Spruce budworm biological and nutritional performance responses to varying levels of monoterpenes
iForest - Biogeosciences and Forestry, Volume 6, Issue 6, Pages 310-314 (2013)
doi: https://doi.org/10.3832/ifor0956-006
Published: Jul 16, 2013 - Copyright © 2013 SISEF
Research Articles
Abstract
Dose effect of six monoterpenes (α-pinene, bornyl acetate, camphene, δ-3-carene, terpinolene, tricyclene) found in the foliage of host trees was tested on sixth-instar spruce budworm (Choristoneura fumiferana (Clem.)) using artificial diet. The larval mortality, growth and food utilization have been observed. Two monoterpenes, α-pinene and δ-3-carene caused 22 and 12% mortality respectively at concentrations found in balsam fir foliage. Bornyl acetate and camphene reduced larval survival when their concentration was higher than the foliage. Terpinolene and tricyclene have no effect on mortality. All six tested monoterpenes reduced larval growth rate. Spruce budworm tried to minimize this negative growth impact by increasing his digestibility in presence of camphene and δ-3-carene, and by increasing his efficiency of conversion of ingested-digested food with α-pinene and bornyl acetate. These results support the traditional theory that monoterpenes are a defense agent against spruce budworm and that each monoterpene has a different mode of action and effects which are not necessarily proportional to its concentration.
Keywords
Introduction
Spruce budworm (Choristoneura fumiferana (Clem.)) is an important defoliator of coniferous forests in eastern North America that can severely damage balsam fir (Abies balsamea (L.) Miller) and three spruce tree species (Picea glauca (Moench) Voss, P. mariana (Mill.) BSP, P. rubens Sarg. - [20]). Monoterpenes are among the foliar secondary metabolites deployed by these host plant species to defend against spruce budworm. Concentrations of these compounds, like others present in the host plant foliage, are subject to spatial and temporal change. It is well known that terpenoid concentrations in foliar tissues generally increase with light intensity ([22], [5], [14]), with nutrient availability ([4]), and following stand thinning, for example, in balsam fir ([2], [16], [12]).
Bauce et al. ([1]) showed that monoterpenes are positively correlated with the strong resistance offered by young balsam fir trees against budworm attack, compared to mature individuals. Kumbasli et al. ([15]) simulated this phenomenon under laboratory conditions using an artificial diet. They found that high monoterpenes concentrations that reflected the levels of monoterpenes found in young balsam fir trees increased spruce budworm mortality, while decreasing spruce budworm growth rate and efficiency of food conversion. The authors used a mixture of ten synthetic monoterpenes that represented levels of foliage monoterpenes (per dry mass), which were encountered in young and old balsam fir trees. While the defensive role of this monoterpene mixture was demonstrated in this study, but it did not provide information regarding the effects of each monoterpene found in the foliage or its most effective concentration. The mode of action varied, depending on the molecule and also its concentration. For example, Cates et al. ([9]) measured dosage effects of bornyl acetate and β-pinene on the western spruce budworm (Choristoneura occidentalis). These authors reported that bornyl acetate reduced both growth and survival, β-pinene increased budworm growth rates by functioning as a feeding stimulant. All these studies show that spruce budworm is affected in its feeding behavior by monoterpenes, but there is little information on the individual effect of these compounds on the nutritional indices and mortality.
We performed an experiment under laboratory conditions using an artificial diet to determine the individual dosage effects of six monoterpenes (α-pinene, bornyl acetate, camphene, δ-3-carene, terpinolene, tricyclene), which were naturally found in the host tree foliage, on food utilization and survival of sixth-instar spruce budworm larvae.
Materials and methods
Tab. 1 - Monoterpenes concentrations measured in A. balsamea foliage and doses used in the experiment. (a) sources: [1], [2], [6], [7], [8].
| Monoterpenes | % dry weight | |
|---|---|---|
| Min and max % observed in foliage (a) |
Doses used | |
| α-pinene | 0.05 - 0.71 | 0 - 0.6 - 1 - 3 |
| Bornyl acetate | 0.019 - 0.49 | 0 - 0.3 - 0.8 - 2.5 |
| Camphene | 0.011 - 0.33 | 0 - 0.2 - 0.5 |
| δ-3-carene | 0 - 0.58 | 0 - 0.2 - 0.5 - 1.5 |
| Terpinolene | 0 - 0.046 | 0 - 0.03 - 0.05 - 0.15 |
| Tricyclene | 0.003 - 0.057 | 0 - 0.04 - 0.09 - 0.3 |
The principal monoterpenes that are produced by host tree foliage and their concentrations have been determined in several previous studies ([1], [2], [6], [7], [8], [15]). Based on these data, six monoterpenes (α-pinene, bornyl acetate, camphene, δ-3-carene, terpinolene, tricyclene - Sigma-Aldrich, Milwaukee, WI, USA) were tested at different concentrations (Tab. 1). Two different levels were selected for each monoterpene in order to test the effect of concentrations within and exceeding their natural range in the foliar tissue. The exact concentrations were determined based on the preliminary study, concentration ranges, and methodology of the laboratory study (i.e., incorporation of monoterpene to the artificial diet). Artificial diet was prepared as described by McMorran ([18]) and each monoterpene concentration was incorporated into the diet by hexane solubilization. A diet without monoterpenes was also prepared and considered as the control for each monoterpene molecule. The artificial diet was poured into 37 mL cups (Solo Cup Company, Chicago, IL, USA) and five additional cups were used to determine the dry mass of food provided to the larvae. Each diet cup was weighed before and after feeding, and the difference was multiplied by the food dry mass ratio to determine the quantity of dry food that had been ingested. We focused upon sixth-instar larvae to understand the effects of monoterpenes because 87% of spruce budworm total food consumption occurs during this larval stage ([19]). Further, Carisey & Bauce ([7]) found that foliar monoterpene concentrations had no effect on nutritional indices calculated for fifth-instar larvae. The monoterpenes used were in a volatile form, with preliminary tests showing that 7 days was a sufficient period of time for deploying the diets without marked decreases in their monoterpene concentrations. Spruce budworm larvae were obtained from the rearing facility of the Great Lakes Forestry Centre, Canadian Forest Service (Sault Ste. Marie, Ontario, Canada). Twenty-five sixth-instar females were individually reared in each monoterpene assay, with larvae being monitored twice a day to record mortality and nutritional indices measurements (initial and final larval mass, ingested food, faeces excreted) over 7 days (duration that covers the last instar of spruce budworm). Insect rearing conditions were maintained at 23 °C, 65% relative humidity, and a 16 h: 8 h LD photoperiod. Nutritional indices were expressed on a dry-mass basis according to Waldbauer ([24]), and included (eqn. 1 to 5):
where RGR is the relative growth rate, RCR is the relative consumption rate, D is the digestibility, ECI is the efficiency of conversion of ingested food, ECD is the efficiency of conversion of digested food, G is the mass gained (= final mass - initial mass), MW is the mean larval mass [= G / log(final mass/ initial mass)], I is the ingested food, F is the faeces.
Nutritional indices were analyzed by a general linear model procedure, i.e., a covariance analysis described by Bauce et al. ([1]). Because RCR and RGR indices are ratios and the variance of a ratio is equal to the variances only when the dominator of the ratio is constant, they were adjusted in accordance with Bauce et al. ([1]). Mortality was treated in a binomial analysis using the GENMOD procedure in SAS ([21]), followed by LSMEANS to detect differences between concentrations.
Results
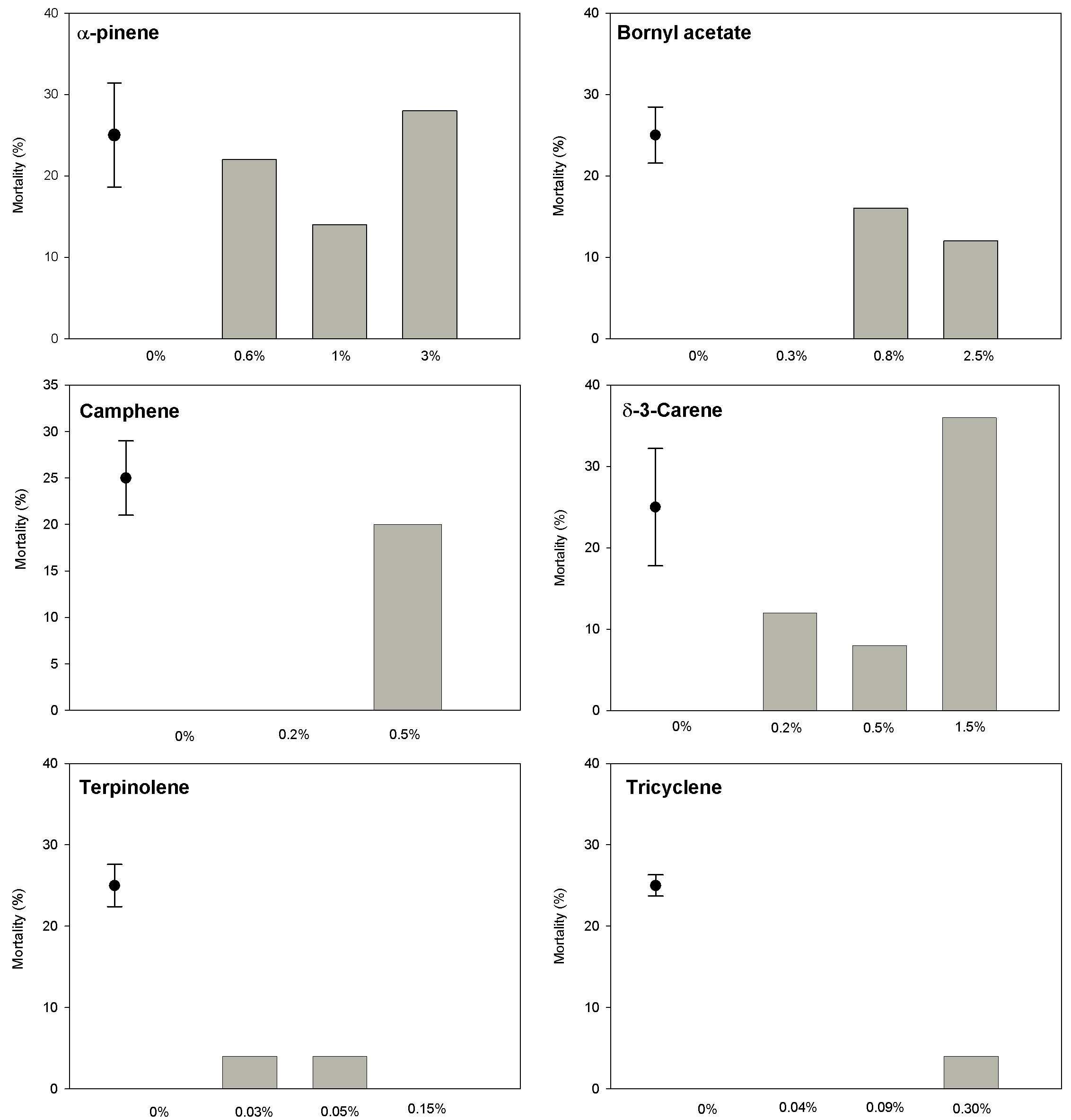
The terpenes α-pinene and δ-3-carene exerted significant mortality effects on sixth-instar budworm larvae. These effects were most pronounced at the maximum concentrations found in the foliage (Fig. 1, Tab. 1). Bornyl acetate and camphene had significant effects when their concentrations were twice those encountered in the foliage. Any effect from terpinolene and tricyclene on mortality was not observed. The greatest mortality (22 %) was observed for α -pinene at a concentration of 0.6%. A slightly lower mortality level was detected for δ-3-carene. All larvae in the control treatments were alive after 7 days, compared to those exposed to each of the monoterpenes (Fig. 1).
Fig. 1 - Mortality after seven days of female sixth-instar spruce budworm fed on artificial diet supplemented differents levels and differents monoterpenes. Bar represents ± 2 SE (n = 25 larvae per treatment).
Results of ANCOVA performed indicated that α-pinene, bornyl acetate, camphene, δ-3-carene and terpinolene significantly affected sixth-instar spruce budworm nutritional indices (Tab. 2). The presence of monoterpenes, except for bornyl acetate, decreased RGR. Digestibility increased with increasing concentrations of camphene and δ-3-carene when compared to the control; decreased digestibility was only observed for α-pinene. ECD and ECI indices increased with increased concentrations of α-pinene and bornyl acetate. ECD decreased in the presence of δ-3-carene. Nutritional indices of sixth-instar spruce budworm that were reared on artificial diets containing different concentrations of monoterpenes are summarized in Tab. 3.
Tab. 2 - The effect of monoterpene concentrations on C. fumiferana nutritional indices. Insects were reared on artificial diet supplemented with synthetic monotepenes. Data were analyzed using ANCOVA. (*): significant effect ( < 0.01); (ns): no significant effect.
| Index | α-pinene | Bornyl acetate | Camphene | δ-3-carene | Terpinolene | Tricyclene | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F(3; 83) | P | F(3; 89) | P | F(2; 65) | P | F(3; 80) | P | F(3; 94) | P | F(3; 94) | P | |
| D | 101.83 | 0.0001* | 1.92 | 0.0131 | 7.88 | 0.0009* | 20.31 | 0.0001* | 1.95 | 0.1270 | 2.63 | 0.0549 |
| ECD | 175.18 | 0.0001* | 3.37 | 0.0221 | 0.33 | 0.7196 | 27.12 | 0.0001* | 2.89 | 0.0394 | 3.65 | 0.0153 |
| ECI | 68.53 | 0.0001* | 5.61 | 0.0014* | 0.26 | 0.7735 | 19.26 | 0.0001* | 1.29 | 0.2809 | 1.13 | 0.3411 |
| RCR | 31.82 | 0.0001* | 6.47 | 0.0005* | 0.91 | 0.4070 | 42.86 | 0.0001* | 2.46 | 0.0678 | 0.28 | 0.8417 |
| RGR | 173.18 | 0.0001* | 5.63 | 0.0014* | 3.57 | 0.0340 | 146.38 | 0.0001* | 6.53 | 0.0005* | 2.83 | 0.0427 |
Tab. 3 - Nutritional indices (mean ± standard deviation) of sixth-instar female spruce budworm larvae fed on artificial diet containing different concentrations of monoterpenes. Values in each column followed by the same letter do not differ significantly at P<0.05 according Duncan’s multiple range test.
| Monoterpene | Concentration (%) |
Nutritional Indices | ||||
|---|---|---|---|---|---|---|
| D (%) | ECD (%) | ECI (%) | RCR (mg mg-1 h-1 10-2) |
RGR (mg mg-1 h-1 10-3) |
||
| α-pinene | 0 | 48.30 ± 0.35 | 50.11 ± 1.15 | 23.27 ± 0.50 | 6.07 ± 0.12 | 13.96 ± 0.19 |
| 0.6 | 38.69 ± 0.50 | 79.99 ± 1.43 | 28.84 ± 0.69 | 4.24 ± 0.13 | 12.80 ± 0.25 | |
| 1 | 44.76 ± 0.68 | 62.61 ± 1.42 | 28.49 ± 0.71 | 4.40 ± 0.13 | 12.42 ± 0.20 | |
| 3 | 63.36 ± 0.79 | 21.73 ± 1.31 | 14.81 ± 0.61 | 4.26 ± 0.18 | 6.53 ± 0.35 | |
| Bornyl acetate | 0 | 44.73 ± 0.40 | 51.02 ± 1.46 | 23.09 ± 0.67 | 5.52 ± 0.21 | 12.44 ± 0.15 |
| 0.3 | 44.06 ± 0.40 | 54.26 ± 1.34 | 23.37 ± 0.57 | 5.24 ± 0.14 | 12.15 ± 0.20 | |
| 0.8 | 46.00 ± 0.45 | 52.94 ± 1.42 | 24.06 ± 0.72 | 4.98 ± 0.22 | 12.75 ± 0.24 | |
| 2.5 | 45.16 ± 0.81 | 57.27 ± 1.46 | 26.56 ± 0.68 | 4.39 ± 0.16 | 11.58 ± 0.21 | |
| Camphene | 0 | 41.57 ± 0.56 | 58.06 ± 1.29 | 24.40 ± 0.67 | 5.57 ± 0.19 | 13.83 ± 0.17 |
| 0.2 | 43.67 ± 0.48 | 56.90 ± 1.20 | 24.86 ± 0.57 | 5.52 ± 0.15 | 13.62 ± 0.16 | |
| 0.5 | 44.66 ± 0.63 | 56.81 ± 1.08 | 24.21 ± 0.74 | 5.26 ± 0.15 | 13.11 ± 0.23 | |
| δ-3-carene | 0 | 43.42 ± 0.47 | 55.40 ± 1.19 | 24.43 ± 0.56 | 5.18 ± 0.21 | 12.45 ± 0.22 |
| 0.2 | 50.14 ± 0.85 | 49.49 ± 0.97 | 24.95 ± 0.50 | 4.88 ± 0.15 | 12.07 ± 0.15 | |
| 0.5 | 51.81 ± 0.83 | 48.18 ± 1.09 | 24.50 ± 0.59 | 4.17 ± 0.23 | 10.07 ± 0.20 | |
| 1.5 | 47.01 ± 1.32 | 38.01 ± 2.01 | 18.09 ± 1.12 | 1.97 ± 0.17 | 6.50 ± 0.24 | |
| Terpinolene | 0 | 40.99 ± 0.49 | 62.27 ± 1.06 | 24.93 ± 0.50 | 5.30 ± 0.19 | 13.09 ± 0.25 |
| 0.03 | 43.26 ± 0.89 | 59.81 ± 1.05 | 25.88 ± 0.58 | 5.22 ± 0.22 | 13.04 ± 0.29 | |
| 0.05 | 41.47 ± 0.79 | 58.39 ± 1.32 | 24.77 ± 0.65 | 5.44 ± 0.17 | 13.29 ± 0.21 | |
| 0.15 | 42.25 ± 0.59 | 62.86 ± 1.43 | 26.21 ± 0.71 | 4.69 ± 0.23 | 11.87 ± 0.24 | |
| Tricyclene | 0 | 42.32 ± 0.66 | 58.14 ± 1.02 | 24.59 ± 0.55 | 5.09 ± 0.17 | 13.49 ± 0.24 |
| 0.04 | 44.27 ± 0.70 | 56.80 ± 1.13 | 25.06 ± 0.56 | 5.00 ± 0.10 | 13.44 ± 0.19 | |
| 0.09 | 44.21 ± 0.47 | 56.52 ± 0.92 | 24.98 ± 0.50 | 5.18 ± 0.15 | 13.89 ± 0.17 | |
| 0.3 | 44.47 ± 0.60 | 53.35 ± 1.10 | 23.72 ± 0.66 | 5.13 ± 0.13 | 13.02 ± 0.22 | |
Discussion
The defensive role of monoterpenes against spruce budworm has been demonstrated in several studies, based on rearing trials either on foliage ([1]) or on artificial diet ([15]). This negative response was supported by results of the present study, given that we detected increased mortality (α-pinene, δ-3-carene) and reduced growth rates (α-pinene, δ-3-carene, terpinolene) as result of exposure to the individual monoterpenes. In addition, this study allowed us to reveal the mode of action for each monoterpene as previous studies used only mixtures of monoterpenes.
Secondary compounds can negatively affect herbivores performance in different ways: (i) by reducing food intake; and once ingested (ii) by reducing the efficiency of food utilization; and (iii) through their direct toxic effects ([11]). Mortality is frequently used to assess the effect of secondary compounds on insect biological performance and the toxicity of secondary compounds is often the main argument advanced for accepting the defensive functions of secondary compounds in plant-herbivore interactions. The higher spruce budworm mortality observed in young balsam fir compared to old trees was attributed to high concentrations of δ-3-carene, terpinolene and bornyl acetate ([1], [15]). Similarly, Bauce & Kumbasli ([3]) found that only resistant white spruce trees (Picea glauca Moench) contain borneol and δ-3-carene. Our results show that α-pinene and δ-3-carene individually provide good protection against herbivory at concentrations that were encountered in the foliage. Exceeding the maximum level found in the foliage does not necessarily increase spruce budworm mortality, especially in the case of α-pinene. We observed mortality levels that were three times higher for δ-3-carene when its concentrations were seven times higher than the maximum routinely found in foliage (from 0.2 to 1.5%). Bornyl acetate and camphene must reach a concentration that is two-fold higher than the foliage to achieve a toxic effect.
In the literature ([23], [10], [13]), α-pinene has been reported as budworm oviposition stimulant, but our results showed that this monoterpene, at concentrations found in the foliage, proved toxic to sixth-instar larvae, incurring 20% mortality. To reduce the toxic effect of α-pinene, spruce budworm decreases the digestibility D (Tab. 3), i.e., it attempts to minimize the amount of α-pinene retained in its body. An opposite strategy is employed for δ-3-carene. To reduce negative effects of δ-3-carene, spruce budworm larvae decrease their RCR by increasing the rate of food transit through the gut.
Bornyl acetate, δ-3-carene, camphene and terpinolene are considered as being toxic to spruce budworm ([17], [1], [15]). Our results show that these monoterpenes are individually not very effective in terms of their toxicity against sixth-instar larvae. This low toxicity can be explained by the absence of other monoterpenes or by the provision of sufficient energy for detoxification by soluble sugars.
Our results on nutritional indices show that the reaction of spruce budworm differed for each monoterpene against which it was tested. We observed a small decrease of RGR in the presence of α-pinene, bornyl acetate and δ-3-carene, while other monoterpenes do not affect growth. To counteract the negative effects of δ-3-carene, the spruce budworm tries to increase its digestibility. Despite this attempt, detoxification of δ-3-carene is an energetically costly process, which results in a reduced efficiency of conversion (typical compensation strategy observed with tannins). This compensation strategy is not validated in the case of α-pinene, where digestibility is reduced compared to the diet without the monoterpene and a correspondingly higher efficiency of conversion. A reduced digestibility compared to control diet can be attributed to the phago-repulsive effects of α-pinene. All of these different strategies used by spruce budworm to reduce negative effects of monoterpenes observed in this study can explain why these molecules are more effective as an ensemble in terms of foliage protection. Using a single strategy is not sufficient to confront and counteract the other monoterpenes present in the foliage.
Carisey & Bauce ([6]) tried to explain the lower efficiency of food conversion and reduced RGR of spruce budworm that were fed on old balsam fir foliage, which they attributed to a lack of water, to nutritional unbalances, and to a high level of some monoterpenes such as bornyl acetate, terpinolene and camphene. Our results showed that these three monoterpenes, when tested individually, reduced neither growth nor the efficiency of food conversion. This demonstrates that the reductions observed in the work of Carisey & Bauce ([6]) were probably due to a lack of water or nutritional imbalance within the old foliage.
We know that factors such as nitrogen or soluble sugar levels may be important in the compensatory mechanism employed by insects to deal with secondary compounds. Cates et al. ([9]) emphasizes the importance of nitrogen concentration on the effect of bornyl acetate and β-pinene. In our study, nitrogen and soluble sugars were maintained at stable levels to assess the effect of each secondary compound. Soluble sugars play an important role during the sixth-instar stage of spruce budworm and it would be interesting to observe the effects of these monoterpenes when combined with different concentrations of soluble sugars.
In conclusion, the negative effects of monoterpenes on spruce budworm performance and the respective variations of in their modes of action were demonstrated in the current study. It has been widely assumed that the effect of secondary compounds on herbivores is proportional to concentrations of the compounds of interest ([17]). Our results do not support this assumption and we can conclude that spruce budworm host plants produce these molecules at concentrations that provide optimal protection.
Acknowledgements
The authors wish to thank Sophie Rochefort, Martin Charest and Richard Bérubé (Université Laval, Quebec, Canada) for their helpful contributions to this study and Dr. W.F.J. Parsons for checking the English. Financial support was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Ministère des Ressources Naturelles et de la Faune du Québec (MRNFQ), and the Scientific and Technological Research Council of Turkey (TÜBITAK) BIDEB-2219 Postdoctoral Research Program. We declare that the experiments comply with the current laws of the country in which they were performed.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Faculty of Forestry, Istanbul University, 34473 Bahçeköy, Istanbul (Turkey)
Département des Sciences du Bois et de la Forêt, Faculté de Foresterie et de Géomatique, Université Laval, G1V 0A6 Québec, QC (Canada)
Corresponding author
Paper Info
Citation
Kumbasli M, Bauce É (2013). Spruce budworm biological and nutritional performance responses to varying levels of monoterpenes. iForest 6: 310-314. - doi: 10.3832/ifor0956-006
Academic Editor
Massimo Faccoli
Paper history
Received: Jan 24, 2013
Accepted: Apr 09, 2013
First online: Jul 16, 2013
Publication Date: Dec 02, 2013
Publication Time: 3.27 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2013
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 52719
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 44372
Abstract Page Views: 3057
PDF Downloads: 3816
Citation/Reference Downloads: 18
XML Downloads: 1456
Web Metrics
Days since publication: 4586
Overall contacts: 52719
Avg. contacts per week: 80.47
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2013): 12
Average cites per year: 0.92
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
The impact of post-defoliation foliage of Pinus halepensis Mill. on the larval performance of Thaumetopoea pityocampa and its relationship with the tree-induced defense
vol. 18, pp. 186-193 (online: 01 July 2025)
Review Papers
Forest health under climate change: impact of insect pests
vol. 17, pp. 295-299 (online: 30 September 2024)
Research Articles
Performances of an expanding insect under elevated CO2 and snow cover in the Alps
vol. 1, pp. 126-131 (online: 27 August 2008)
Research Articles
Effects of artificial defoliation and simulated insect damage on the growth of Betula pendula saplings
vol. 9, pp. 95-100 (online: 15 July 2015)
Research Articles
Estimating the potential threat of increasing temperature to the forests of Turkey: a focus on two invasive alien insect pests
vol. 15, pp. 444-450 (online: 03 November 2022)
Research Articles
Monitoring of damage from cedar shoot moth Dichelia cedricola Diakonoff (Lep.: Tortricidae) by multi-temporal Landsat imagery
vol. 7, pp. 126-131 (online: 13 January 2014)
Review Papers
Leaf volatile isoprenoids: an important defensive armament in forest tree species
vol. 5, pp. 13-17 (online: 14 February 2012)
Research Articles
Influence of soil and topography on defoliation intensity during an extended outbreak of the common pine sawfly (Diprion pini L.)
vol. 10, pp. 164-171 (online: 19 November 2016)
Research Articles
Arbuscular mycorrhizal colonization in black poplar roots after defoliation by a non-native and a native insect
vol. 9, pp. 868-874 (online: 29 August 2016)
Research Articles
Discovering interaction between oaks and carabid beetles on a local scale by point pattern analysis
vol. 9, pp. 618-625 (online: 06 May 2016)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword