5]) and Ort ([26]). In addition to increasing the efficiency of light absorption and primary photosynthetic reactions, the maximum photosynthetic capacity could be influenced by stomatal and mesophyll resistance to CO2 diffusion ([10], [31]). Furthermore, it has been reported that sun-adapted species show stomatal and mesophyll conductance much higher than shade-adapted species grown under identical conditions with artificial light ([15]) and throughout the seasons ([22]). It is likely that CO2 diffusion is a crucial factor in the photosynthetic differences between shade and sun species.
In this study, we investigated whether habitat of origin has any effect on photosynthetic acclimation to light. For this purpose, we examined the ability to adjust carbon assimilation in response to higher growth irradiance in cork oak (Quercus suber L.) seedlings originating from two Tunisian populations representing a marked climate gradient.
Materials and Methods
Origin and experimental design
In October 2010, Q. suber L. acorns were collected from two populations originating from contrasting environments in the northwestern provinces of Tunisia (Tab. 1). The first site, the National Park of Feija (36° 30′ 00″ N, 8° 20′ 00″ E), is located in the northern extent of the Kroumirie Mountains and is characterized by a cold and humid climate. The average temperature is 7 °C in January and can drop to 0 °C, which allows for year-round snowfall. The second site is located at Gaafour (36° 32′ 19″ N, 9° 32′ 40″ E) in the southern hills and plains around Siliana, and it is characterized by a semiarid climate with moderate winters and hot dry summers. Immediately after collection, acorns were planted in the greenhouse of the National Research Institute for Rural Engineering, Waters, and Forestry under low light conditions (LL, 15% of full sunlight). On October 1, 2013, low-light adapted seedlings that had been grown in 5 L pots containing a mixture of equal parts of soil and compost were randomly assigned to one of three light treatments for one month: (1) high light (100% natural incident irradiance, HL); (2) medium light (43% of full sunlight, ML); and (3) low light (LL, 15% of full sunlight). In the ML and LL treatments, light levels were obtained through the use of layers of neutral shade-cloth, while seedlings were left uncovered in the HL treatment. Photosynthetically active radiation was measured (Li-190, Li-Cor Bioscience, Lincoln, NE) at midday on October 23, 2013, and it ranged between 1630-1810 µmol m-2 s-1, 634-766 µmol m-2 s-1 and 215-315 µmol m-2 s-1 in the HL, ML and LL treatments, respectively. Differences in maximum and minimum daily temperatures between treatments did not exceed 4°C (data not shown). Irrigation to saturation was provided manually each day. To ensure homogeneity of soil humidity between light treatments and provenances during the experiment, soil water content was monitored weekly by time domain refractometry (TDR, Trase system I, Soil moisture Equipment Corp., USA) in a subsample of pots. Mean volumetric soil water content of the pots containing seedlings in all three light treatments was approximately 25-30%. Each block containing 20 seedlings per light treatment was periodically moved to minimize the possible effects of within-block light variability. All experiments were carried out on fully expanded, mature leaves that had developed before the light treatment.
Tab. 1 - Climatic characteristics of the seed source locations and mean seedling height (n = 9) of the studied cork oak populations.
| Provenance | Altitude (m) | Mean annual rainfall (mm) |
Mean annual temperature (°C) |
Seedlings mean height (cm) |
|---|---|---|---|---|
| Feija | 550-1555 | 1217 | 14.3 | 74.66 ± 4.17 |
| Gaafour | 276 | 480 | 17.6 | 68.77 ± 1.90 |
Gas exchange measurements
The photosynthetic response of the leaves to varying levels of photosynthetic photons flux density (PPFD) was measured at the ambient CO2 concentration (400 ppm) with an open infrared gas analysis system (Li-Cor 6400-40 equipped with a red-blue LED source; Li-Cor Inc., Lincoln, NE, USA). Light response curves were measured for attached leaves at 25 °C at an airflow rate of 300 cm3 min-1. The vapor pressure deficit was kept at 1.2 ± 0.2 kPa. Each cork oak leaf was allowed to reach a steady state at an incident light level of 1200 µmol m-2 s-1 PAR. We then changed the PPFD, and net photosynthesis (An) was recorded at each PPFD level once it became stable. Stomatal conductance (gs) and transpiration (E) were also recorded concurrently. Instantaneous water-use-efficiency (WUE) was calculated as An/E (µmol CO2 µmol-1 H2O). Photosynthetic capacity was estimated by light-saturated net photosynthetic rate (Amax) as determined by fitting An/PPFD curves using a three component exponential function (see [23] - eqn. 1):
where An is the net photosynthetic rate, and Amax is the maximum photosynthetic rate. For each light curve, the apparent quantum yield on the basis of incident light (Φ) was calculated as the initial slope at the 3 lowest PPFD values (between 0 and 100 µmol m-2 s-1). The light compensation point (LCP) was estimated from the x-axis intercepts.
Chl fluorescence measurements
In vivo chlorophyll a fluorescence emissions in 30-min dark-adapted leaves were measured with a portable modulated chlorophyll fluorometer (Opti-Sciences, OS-30p+) at predawn and midday. After adaptation to the dark, the modulated fluorometer allows the accurate measurement of minimum fluorescence (Fo) using a weak, modulated light that is too low to induce photosynthesis. In this state, photosystem II is maximally oxidized. A subsequent saturating flash of white light (3500 µmols) reduces all available PSII reaction centers, and the maximum fluorescence (Fm) during the saturating light radiation is recorded. The maximum photochemical efficiency of PSII was calculated as the ratio of the light-induced variable and the maximum fluorescence of chlorophyll, Fv/Fm (eqn. 2):
Photosynthetic pigments
The same leaves used for the measurement of gas exchange were sampled for the assessment of the total content of chlorophyll and carotenoids. Leaf tissue of known area (measured using a leaf area meter, Portable Laser, Model Cl-202) and fresh weight was incubated in 80% acetone until all of the chlorophyll was visibly extracted. Spectrophotometer readings of the extracts were obtained at 750, 663, 645 and 453 nm to determine the chlorophyll a and b and total carotenoid contents using the equations of Arnon ([3]). Chlorophyll and carotenoid concentrations were estimated on the basis of fresh weight and leaf area.
All experiments were repeated at least 3 times, and the mean values and standard errors of the data are shown. Statistically significant differences at the 5% level were calculated with the Student’s t-test using the software package SigmaPlot® (Systat Software Inc., San José, CA, USA).
Results
Photosynthetic pigments
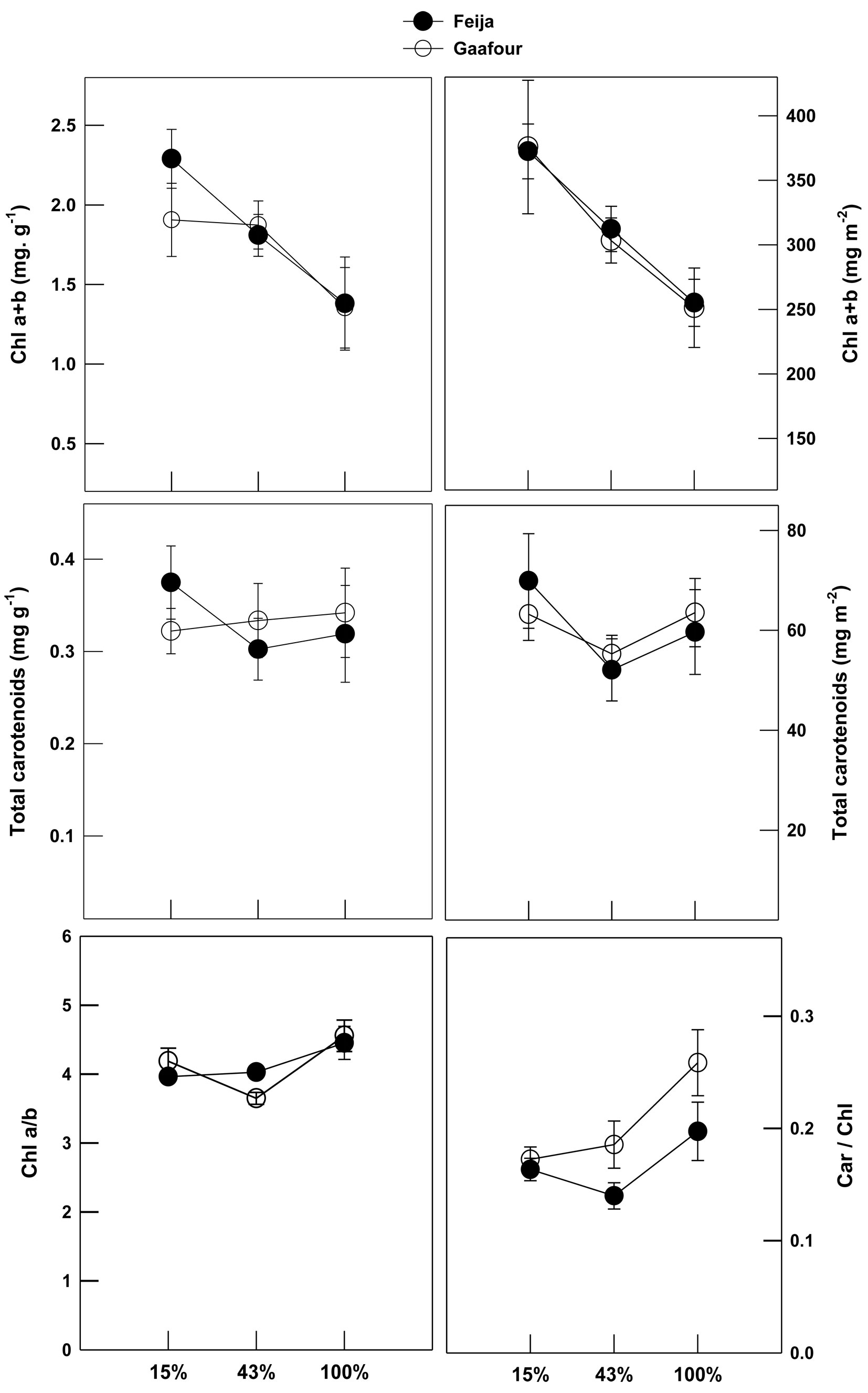
Total chlorophyll concentration on the basis of fresh mass basis and leaf area declined significantly as growth irradiance increased for both populations (Fig. 1). The area and mass-based carotenoid concentrations were not affected by the different light treatments for either provenance. The ratio of chlorophyll a/b remained unchanged at different light treatments in the Feija population, but it decreased in Gaafour leaves at 43% sunlight. The Car/Chl ratio did not vary with increasing light in the Feija populations. In contrast, there was a significant increase in the Car/Chl ratio in HL in the Gaafour population.
Fig. 1 - Leaf pigments content: Chl a+b and total carotenoid concentrations per unit leaf area and fresh weight and chlorophyll a/b and carotenoids/total chlorophyll (Car/Chl) ratios. Values shown represent the mean of at least three replicates with standard errors.
Photosynthetic response to growth under different light levels
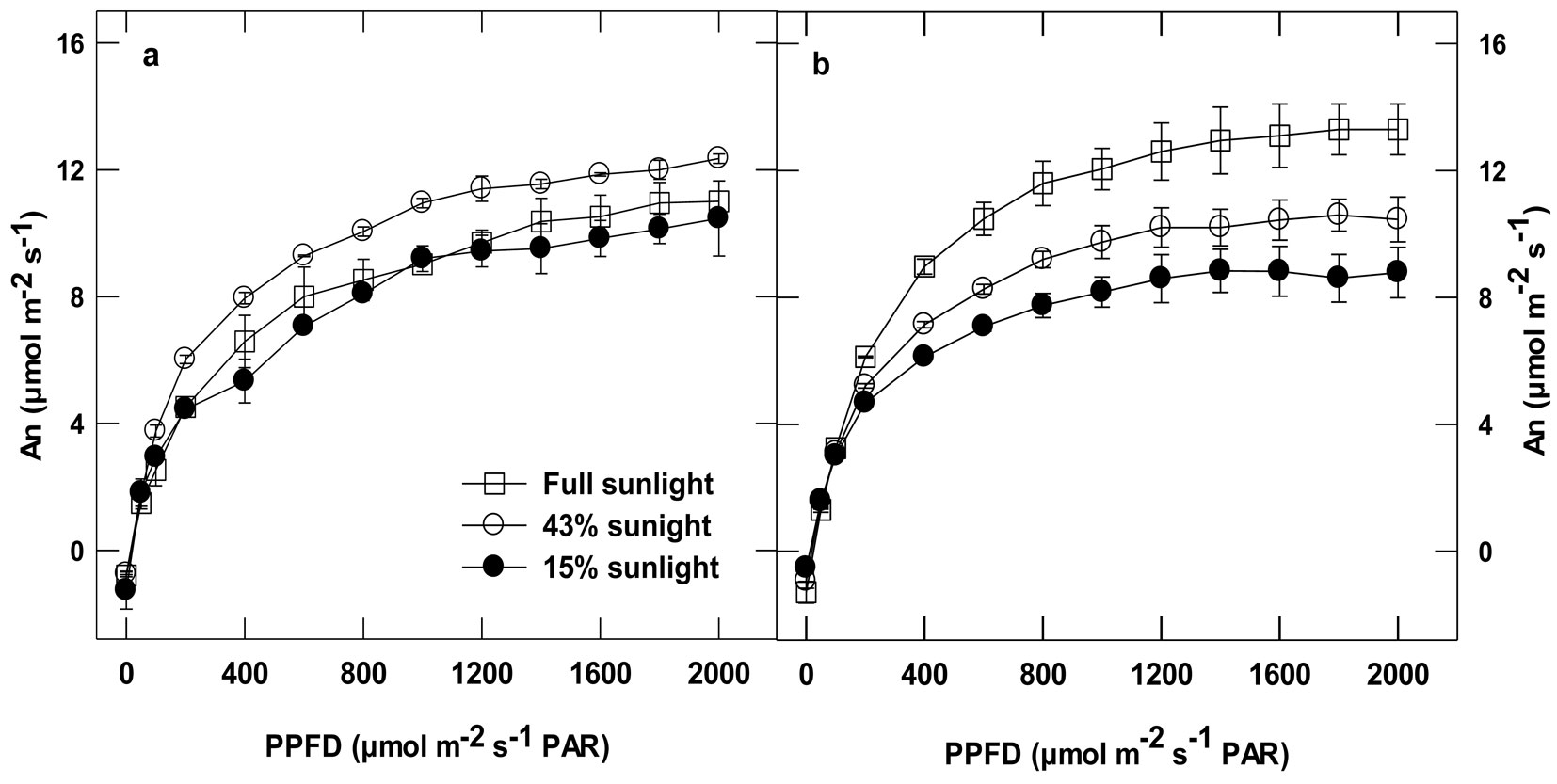
Photosynthesis light curves showed a marked acclimation response to the growth irradiance in the Gaafour but not in the Feija leaves (Fig. 2). Net carbon uptake in the Gaafour leaves at full sunlight was higher than in those grown at LL and ML, light intensities ≥ 200 µmol m-2 s-1 PAR. There was no significant change in the light compensation point (LCP) in the Feija leaves (Tab. 2). In the Gaafour leaves, LCP increased proportionally to the intensity of the growth light. Apparent quantum yield (Φ) only increased for HL-grown plants of Gaafour provenance compared to the counterparts grown in low light (Tab. 2). In contrast, the apparent quantum yield increased at ML only in plants of Feija provenance in comparison with plants grown at LL. Furthermore, maximum applied PPFD completely saturated photosynthesis only in the Gaafour population under LL and ML conditions. As shown in Fig. 2, net photosynthesis rate (An) was higher in Feija than Gaafour leaves grown at low and medium light but significantly lower when grown at high light. This suggests that the capacity to acclimate to high light was different between provenances because the assimilation rate across light regimes only increased in Gaafour seedlings.
Fig. 2 - Light response curves: Net carbon assimilation (An) of attached leaves measured at different PPFD levels in an atmosphere of 400 ppm CO2 and 25°C from Feija (a) and Gaafour (b). Values are means ± SE of at least three independent measurements.
Tab. 2 - Light-saturated photosynthesis (Amax), light compensation point (LCP) and apparent quantum yield (Φ) in leaves of Gaafour and Feija provenances grown under three light regimes. Different uppercase letters indicate a significant difference between provenances in the same light environment, whereas lowercase letters indicate significant differences between light environments in the same provenance (p ≤ 0.05, Student’s t-test).
| Parameter | Feija | Gaafour | ||||
|---|---|---|---|---|---|---|
| LL (15%) | ML (43%) | HL (100%) | LL (15%) | ML (43%) | HL (100%) | |
Amax (µmol CO 2 m -2 s -1 ) |
10.36 ± 0.8 Aa | 11.92 ± 0.27 Ab | 11.20 ± 0.29 Aa | 8.84 ± 0.77 Aa | 10.45 ± 0.71 Ba | 13.30 ± 1.10 Bb |
LCP (µmol photons m -2 s -1 ) |
19.30 ± 2.00 Aa | 14.60 ± 1.60 Aa | 19.60 ± 2.00 Aa | 12.33 ± 1.40 Ba | 18.30 ± 2.60 Ab | 23.30 ± 2.30 Ab |
Φ (mol CO 2 mol -1 photon) |
0.034 ± 0.001 Aa | 0.046 ± 0.001 Bb | 0.032 ± 0.003 Aa | 0.035 ± 0.001 Aa | 0.036 ± 0.004 Ba | 0.045 ± 0.002 Bb |
In summary, net carbon assimilation was comparable in Feija leaves grown under both low and high light conditions, which suggests that there was no stimulation of An at HL compared to LL. Obviously, the Gaafour population is able to acclimate its photosynthetic activity to high light whereas the Feija plants cannot.
Variations of gs, E and WUE in response to light
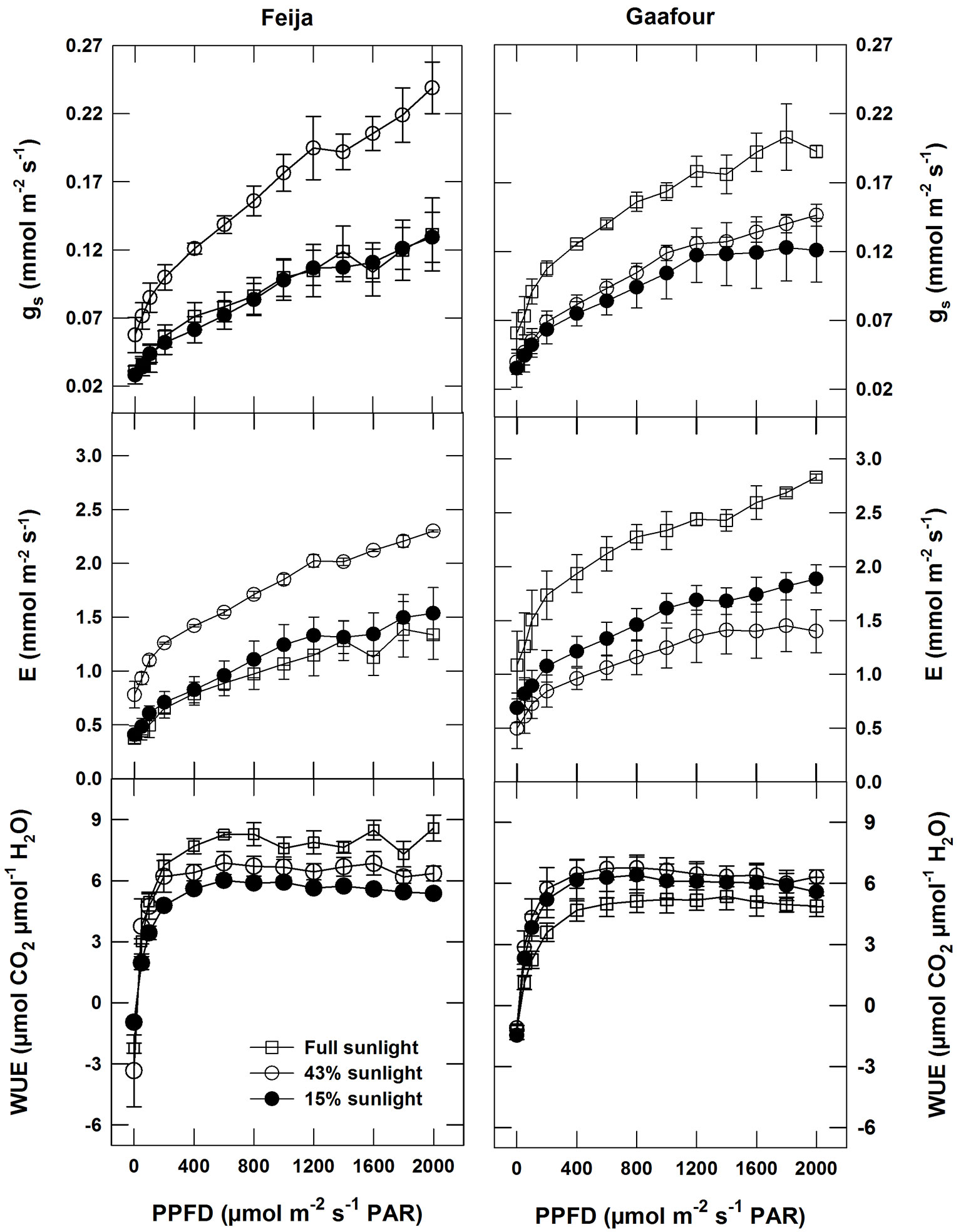
Stomatal conductance (gs) and transpiration (E) changed similarly as PPFD increased in plants of both Feija and Gaafour provenance. Under full sunlight, gs and E increased in Gaafour plants compared with those grown under low and medium light (Fig. 3). For the Feija plants, the conductance and transpiration curves of leaves grown in medium light (with the highest carbon assimilation) were above the curves of the low- and high-light leaves (Fig. 3).
Fig. 3 - Response of gas exchange to light: Stomatal conductance (gs), transpiration rate (E) and instantaneous water use efficiency (WUE) as a function of PPFD in an atmosphere of 400 ppm CO2 and 25 °C. Values are means ± SE of at least three independent measurements.
Intrinsic water use efficiency (WUE) was calculated from the gas exchange measurements. For the three light treatments, WUE was completely saturated at low light intensities (< 400 µmol m-2 s-1) in both Feija and Gaafour plants. A remarkable increase in WUE in full sunlight was detected in the Feija population (Fig. 3), but there was a slight decrease in the Gaafour group (Fig. 3). Plants of both Gaafour and Feija provenance had the same WUE under LL and ML conditions.
Chl fluorescence
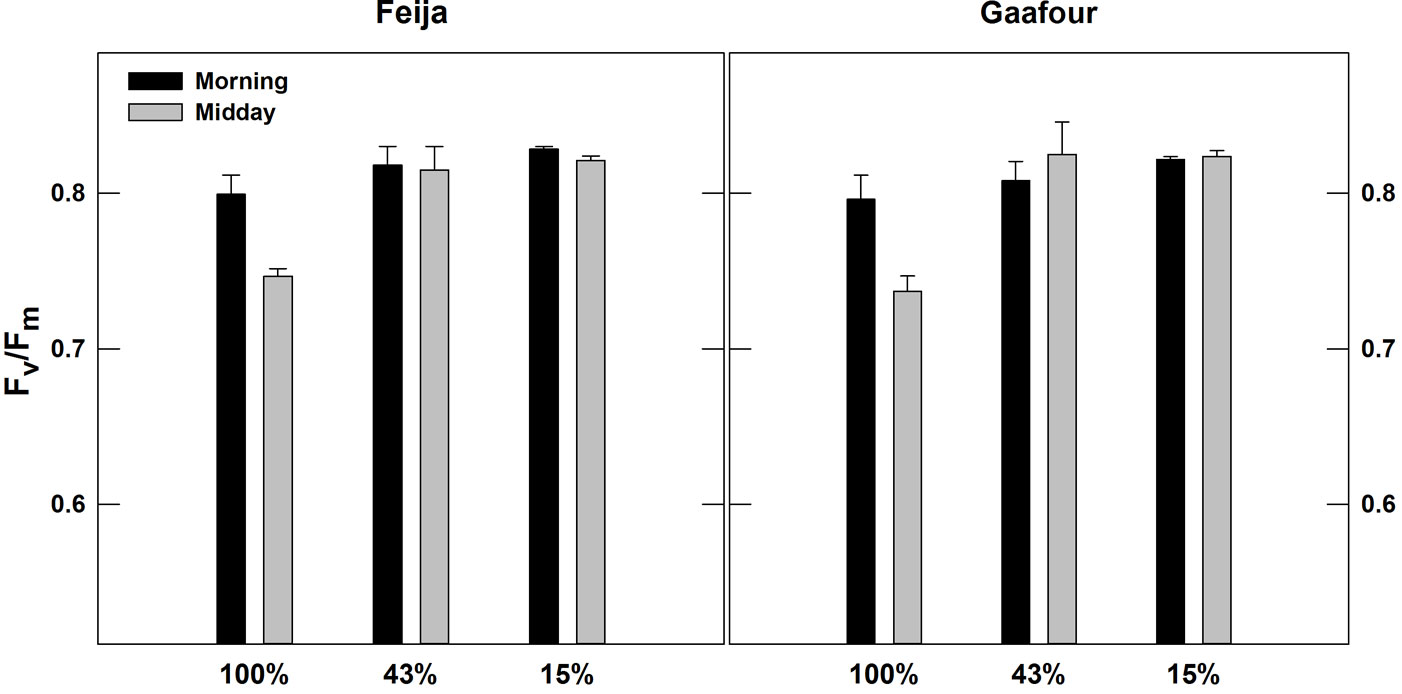
The diurnal evolution of the maximum photochemical efficiency of PSII (given by Fv/Fm in dark-adapted leaves) is shown in Fig. 4. The Fv/Fm ratio remained constant at approximately 0.8 in LL and ML in both Feija and Gaafour leaves. The growth in HL decreased Fv/Fm significantly (p < 0.05) by 7.6 and 8.4% at midday in the plants of Feija and Gaafour provenance, respectively.
Fig. 4 - Chlorophyll fluorescence: maximal photochemical efficiency of PSII (Fv/Fm) measured in the morning (07:00) and at midday (12:00). Values are means ± SE of five independent measurements.
Discussion
Intraspecific differences in response to high light
This study shows that the sensitivity of photosynthetic capacity to light availability was distinguishable between two populations belonging to different natural habitats. The Feija population was the least tolerant to HL, exhibiting severe restrictions to gas exchange and performance of photosynthesis. The plants of Gaafour provenance were the most tolerant to the high levels of irradiance but showed low photosynthetic capacity when treated with medium and low light. Interspecific differences in acclimation to HL among Mediterranean species have been studied previously ([18], [17], [39], [40], [12], [33], [30]). It has been suggested that species acclimate to full sunlight conditions by increasing their photosynthetic capacity ([21]). However, interpopulation differences in the response to the light environment have been analyzed less frequently. In cork oak, some studies have investigated the possible existence of intraspecific variability as an adaptation to contrasting regional climate conditions. For example, Staudt et al. ([35]) examined intraspecific variability in the emission of volatile organic compounds during drought in Tunisian cork oak populations originating from contrasting climatic conditions. More recently, Ramirez-Valiente et al. ([29]) tested the phenotypic plasticity of 13 contrasting European cork oak populations under conditions of differential water availability. Additionally, the interpopulation variability in temperature response was investigated for other cork oak populations ([2]). The results found in this study show that the ecological origin of the populations determined their capacity for light acclimation. Blaguer et al. ([4]) also found that populations of Q. coccifera, an evergreen oak originating from different localities in the Mediterranean, differ in their plastic response to light intensity. They concluded that differences among populations suggested an ecotypic differentiation towards less phenotypic plasticity in the most homogeneous light environments ([4]). More recently, Aranda et al. ([2]) found differences in cold tolerance among populations of cork oak. Our findings indicate a divergence in the plasticity of Q. suber populations in response to the light environment. In this species, a change in plasticity may be a consequence of habitat-based selection, as proposed by Blaguer et al. ([4]).
Light acclimation of chlorophyll content and ratio
Light acclimation is commonly associated with an increase in the Chl a/b ratio ([1]), which reflects a relatively lower ratio of light harvesting complexes (LHCs) to reaction centers ([27]). However, in our study, the Chl a/b ratio was not significantly affected by high light treatment in plants of either provenance (Fig. 1). This result is in accordance with several studies of other species that suggest that the Chl a/b ratio presents low plasticity in response to light ([38], [22]). In contrast, Gomez-Aparicio et al. ([12]), who studied the light response of several Mediterranean tree saplings, showed that area- and mass-based carotenoid concentrations and Chl a/b ratio increased with increasing light intensity. In shade leaves, the increased Chl concentration has been interpreted as an adjustment to low light regimes ([22], [6]). This marked decrease in total chlorophyll content with increasing light was shown on both area and dry mass basis by Valladares et al. ([38]) in two other oak species (Quercus ilex and Q. coccifera). We suggest that the inability of the Feija population to adjust its rate of photosynthetic carbon assimilation to higher growth irradiance was not related to lower light acclimation of the chlorophyll content; it seems to be linked to other factors.
Apparent quantum yield and maximum photochemical efficiency of PSII
The maximum photochemical efficiency of PSII (given by Fv/Fm in dark-adapted leaves) in leaves grown in low and medium light remained constant during the day in both populations. However, it decreased slightly but significantly (p < 0.05) at the midday depression in leaves grown in full sunlight (Fig. 4). It was demonstrated that Fv/Fm of cork oak sun leaves exhibited a slight, reversible midday depression that co-occurred with the increase of light and temperature ([8]). This slight reduction in the midday Fv/Fm in seedlings of both populations exposed to high light may be due to the light-induced downregulation of PSII ([25], [24]). Faria et al. ([8]) showed that Q. suber leaves have the ability to dissipate excessive light energy at midday through a non-photochemical mechanism. Cork oak, a widely distributed forest tree species in the Mediterranean basin, is not well adapted to low temperatures, and a large decline in Fv/Fm in populations originating from warmer sites was observed ([2]). However, the species is able to maintain maximum photochemical efficiency of PSII during periods of drought ([9]). Additionally, Ghouil et al. ([11]) demonstrated its tolerance to high temperatures.
The highest maximum photosynthetic rates and apparent quantum yields in Feija plants were achieved under the ML condition (Tab. 2). However, plants of Gaafour provenance acclimate better to the HL condition. The apparent quantum yield reflects “photochemical efficiency” ([13]), which means that under the HL condition, 31 and 22 photons are required for the assimilation of one molecule of CO2, in the Feija and Gaafour populations, respectively. As indicated, the lower Φ can explain the poor performance of Feija plants in comparison to Gaafour at full sunlight. Generally, light-demanding species have a higher light-saturated leaf photosynthetic rate than shade-tolerant species ([14]). This observed difference in photosynthetic plasticity between the two study populations suggests a limited ability of the Feija seedlings to benefit from high light conditions.
Stomatal resistance to CO2 diffusion may become limiting in leaves of Feija provenance grown in high light
The highest stomatal conductance (gs) values and transpiration rates (E) were found in leaves grown in high light and medium light for the Gaafour and Feija populations, respectively, which correlates well with the highest Amax recorded in both groups. This is consistent with the results of Marenco et al. ([21]) who found higher gs and E in Swietenia sun leaves, suggesting that this species is more efficient than Dipteryx species in controlling water loss and CO2 uptake in open fields.
It is well known that the opening and closing of the stoma enables terrestrial plants to adjust their gas exchange (uptake of CO2 and evaporation of water) in response to environmental and physiological conditions ([34]). The results of this study show that only Gaafour plants could use the additional supplied light for increased CO2 fixation under the HL condition, as indicated by its enhanced assimilation rates. Feija plants may dissipate more energy via non-photochemical quenching, which would be an interesting subject for further study. For plants of this provenance, high light intensity affected the efficiency of CO2 diffusion into intercellular spaces, so the photosynthetic performance of the leaves declined. Considering the often observed, coordinated regulation of leaf hydraulic conductance (Kleaf), gs and photosynthetic capacity ([16], [32], [36]) in response to the light environment, we suggest that the limitation of photosynthetic rates in the Feija plants under HL compared to the Gaafour plants can be attributed to their lower gs. It was shown that, in the sun leaves of Myrtus communis, CO2 uptake was improved by increased stomatal density ([22]). Additionally, Evans ([7]) results show that, under high irradiance, the lower assimilation rate of sclerophyllous leaves was accompanied by a lower concentration of chloroplastic CO2 (Cc) compared to mesophyllous leaves.
The response of WUE to the light treatments was different to the response of An. A positive relationship was observed between WUE and growth irradiance in the population from Feija. However, the seedlings of Gaafour provenance had the lowest WUE under the HL condition, to which they were better acclimated. This result is in contrast with conclusions from previous studies suggesting that increasing WUE is an important aspect of plant acclimation to high light ([14]). In species of the genus Acer, acclimation to high light can be characterized by traits that maximize WUE rather than only maximizing leaf net carbon gain ([22]). In our study, Gaafour seedlings grown under HL have higher Amax and gs, which is generally found in light-demanding species ([41], [23]). However, there was a trend toward higher stomatal control of water loss in HL Feija seedlings.
In conclusion, a distinct difference in photosynthetic plasticity was observed in response to the light environment between both of the studied populations. Gaafour seedlings exhibited the highest photosynthetic rate in high light, and there was a difference in carbon assimilation across light regimes that is consistent with high physiological plasticity. However, Feija seedlings were better acclimated to low and medium-light environments. This suggests that populations originating from semi-arid sites can benefit more from high light conditions than populations from humid sites. Additional studies of more populations are necessary to corroborate this hypothesis.
List of abbreviations
Amax, light-saturated carbon assimilation; HL, high light; LL, low light; ML, medium light; PAR, photosynthetic active radiation; An, net photosynthesis; PPFD, photosynthetic photon flux density; LED, light emitting diode; Ca and Ci, external and intercellular CO2 molar fractions, respectively; gs, stomatal conductance; E, transpiration; WUE, water use efficiency; LCP, light compensation point; Φ, apparent quantum yield; Fo, minimum fluorescence yield; Fm, maximum fluorescence yield; Fv/Fm, ratio of variable to maximum fluorescence called the “maximum quantum yield of PSII photochemistry”; Car, carotenoid; Chl, chlorophyll.
Acknowledgments
This work was supported by the National Research Institute for Rural Engineering, Waters, and Forestry in Tunis, Tunisia. We wish to thank Dr. Peter Streb (Université Paris-Sud 11, Ecologie, Systématique et Evolution, UMR-CNRS 8079, Orsay, France) and Dr. Kumud Bandhu Mishra (CzechGlobe - Global Change Research Center, Academy of Sciences of the Czech Republic) for carefully reading the manuscript. The constructive comments of two anonymous referees are gratefully acknowledged.
References
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Habiba Khiari
Khaoula Ben Baaziz
Zouheir Nasr
National Research Institute for Rural Engineering, Waters, and Forestry, Box 10, Ariana 2080 (Tunisia)
Field Crop Laboratory, Carthage University, National Institute for Agricultural Research of Tunisia (INRAT), Rue Hédi Karray, 2080 Ariana (Tunisia)
EL Manar University, Faculty of Science, Department of Biology, Tunis (Tunisia)
Corresponding author
Paper Info
Citation
Rzigui T, Khiari H, Abbes Z, Baaziz KB, Jaouadi I, Nasr Z (2015). Light acclimation of leaf gas exchange in two Tunisian cork oak populations from contrasting environmental conditions. iForest 8: 700-706. - doi: 10.3832/ifor1306-007
Academic Editor
Silvano Fares
Paper history
Received: Apr 03, 2014
Accepted: Sep 04, 2014
First online: Jan 08, 2015
Publication Date: Oct 01, 2015
Publication Time: 4.20 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 56215
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 47398
Abstract Page Views: 3511
PDF Downloads: 3895
Citation/Reference Downloads: 21
XML Downloads: 1390
Web Metrics
Days since publication: 4042
Overall contacts: 56215
Avg. contacts per week: 97.35
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 4
Average cites per year: 0.36
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Adjustment of photosynthetic carbon assimilation to higher growth irradiance in three-year-old seedlings of two Tunisian provenances of Cork Oak (Quercus suber L.)
vol. 10, pp. 618-624 (online: 17 May 2017)
Research Articles
Drought tolerance in cork oak is associated with low leaf stomatal and hydraulic conductances
vol. 11, pp. 728-733 (online: 06 November 2018)
Research Articles
Photosynthesis of three evergreen broad-leaved tree species, Castanopsis sieboldii, Quercus glauca, and Q. myrsinaefolia, under elevated ozone
vol. 11, pp. 360-366 (online: 04 May 2018)
Research Articles
Gas exchange characteristics of the hybrid Azadirachta indica × Melia azedarach
vol. 8, pp. 431-437 (online: 17 December 2014)
Research Articles
A new approach to ozone plant fumigation: The Web-O3-Fumigation. Isoprene response to a gradient of ozone stress in leaves of Quercus pubescens
vol. 1, pp. 22-26 (online: 28 February 2008)
Research Articles
Stomatal morphometry of Andean species and their relationship with spatial variation
vol. 18, pp. 327-334 (online: 03 November 2025)
Research Articles
Growth and physiological acclimation to shade in young plants of Adesmia bijuga Phil., a critically endangered species in central Chile
vol. 14, pp. 307-312 (online: 01 July 2021)
Short Communications
Variation in growth, photosynthesis and water-soluble polysaccharide of Cyclocarya paliurus under different light regimes
vol. 10, pp. 468-474 (online: 04 April 2017)
Research Articles
Developing stand transpiration model relating canopy conductance to stand sapwood area in a Korean pine plantation
vol. 14, pp. 186-194 (online: 14 April 2021)
Research Articles
Role of photosynthesis and stomatal conductance on the long-term rising of intrinsic water use efficiency in dominant trees in three old-growth forests in Bosnia-Herzegovina and Montenegro
vol. 14, pp. 53-60 (online: 28 January 2021)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword