Effects of artificial defoliation and simulated insect damage on the growth of Betula pendula saplings
iForest - Biogeosciences and Forestry, Volume 9, Issue 1, Pages 95-100 (2015)
doi: https://doi.org/10.3832/ifor1522-008
Published: Jul 15, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
One-year-old silver birch (Betula pendula Roth) saplings were subjected to artificial insect damage and defoliations of varying intensities, and subsequent growth indexes, biomass allocation patterns and photosynthesis were monitored during a 60-day period. Seven treatments were conducted in which the leaves of saplings were perforated with three or six holes per each leaf, and damaged by clipping one-third of each leaf, or they received 25, 50 and 75% defoliations during a single growing season (from April to August of 2014). Simulated insect damage and artificial defoliation decreased growth. The 75% defoliation significantly reduced the total dry mass of birch saplings at harvest by 30%, while such reduction did not influence the total productivity. The dry mass of leaves was reduced by 45% when saplings were defoliated by 75% compared to not defoliated saplings. Moreover, the total production of leaves significantly increased in the 75% defoliated saplings compared with control saplings. Artificial defoliation increased the relative biomass allocation to foliage, and this was more evident in defoliated than in mechanically insect-damaged saplings. Despite losing 25, 50 or 75% of leaf mass due to clipping, defoliated birch saplings recovered similar dry masses and root/shoot ratios by harvest as the non-defoliated saplings. Perforation and clipping parts of the leaves, as well as the artificial defoliations, caused the regrowth of biomass that did not significantly change compared to healthy silver birch saplings, and this phenomenon could be assessed as equal-compensatory growth.
Keywords
Betula pendula, Artificial Defoliation, Artificial Insect-Damage, Growth Compensation
Introduction
An increasing interest in various aspects of climate change impacts on forest ecosystems provides a basis for discussions regarding the increased abundance of insect pests and, furthermore, increased deterioration in forest health. Initially, defoliation or insect-caused damage reduces the photosynthetic area per plant, which may eventually result in a considerable loss of leaf area. Therefore, the expected implications of insect damage include reductions in height, biomass and growth rates, as well as changes in photosynthetic activity ([27], [12]).
Generally, the effect on biomass production depends on the compensation potential of individual species and damage scenarios. There are two opposite growth responses to defoliation. In the first, the final vegetative biomass of defoliated plants decreases after the removal of biomass, while in the second the defoliated plants partially or fully compensate for the removal of biomass ([3], [19], [22], [5], [6]). Meanwhile, root growth decreases or stops post-defoliation ([5]).
With regard to the compensatory hypothesis ([17]), over-, under- or equal-compensatory rates could occur under different site and defoliation conditions. For example, Zhao et al. ([31]) found a greater reduction in the growth of aboveground biomass when an intense clipping of 80% was applied. Data from several sources have identified cases of partial compensation, especially for insect-damaged trees ([25], [13], [14]). Some studies investigating growth changes under various insect damage scenarios have focused exclusively on birch species ([10], [8], [21], [1], [11]).
It has been demonstrated that the greater ability of birches to exhibit compensatory growth and recover after severe defoliation occurs faster after increasing the nutrient supply ([26], [21]). Subsequently, Anttonen et al. ([1]) reported that nutrient availability was significant only in determining how the total biomass responded to defoliation, and no interaction between defoliation and fertilization was found in the second growing season after the defoliation.
Previous research has indicated that photosynthetic rates increase in damaged leaves, or even in healthy leaves adjacent to damage ([4], [20], [29], [28]).
Numerous studies have attempted to explain the different consequences of defoliation for relative biomass allocation ([19], [1], [31], [28]). Markkola et al. ([16]) showed that the loss of foliage decreases the root-to-shoot ratio, i.e., changes in the partitioning between above- and below-ground biomass occur, as shoot growth is favored at the expense of root growth ([22]).
The present study was designed to investigate the effects of different artificial defoliation and simulated insect-damage regimes on the growth characteristics of silver birch saplings. The first objective of this study was to determine possible changes in dry mass and relative biomass allocation across different compartments of birch saplings. We also conducted a pilot study on foliar gas-exchange, aiming to investigate the effect of simulated insect damage and partial defoliation on the photosynthetic characteristics of the remaining leaves.
Materials and Methods
Site description
This research was conducted in southwestern Lithuania (54° 51′ N, 24° 03′ E) at the Dubrava Experimental and Training Forest Enterprise. The mean annual temperature in this area was 6.5 °C, ranging from 5.0-13.7 °C in April, 8.8-17.1 °C in May, 13.2-16.8 °C in June, 19.3-22.8 °C in July and 13.7-22.5 °C in August. The mean monthly temperature was higher by 1-1.5 °C (May) to 3-4.5 °C degrees (July) compared to the standard climatic norm during the vegetation season. The mean annual precipitation was 686 mm, with 21 mm in April, 84 mm in May, 57 mm in June, 71 mm in July and 112 mm in August. The mean monthly precipitation was close to the standard climatic norm.
Plant material and study design
In this study, a pot-cultivated experiment of silver birch (Betula pendula Roth) saplings was conducted. Initially, all experimental material was approximately equal in size, averaging 40.6 ± 0.14 cm of height. Before treatment, the growth of birch saplings did not differ among treatments (Kruskal-Wallis ANOVA: p<0.05).
The soil for planting was prepared by mixing sand and neutralized peat in a 1:5 ratio.
Saplings were planted into individual 3-L plastic pots filled with the mixed substrate. The mean pH value of the soil in the pots was 5.0. The total nitrogen and potassium contents of the soil were 0.4 and 40 mg l-1, respectively. The soil received no fertilizer. Throughout the experiment, trees were watered as needed. Pots had 16 equidistant perforations at the base to allow excess water to drain.
One-year-old silver birch saplings were planted on April 2, 2014. Each sapling was planted in its own pot and numbered. Artificial defoliation of 25, 50 and 75% and three different types of simulated insect damages, i.e., three and six holes per each leaf, and clipping one-third of each leaf, were conducted on June 17, 2014. No visible injury caused by biotic and abiotic agents was fixed during the experimental period. In total, seven treatments were conducted, including a non-defoliated control. Each treatment had 20 replicates. Potted trees were placed adjacent to each other in rows, the treatments were mixed. The intensities of the defoliation treatments were designed to approximate insect defoliation levels during the active vegetation period in Lithuania. The simulated damages showed the different insect feeding intensity during outbreaks. The artificial defoliation was the basis for the potentially different effects of the remaining parts of the birch saplings.
The defoliation was conducted using scissors, cutting each leaf perpendicular to the midrib near its base. Each leaf was damaged with 3 or 6 non-overlapping holes (0.33 cm2) using a steel hole-punch, or one-third of each leaf was cut using scissors. Defoliation treatments were applied only once, at the beginning of the experiment.
Growth measurements and biomass harvest
Height measurements
The individual height of birch saplings before each treatment was measured on April 4, April 24, May 15 and June 4 to calculate the relative growth rates in different periods. The height increment was measured 1, 5 and 8 weeks post-defoliation on June 25, July 25 and August 18, respectively.
Biomass harvest
At the end of experiment, aboveground biomass was sampled on August 18 or 8 weeks post-defoliation. For the determination of total dry mass, samples were removed from the following aboveground tree compartments: leaves, living and dead shoots, branches and stems.
The samples were transported to the laboratory in paper bags. The bags were stored in a clean and ventilated room, and left open prior to oven drying. The oven drying took place in the weeks immediately after sampling. The removed biomass from each treatment, as well as the final live biomass, was collected and oven-dried to a constant weight at 60°C to determine the accumulated aboveground biomass. Roots were then harvested, rinsed of soil and oven-dried to determine the total belowground biomass.
Post-harvest measurements
The leaf areas of 3 leaves, taken randomly from each sapling per each treatment, were estimated using a scanner using leaf area analysis software (WinFOLIA 2004a, Regent Instruments Inc., Quebec, Canada). Later, specific leaf area was interpreted as the projected leaf area/leaf dry mass ratio and used for re-calculation of the photosynthetic rate per square centimeter per treatment. The diameter of air-dried main stems was measured at a 2 cm distance above the stump base for each individual sapling using an electronic digital caliper. The length of the roots of each sapling was measured from the root base to the bottom, straightening the root before measurement.
Gas-exchange measurements
The net photosynthetic rate of the remnant leaves was measured with a portable photosynthetic system LCpro-SD (ADC BioScientific Ltd, Hoddesdon, England). Measurements were taken 1 day, 4 weeks and 7 weeks (June 18 to August 4) after the clippings. To avoid strong sunlight and wind, the measurements were conducted between 10:00 AM and 1:00 PM.
For the first measurement, five systematically selected birch saplings were taken from four treatments: the non-defoliated control, the simulated insect damages of three or six holes per leaf, and the clipped third of the leaf. The photosynthetic rate was measured for three leaves from each sapling, yielding 15 leaves per treatment. For the measurements conducted after 4 and 7 weeks, the saplings from all treatments were measured following the same selection scheme. We measured mature, sunlit, naturally undamaged leaves in each treatment.
The chlorophyll a and b contents of the remnant leaves were determined by a spectrophotometric method using ethanol extraction. The leaf sampling was conducted 7 weeks after the treatment (on August 7). We measured leaves sampled from the non-defoliated control, 50%-defoliated saplings and saplings with 6 hole-damaged leaves. From each treatment, nine leaves were sampled from the middle crown. Later, the samples were combined into 3 samples. We calculated total chlorophyll, and chlorophyll a and b concentrations (mg l-1) based on the following formulas (eqn. 1, eqn. 2, eqn. 3):
where D is the sample absorbance at 644 nm wavelength. The chlorophyll content of leaves was expressed in milligrams per gram of fresh weight of leaves as follows (eqn. 4):
where A is the chlorophyll content of the fresh weight of leaves (mg g-1), C is the chlorophyll concentration (mg l-1), V is the volume of chlorophyll extract (ml) and P is the weight of plant material (g).
Calculations and statistical analysis
The total aboveground biomass of each tree was calculated by the summation of the mass of dried shoots, branches and leaves, in grams. The total biomass of each tree was calculated by the summation of the aboveground and belowground biomass, in grams. Leaf, aboveground and total production or cumulative dry mass were calculated by summing the dry mass at harvest plus the leaf mass removed during insect damage and in the defoliation events. Root biomass was expressed in relation to the aboveground biomass, and the root-to-shoot ratio (root/shoot ratio) was calculated. Cumulative leaf dry mass in relation to the root was calculated, and the ratio of leaf production-to-root ratio (leaf production/root ratio) was obtained.
Departure from normal distribution of the variables was tested by Lilliefors and Kolmogorov-Smirnov tests. However, the normality hypothesis was rejected for all observed variables (α = 0.05).
The Kruskal-Wallis analysis of variance (ANOVA) test was used to ascertain the significant differences in dry mass between the different treatments (7 groups: control, 3 and 6 holes per leaf, clipped one-third of leaf, and 25, 50 and 75% defoliations). The test was taken as a non-parametric alternative to between-groups one-way ANOVA, as the data were non-normally distributed. Throughout the study, the means are presented with the standard error of the mean (± SE). Statistical analyses were conducted using the software STATISTICA® 7.0 (StatSoft, Tulsa, OK, USA), and a level of significance of α = 0.05 was chosen in all cases.
Results
Growth and biomass allocation
At the end of the experiment, the largest height increment of 6.0-6.5 cm was found in the 75%-defoliated saplings and in those with clipped one-third of leaf compared to their height before treatment (Tab. 1). Other treatments did not show any remarkable difference compared to not defoliated saplings.
Tab. 1 - Height (cm) of B. pendula saplings 1, 5 and 8 weeks after treatment. Values are given as the mean ± standard error (SE, n=20). Non statistically significant differences between treatments are indicated in all cases at p<0.05.
| Treatment | Time after treatment | ||
|---|---|---|---|
| 1 week | 5 weeks | 8 weeks | |
| Control | 53.4 ± 1.0 | 55.2 ± 1.2 | 56.3 ± 1.3 |
| 3 holes per leaf | 54.5 ± 1.0 | 55.8 ± 1.1 | 56.4 ± 1.1 |
| 6 holes per leaf | 55.4 ± 1.1 | 57.5 ± 1.3 | 58.0 ± 1.3 |
| Clipped one-third of leaf | 55.0 ± 0.9 | 57.5 ± 1.1 | 58.5 ± 1.1 |
| 25% defoliation | 52.4 ± 1.0 | 53.9 ± 1.1 | 54.7 ± 1.1 |
| 50% defoliation | 53.8 ± 0.9 | 56.0 ± 0.9 | 56.5 ± 0.9 |
| 75% defoliation | 53.4 ± 1.0 | 56.0 ± 1.3 | 57.1 ± 1.3 |
In all cases, simulated insect damage and defoliation decreased growth (Tab. 2). The 75% defoliation had a significant impact on the biomass present at harvest. The dry mass of silver birch saplings at harvest was by 30% lower due to the 75% defoliation, while, when included leaf lost in defoliation, total productivity was not significantly reduced. At harvest, the dry mass of leaves was by 45% lower in the 75% defoliated saplings than non-defoliated control. However, total leaf production was by 21% higher in the 75% defoliated saplings than in the control saplings.
Tab. 2 - Dry mass (g) of leaves, shoots, stems and roots of B. pendula saplings at harvest. Values are given as the mean ± SE (n=20). Different letters within a row indicate statistically significant differences in dry mass between treatments (p<0.05). (1): Leaf production include leaf lost in defoliation (cumulative dry mass); (2): total production value was obtained by summing dry mass at harvest plus leaf mass removed in defoliation events; (3): Gram roots / (gram stems + gram shoots + gram leaf); (4): Gram leaf production / gram roots.
| Variable | Control | Simulated insect damage | Artificial defoliation | ||||
|---|---|---|---|---|---|---|---|
| 3 holes per leaf |
6 holes per leaf |
Clipped 1/3 of leaf |
25% | 50% | 75% | ||
| Leaf mass (g) | 2.19 ± 0.16d | 1.97 ± 0.12bcd | 1.84 ± 0.11bcd | 1.91 ± 0.12bcd | 1.46 ± 0.10abc | 1.53 ± 0.12ab | 1.21 ± 0.10a |
| Leaf production (g)1 | 2.19 ± 0.16ab | 2.19 ± 0.12ab | 2.27 ± 0.11ab | 2.48 ± 0.12ab | 1.98 ± 0.10a | 2.67 ± 0.12b | 2.66 ± 0.10b |
| Shoot mass (g) | 0.44 ± 0.05a | 0.43 ± 0.05a | 0.45 ± 0.05a | 0.41 ± 0.03a | 0.40 ± 0.04a | 0.37 ± 0.04a | 0.35 ± 0.03a |
| Stem mass (g) | 4.06 ± 0.23b | 3.95 ± 0.21b | 3.79 ± 0.22ab | 3.60 ± 0.13ab | 3.44 ± 0.16ab | 3.53 ± 0.15ab | 3.09 ± 0.14a |
| Root mass (g) | 5.27 ± 0.28b | 4.88 ± 0.29ab | 4.74 ± 0.31ab | 4.75 ± 0.25ab | 4.13 ± 0.25ab | 4.54 ± 0.26ab | 3.81 ± 0.19a |
| Total mass (g) | 11.96 ± 0.68b | 11.24 ± 0.63b | 10.82 ± 0.63ab | 10.66 ± 0.48ab | 9.42 ± 0.52ab | 9.97 ± 0.52ab | 8.45 ± 0.41a |
| Total production (g)2 | 11.96 ± 0.68a | 11.45 ± 0.63a | 11.25 ± 0.63a | 11.24 ± 0.48a | 9.94 ± 0.52a | 11.11 ± 0.52a | 9.91 ± 0.41a |
| Root/shoot ratio3 | 0.80 ± 0.02a | 0.77 ± 0.02a | 0.79 ± 0.03a | 0.81 ± 0.03a | 0.78 ± 0.02a | 0.84 ± 0.03a | 0.83 ± 0.03a |
| Leaf production/ root ratio4 | 0.41 ± 0.02a | 0.46 ± 0.02ab | 0.49 ± 0.02abc | 0.53 ± 0.02bc | 0.49 ± 0.01abc | 0.60 ± 0.02cd | 0.72 ± 0.03d |
Lower defoliation intensities (25 and 50%) had, to some extent, lower impacts on the total dry mass of defoliated saplings at harvest. The dry mass of saplings was approximately by 20% lower due to the 25 and 50% defoliation, while total productivity did not differ from controls. However, the total dry mass of leaves was significantly reduced by 30-33%. At harvest, the total production of leaves was significantly by 22% higher in the 50% defoliated saplings compared with the control. This response was similar to that of the 75%-defoliated saplings.
In almost all cases, different intensities of simulated insect damages (3 and 6 holes, 1/3 clipped leaf) did not significantly affect the dry mass of damaged saplings. For example, the total leaf dry mass of saplings damaged with 6 holes per each leaf was lower by 16% than that of the control, while the total dry mass at harvest remained similar to that of the controls, with a slight, increasing tendency.
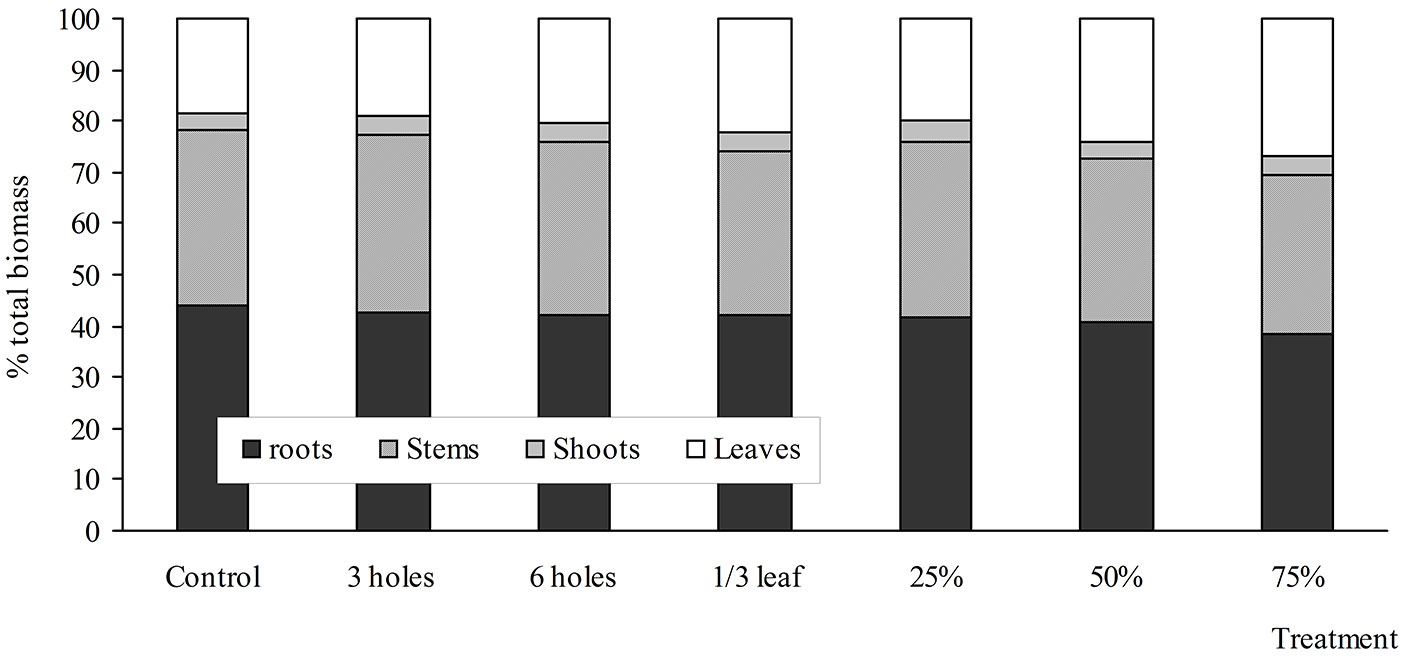
Similar to the response of aboveground dry mass, root dry mass was also significantly reduced by 28% in the 75% defoliated saplings. The root dry mass in the other defoliation treatments did not respond significantly. The differences in biomass partitioning enabled artificially insect-damaged and defoliated saplings to recover root/ shoot ratios that were similar to that of the control saplings (Tab. 2). Relative biomass allocation across the compartments of birch saplings at harvest was affected mainly by defoliation treatments as compared with the simulated insect damage (Tab. 2, Fig. 1).
Fig. 1 - Biomass allocation to stem, leaves, and branches of B. pendula saplings in the different treatments of simulated insect damage and artificial defoliation 60 days after treatment.
Defoliation increased the relative biomass allocation to leaves. In comparison with the control saplings, 75%-defoliated saplings allocated a greater proportion of their biomass to leaf production: the ratio of total leaf production relative to root production was 0.72 in the 75%-defoliated saplings and 0.41 in the controls (Tab. 2). The 25- and 50%-defoliated saplings allocated a lower proportion of mass to leaf production than the 75%-defoliated saplings (Tab. 2). However, the ratio of total leaf production to root production was higher than in the controls (0.41), i.e., it was 0.49 and 0.60 in the 25- and 50%-defoliated saplings, respectively. Simulated insect damage did not affect the leaf production-to-roots ratio.
Effects on photosynthetic indes
Leaf photosynthesis responded inconsistently to different defoliation regimes or damage levels (Tab. 3). In June, one day after treatment, the photosynthetic rate was lower in damaged than control saplings (data not shown). A significant increase in the photosynthetic rate in response to clipping one-third of each leaf was observed 7 weeks post-treatment. In contrast, reduced photosynthetic rates were obtained after weak artificial insect-damage (leaves damaged with 3 holes) and the moderate 25 and 50% defoliation regimes, although these differences were not significant.
Tab. 3 - Photosynthetic rate (μmol m-2 s-1) of the remnant leaves of B. pendula saplings one day, 4 and 7 weeks after treatment. Values are given as the mean ± SE (n=15). Different letters indicate statistically significant differences in dry mass between treatments (p<0.05).
| Treatment | Time after treatment | |
|---|---|---|
| 4 weeks | 7 weeks | |
| Control | 1.22 ± 0.20 | 1.80 ± 0.25ab |
| 3 holes per leaf | 0.98 ± 0.13 | 1.28 ± 0.23a |
| 6 holes per leaf | 1.58 ± 0.49 | 2.05 ± 0.36ab |
| Clipped one-third of leaf | 0.72 ± 0.13 | 2.99 ± 0.30b |
| 25% defoliation | 1.19 ± 0.16 | 1.07 ± 0.19a |
| 50% defoliation | 1.81 ± 0.48 | 1.41 ± 0.16a |
| 75% defoliation | 1.24 ± 0.16 | 1.83 ± 0.16ab |
The pilot study of foliar chlorophyll content showed that simulated insect-damage with 6 holes per each leaf and 50% defoliation significantly (p=0.007-0.02) decreased the content of chlorophyll a from 2.41 ± 0.15 in the controls to 1.57 ± 0.13 and 1.63 ± 0.11 mg g-1, respectively. The chlorophyll b content decreased by 17% and total chlorophyll - by 30% in the saplings damaged with 6 holes per each leaf and 50% defoliation compared with controls 7 weeks after treatment (data not shown).
Effects on some indes of leaves, stem and roots
The 75% defoliation significantly decreased the diameter of the main stem compared with that of the control (p=0.007 - Tab. 4). The other defoliation regimes, as well as the simulated insect damages, had no significant effect on the diameter of the main stem.
Tab. 4 - Leaf area (cm2), diameter of main-stem (mm) and length of main-root (cm) of B. pendula saplings 8 weeks after treatment. Values are given as the mean ± SE (n=20). Different letters indicate statistically significant differences in dry mass between treatments (p<0.05).
| Treatment | Leaf area (cm2) |
Diameter of main-stem (mm) |
Length of main-root (cm) |
|---|---|---|---|
| Control | 8.75 ± 0.35b | 5.66 ± 0.13b | 43.55 ± 2.20b |
| 3 holes per leaf | 9.13 ± 0.30b | 5.65 ± 0.15b | 43.75 ± 1.94b |
| 6 holes per leaf | 8.02 ± 0.35b | 5.48 ± 0.15b | 38.40 ± 1.34a |
| Clipped one-third of leaf | 6.90 ± 0.30a | 5.31 ± 0.10b | 39.15 ± 1.68ab |
| 25% defoliation | 8.83 ± 0.30b | 5.44 ± 0.12b | 37.20 ± 1.68a |
| 50% defoliation | 8.02 ± 0.35b | 5.37 ± 0.10b | 37.26 ± 1.06a |
| 75% defoliation | 7.95 ± 0.40b | 4.99 ± 0.10a | 39.45 ± 1.66ab |
At the final harvest, the clipped leaves treatment significantly decreased the leaf area by 20% (Tab. 4). Decreases of 8-9% were found after the 6 holes per leaf and 50 and 75% defoliation treatments.
The results obtained from the root analysis showed that the 25 and 50% defoliations caused the highest reductions, up to 15%, in the length of the main root. About a 10-12% significant reduction was found in the 6 holes per leaf treatments.
Discussion
This study was set out with the aim of assessing the effects of different simulated insect damage levels and defoliation regimes on the growth characteristics of silver birch saplings. Our results confirmed that defoliated plants partially or fully compensate for the removal of biomass ([3], [23], [22], [6]). The main growth indexes of defoliated saplings grown under the same nutrient conditions most often showed the compensatory response in the case of total productivity. However, no data showed that the stimulation of productivity resulted in overcompensation, as demonstrated for example by Oesterheld & McNaughton ([24]).
Saplings were able to tolerate the defoliation of all studied regimes and all damage levels with slight effects on height growth. This study was unable to demonstrate a reduction in height growth in response to the 50 and 75% defoliation treatments, as found by Augner et al. ([2]).
In our study, a single 75% defoliation event had a significant effect on leaf and stem biomass. All treatments non-significantly decreased the total aboveground and belowground biomass at harvest, as well as the masses of leaves and stems compared with controls. These changes were similar to those obtained by McGraw et al. ([18]). Nevertheless, the cumulative dry mass of birch leaves increased in response to the 50 and 75% defoliation intensities.
Despite losing a considerable part of leaf mass due to clipping, 25-, 50- or 75%-defoliated birch saplings recovered similar dry masses and root/shoot ratios by harvest as the control saplings. This finding supports previous research by Oesterheld & McNaughton ([23]), who reported that the root/ shoot ratio recovered by the end of the experiment after a previously drastic increase by clipping.
Other studies confirm that 50%-defoliated plants recovered similarly to non-defoliated plants, and those defoliated by 25% even overcompensated their biomass compared with controls ([26]). In our case, saplings that lost 50% of their foliage increased their growth rate by 40% to achieve a biomass that was similar to that of the controls. Similarly, the growth rate of saplings that were damaged by the 75% defoliation was even greater, i.e., their relative growth rate was 2-fold higher than that of controls, which enabled their biomass to reach a level that was similar to that of the controls. Surveys, such as that conducted by Reich et al. ([26]), have shown that plants defoliated by 75% were only able to partially compensate biomass growth and recover. Our findings are not fully consistent with these results, as we found full compensation, or even a slight overcompensation, in the case of leaf productivity.
The leaf areas of defoliated saplings did not differ from that of the control; therefore, we could assume that their recovery was obvious in terms of leaf area, which was much changed during the single artificial defoliation and simulated insect damage events. We confirm the findings that specific leaf area was unaffected by defoliation treatment ([26]), although the leaf weight ratio decreased under the 25-75% defoliation events.
Allocation refers to the amount of biomass apportioned to each above-ground compartment (leaves, shoots and stems) and roots over the duration of the experiment or 8 weeks post-defoliation. Moderate and severe defoliation regimes correlated with greater biomass allocations to leaves. The findings of Reich et al. ([26]), who examined biomass partitioning in red pine, showed that biomass shifted towards foliage. Changes in allocation that resulted in a higher production of new leaf area were also obtained by Oesterheld & McNaughton ([23]). Otherwise, our findings are not comparable with those of Honkanen et al. ([9]), who found a biomass allocation to stems.
Other existing studies also show a similar tendency, but a higher-nutrient level was given as a specific condition ([28]). Fertilization increased the growth of silver birch saplings at all defoliation levels, demonstrating that nutrient availability limited growth in the experimental field. However, we did not use additional fertilizers, and, therefore, our results differed from those of some other studies (e.g., [17], [26], [1], [28]). It could only be assumed that the increased growth compensation was revealed after the additional input of nutrients ([17], [31]). The same tendency could occur if pot size limits root growth, as stated by Oesterheld ([22]).
The current study found significantly increased photosynthetic rate only in the clipped leaf treatment seven weeks post-defoliation. Otherwise, changes in the photosynthetic area per plant were small over a relatively short period of time. Moreover, despite the significant increase in leaf productivity, the severe 75% defoliation did not cause any changes in the photosynthetic rates compared to the control. However, the moderate defoliation events reduced the photosynthetic rate.
To a certain extent, an atypical response of photosynthesis was observed - it did not increase with increasing defoliation intensity. The reduction of photosynthetic activity after partial leaf removal was presented by Zangerl et al. ([30]). Otherwise, Huttunen et al. ([12]) discussed that the ability to photosynthesize decreases in low nutrient soils, which, therefore, could have an impact on growth. In our experiment, the soil could be characterized as a nutrient-poor substrate. McGraw et al. ([18]) found that defoliation alone did not have a significant effect on photosynthesis, as such effects varied with the watering regime.
In terms of diameter growth, saplings reduced their growth under the highest defoliation intensity at harvest, but did not respond to other treatments. Indeed, such a quick response is quite a good outcome of this experiment compared with some other studies, which found the response only after a longer period ([10]).
The artificial holes and defoliation may not exactly repeat the natural insect feeding ([15]). Some of simulated indexes including the size of the treated area or the amount of removed foliage caused by artificial and natural agents could be similar. However, the plant possibly recognize a sustained damage by feeding insects from the artificial damage which was conducted with own damage strategy and location in the plant only once per vegetation season. A study of different damage types by Hartley & Lawton ([7]) reported that defoliation induced by natural insect herbivory caused stronger responses compared to the artificial damage. However, we have not succeeded to find any published material of similar investigations conducted in naturally growing birch stands. The information regarding natural birch damages induced by insects is available only from the permanent observation plots established in the birch stands and monitored for almost 20 years in Lithuania. Still, it only points that no visible insect damages was registered (unpublished).
Returning to the question posed at the beginning of this study, we assume that the obtained defense strategies of birch trees are significant in determining spatial variation and temporal of this species. This should be important for the determination of competitive abilities following herbivory, especially given the fact that the area of birch stands is currently expanding in Lithuania, at the same time the frequency of insect outbreaks increases.
Conclusions
We found that silver birch saplings were able to recover from moderate and severe (75%) defoliation in one growing season. In the case of leaf production, even growth overcompensation could be observed. Different levels of simulated insect damage, perforations of 3 or 6 holes per leaf, gave weaker responses than the defoliation regimes, and the 6 hole-treatment responded similarly to the 25% defoliation. The recalculation of the dry masses of different compartments to the total production showed that all saplings recovered 8 weeks post-treatment, regardless of the defoliation intensity.
Our results suggest that different defoliation regimes play a considerable role in determining whether silver birch compensates for defoliation. The discrepancy of our findings in the case of photosynthesis could be attributed to specific meteorological conditions, and these findings need to be replicated. According to the present data, the conclusions must be interpreted with caution, and further studies are necessary to explain whether a short time for recovery after artificial defoliation or simulated insect damage and a limited soil nutrient supply could cause any other specific responses.
Acknowledgments
The paper presents research findings obtained through the long-term research programme “Sustainable forestry and global changes”, implemented by the Lithuanian Research Centre for Agriculture and Forestry.
References
Authors’ Info
Authors’ Affiliation
Valda Araminiene
Vidas Stakenas
Institute of Forestry, Lithuanian Research Centre for Agriculture and Forestry, Liepu str. 1, Girionys, LT-53101, Kaunas district (Lithuania)
Corresponding author
Paper Info
Citation
Varnagiryte-Kabašinskiene I, Araminiene V, Stakenas V (2015). Effects of artificial defoliation and simulated insect damage on the growth of Betula pendula saplings. iForest 9: 95-100. - doi: 10.3832/ifor1522-008
Academic Editor
Massimo Faccoli
Paper history
Received: Dec 04, 2014
Accepted: Mar 10, 2015
First online: Jul 15, 2015
Publication Date: Feb 21, 2016
Publication Time: 4.23 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 51568
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 43255
Abstract Page Views: 3103
PDF Downloads: 3934
Citation/Reference Downloads: 26
XML Downloads: 1250
Web Metrics
Days since publication: 3886
Overall contacts: 51568
Avg. contacts per week: 92.89
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 4
Average cites per year: 0.40
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Response of artificially defoliated Betula pendula seedlings to additional soil nutrient supply
vol. 10, pp. 281-287 (online: 13 December 2016)
Research Articles
Arbuscular mycorrhizal colonization in black poplar roots after defoliation by a non-native and a native insect
vol. 9, pp. 868-874 (online: 29 August 2016)
Research Articles
Individual tree mortality of silver birch (Betula pendula Roth) in Estonia
vol. 9, pp. 643-651 (online: 04 April 2016)
Research Articles
Artificial intelligence associated with satellite data in predicting energy potential in the Brazilian savanna woodland area
vol. 13, pp. 48-55 (online: 05 February 2020)
Research Articles
Analyzing regression models and multi-layer artificial neural network models for estimating taper and tree volume in Crimean pine forests
vol. 17, pp. 36-44 (online: 28 February 2024)
Research Articles
Monitoring of damage from cedar shoot moth Dichelia cedricola Diakonoff (Lep.: Tortricidae) by multi-temporal Landsat imagery
vol. 7, pp. 126-131 (online: 13 January 2014)
Research Articles
Influence of soil and topography on defoliation intensity during an extended outbreak of the common pine sawfly (Diprion pini L.)
vol. 10, pp. 164-171 (online: 19 November 2016)
Research Articles
Sensitivity analysis of RapidEye spectral bands and derived vegetation indices for insect defoliation detection in pure Scots pine stands
vol. 10, pp. 659-668 (online: 11 July 2017)
Review Papers
Problems and solutions to cork oak (Quercus suber L.) regeneration: a review
vol. 16, pp. 10-22 (online: 09 January 2023)
Research Articles
Influence of climate on tree health evaluated by defoliation in the ICP level I network (Romania)
vol. 10, pp. 554-560 (online: 05 May 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword