Estimating the potential threat of increasing temperature to the forests of Turkey: a focus on two invasive alien insect pests
iForest - Biogeosciences and Forestry, Volume 15, Issue 6, Pages 444-450 (2022)
doi: https://doi.org/10.3832/ifor3960-015
Published: Nov 03, 2022 - Copyright © 2022 SISEF
Research Articles
Abstract
Rising temperature can affect forests negatively through its impact on insect pests. The present study focused on two invasive alien insect species (Dryocosmus kuriphilus and Leptoglossus occidentalis) to understand how rising temperature might affect their damage in Turkish forests. For D. kuriphilus, the timing of chestnut budburst, gall induction and emergence of its introduced parasitoid, Torymus sinensis, were monitored between 2015 and 2019, and each phenological event was compared annually with fluctuations in temperature to observe the parasitoid-host synchrony. For L. occidentalis, cumulative degree days (CDD) were calculated, and the possible number of generations produced in 2020 in different regions of Turkey were predicted. The CDD calculations were repeated under increasing temperature and different photoperiod-diapause induction scenarios. Evaluation of the monitoring data on the D. kuriphilus system showed that gall induction occurred at the same time as budburst, whereas T. sinensis emergence was independent from the budburst, and that the parasitoid-host synchrony was disrupted after the abnormally warm winter in 2018. The CDD calculations estimated that L. occidentalis produced one to five generations from north to south in 2020. They also suggested a significant increase in the number of generations in the southern Turkey under temperature increase scenarios. Including photoperiod as a time-limiting factor reduced the highest possible number of generations from five to two. In conclusion, rising temperature has a potential to threaten the biocontrol against D. kuriphilus, and it can increase voltinism in L. occidentalis.
Keywords
Dryocosmus kuriphilus, Leptoglossus occidentalis, Asynchrony, Voltinism, Climate Change
Introduction
Possible impacts of climate change on forest insect pests can affect their range, winter survival, phenology ([3]), host-parasitoid dynamics and biocontrol ([19], [37]) and number of generations produced per year ([26]). In any case, at least some of the pest problems in forests are expected to escalate in the near future (for opposite scenarios see [30]). Extensive tree deaths in temperate forests such as those in California, USA ([45]), Italy and Spain ([12]) or Turkey (A. Emin, pers. com.) are already being witnessed due to a variety of factors related to the recent climate change.
Turkey, located in the northeast of the Eastern Mediterranean basin, has been experiencing a significant raise in temperature and a decrease in precipitation since early 2000s ([13]). The majority of highest temperatures that were recorded between 1928 and 2018 in the country, particularly the warmest winters, occurred after 2000. The highest winter temperatures to date were recorded in 2011 and 2018 ([31]). According to future projections, overall air temperature in Turkey will rise by 1-2 °C until 2040 and 6 °C at the end of the century ([11]).
Although an increasing number of studies provide many insights related to the impact of climate change at a local scale in Turkey, we are far from understanding how forest pest insects may respond locally to the changing climate and what precautions should be taken in Turkish forests. The present study focuses on two invasive alien forest pest insects, in order to understand possible relationships between climate change and these pests.
The Asian chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu 1951 (Hymenoptera; Cynipidae), is the most important insect pest of chestnut worldwide ([36]). It was accidentally introduced to Europe in 2002 ([7]), and first recorded in Yalova, Turkey, in 2014 ([9]). The Turkish General Directorate of Forestry (OGM) transported the host-specific parasitoid of the gall wasp, Torymus sinensis Kamijo 1982 (Hymenoptera; Torymidae), a biocontrol agent of proven success ([10]) from Italy and initiated a gall wasp management program in 2015 under supervision of the author ([23]). After the warmer winter in 2018, an anomaly was noticed in the timing of T. sinensis emergence, which necessitated human intervention to synchronize T. sinensis emergence in the laboratory with the gall induction in nature. It is of paramount importance to understand how phenological asynchrony between the parasitoid and the gall wasp can influence the classical biological control against D. kuriphilus in Turkey.
The western conifer seed bug, Leptoglossus occidentalis Heidemann 1910 (Hemiptera; Coreidae), is an invasive pest causing significant seed damage on almost all coniferous species including economically important stone pine, Pinus pinea L. 1753 ([14]). Being native to northwestern USA, it was first recorded in 1999 in Europe ([44]), and in 2009 in Turkey ([1], [15]). Since then, it rapidly expanded its range and invaded the most important pine nut production spot of the country, the Aegean region, which triggered several research and management programs by the OGM and the local municipalities ([24]). The species is univoltine in North America ([28]), whereas it produces up to three generations in Europe ([43]). The exact or possible number of generations in Turkey are not known, although it is an important information not only to understand the possible impact of the pest on forests in different regions but also to be used in pest management planning.
The present study focuses on the following questions to resolve the above-described problems associated with the two alien pest species: (i) what is the extent and pattern of the discordance among the timing of chestnut budburst, D. kuriphilus gall induction, and T. sinensis emergence? (ii) How many generations per year may L. occidentalis produce potentially in different regions of Turkey? (iii) How may the number of L. occidentalis generations in different regions of Turkey change under different temperature increase and photoperiod scenarios? While the first question was handled according to the results obtained from field observations, the last two questions were interpreted in a theoretical framework and only cover a small part of possible scenarios.
Materials and methods
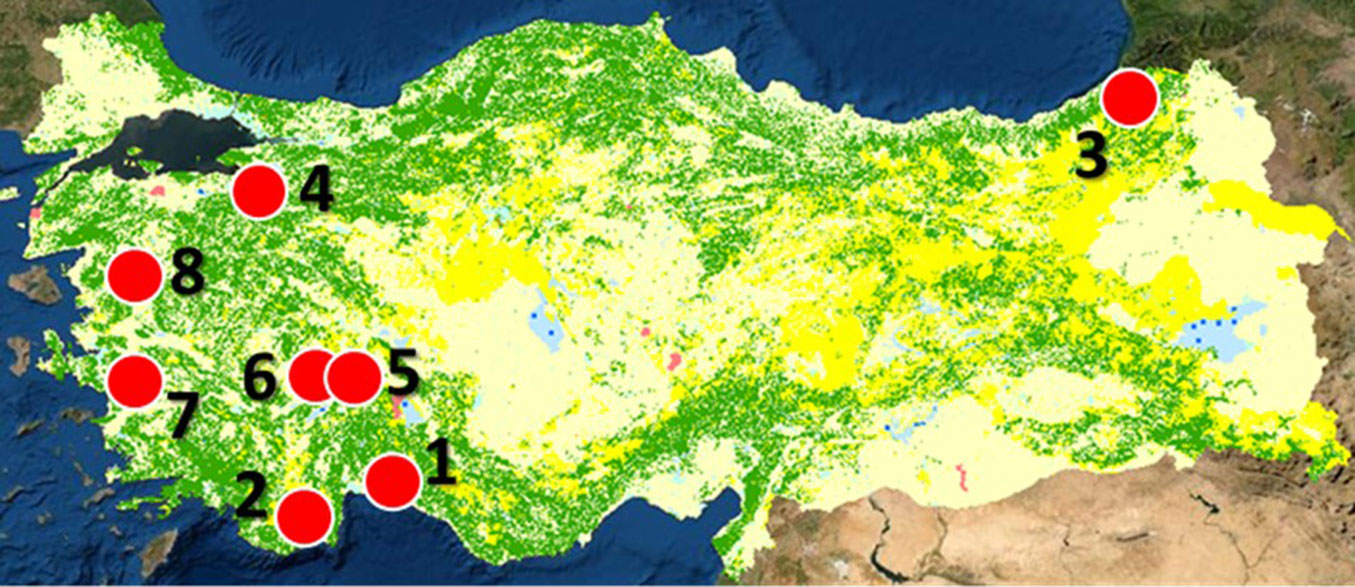
The annual temperature data between 2015 and 2019 was collected from eight meteorological stations throughout Turkey (Tab. 1, Fig. 1). Among those, data from the Bursa station (no. 4 - Tab. 1, Fig. 1) was used for analyses concerning D. kuriphilus, and the others were used to estimate the possible number of generations of L. occidentalis as described below.
Tab. 1 - The meteorological stations where the temperature data were obtained.
| Meteorological Station | Geographic Coordinates |
Altitude (m a.s.l.) |
|---|---|---|
| (1) Antalya-Alanya | 36° 33′ 03″ N - 31° 58′ 49″ E | 15 |
| (2) Antalya-Finike | 36° 18′ 09″ N - 30° 08′ 45″ E | 515 |
| (3) Artvin-Centrum | 41° 10′ 31″ N - 41° 49′ 07″ E | 610 |
| (4) Bursa-Osmangazi | 40° 13′ 51″ N - 29° 00′ 48″ E | 100 |
| (5) Isparta-Centrum | 37° 47′ 06″ N - 30° 34′ 05″ E | 1000 |
| (6) Isparta-Gölcük National Park | 37° 43′ 18″ N - 30° 29′ 35″ E | 1410 |
| (7) Izmir-Selçuk | 37° 58′ 41″ N - 27° 20′ 35″ E | 5 |
| (8) Izmir-Kozak | 39° 13′ 44″ N - 27° 01′ 26″ E | 505 |
Fig. 1 - Geographic location of the meteorological stations from which temperature data were obtained, and the vegetation cover map of Turkey. (Green): forests; (yellow): grasslands and agricultural fields; (red circle): meteorological stations. Vegetation data from OGM ([34]).
Dryocosmus kuriphilus
In order to initiate the Chestnut Biocontrol Program in Turkey, 7000 and 7850 dry galls were transferred from Veneto Region, Italy to Turkey in the winters of 2015 and 2016, respectively. The galls were stored in cardboard boxes at the outdoor booth of the OGM Chestnut Gall Wasp Biological Control Laboratory in Gacik, Yalova Province (northwestern Turkey), and emerging T. sinensis adults were released to the following sites: Site 1, 40° 36′ 16″ N - 29° 20′ 11″ E; Site 2, 40° 36′ 12″ N - 29° 19′ 59″ E; Site 3, 40° 36′ 09″ N - 29° 20′ 24″ E; Site 4, 40° 35′ 55″ N - 29° 20′ 20″ E; Site 5, 40° 35′ 46″ N - 29° 20′ 12″ E; Site 6, 40° 36′ 01″ N - 29° 20′ 30″ E; Site 7, 40° 35′ 57″ N - 29° 20′ 48″ E; Site 8, 40° 36′ 00″ N - 29° 21′ 08″ E; Site 9, 40° 35′ 31″ N - 29° 10′ 23″ E; and Site 10, 40° 35′ 49″ N - 29° 10′ 50″ E. In 2017 and 2018, a total of 194.850 and 301.300 dry galls were collected, respectively, between autumn and winter from these sites for further T. sinensis rearing, and they were stored in cardboard boxes at the outdoor booth. After the unexpectedly early T. sinensis emergence in 2018, a climate chamber was established next to the outdoor booth. In 2019, a total of 344.000 dry galls were collected and stored in cardboard boxes in the climate chamber, while the boxes including totally 6000 dry galls were kept at the outdoor booth (Tab. 2). Timing and number of T. sinensis emergences from the cardboard boxes were recorded between 2015 and 2019. Additionally, regular field trips were conducted between 20 March and 20 April on a weekly basis from 2015 to 2019 in Yalova to record dates of chestnut budburst and gall induction at the study sites on randomly selected 30 chestnut trees in each site. Finally, monthly average temperatures of the region between 2015 and 2019 were obtained to compare temperature anomalies and timing of gall induction along with T. sinensis emergence. Especially since the early T. sinensis emergence was detected in 2018, a comparison was made between the annual temperatures of 2018 and the averages of the other study years. For this purpose, average annual temperatures for the period between 2015 and 2019 (excluding 2018) were compared to the annual temperature of 2018 by Kruskal-Wallis test conducted in R environment ([39]). In order to display monthly differences in temperature, monthly average temperatures between 2015 and 2019 (excluding 2018) were plotted against those in 2018.
Tab. 2 - Number of galls collected (NG) from Italy in 2015 and 2016, and from Turkey in 2017, 2018, and 2019; number of Torymus sinensis emerged from the collected galls (NTs); the first chestnut budburst dates (CB); the first gall induction (G) and adult T. sinensis emergence (Ts) dates recorded in Yalova, Turkey. (*): data from the climate chamber.
| Year | NG | NTs | CB | G | Ts |
|---|---|---|---|---|---|
| 2015 | 7.000 | 1.800 | 3 April | 3 April | 6 April |
| 2016 | 7.850 | 4.224 | 4 April | 4 April | 10 April |
| 2017 | 194.850 | 552 | 7 April | 7 April | 23 March |
| 2018 | 301.300 | 3.250 | 28 March | 28 March | 3 February |
| 2019 | 6.000 (344.000)* |
526 (7.255)* |
10 April | 10 April | 27 March (3 April)* |
Leptoglossus occidentalis
In order to determine the possible number of generations of L. occidentalis in Turkey, cumulative degree-days (CDDs) were calculated based on the maximum and minimum daily air temperatures recorded in 2020 by the Turkish Meteorological Service in the following localities (from lower to higher altitude for the same cities): Antalya-Alanya (southern Turkey - no. 1 in Tab. 1, Fig. 1), Antalya-Finike (southern Turkey - no. 2), Artvin-Centrum (northeastern Turkey - no. 3), Isparta-Centrum (southwestern Turkey - no. 5), Isparta-Gölcük National Park (southwestern Turkey - no. 6), Izmir-Selçuk (western Turkey - no. 7). Data belong to Izmir-Kozak (western Turkey - no. 8) were obtained from the meteorological station of the Izmir Metropolitan Municipality. The CDDs were calculated by using the following formula (eqn. 1):
where Tmax is daily maximum temperature, Tmin is daily minimum temperature, and LDT is lower developmental threshold. Barta ([4]) calculated the LDT for the embryonic and post-embryonic development of L. occidentalis as 13.4 °C and 14.4 °C, respectively. Using separate thresholds did not significantly change the results in preliminary analyses; therefore, 14.4 °C was used for both embryonic and post-embryonic development for simplicity.
In order to estimate possible number of L. occidentalis generations per year per locality, the annual CDDs were divided by the number of degree-days required by L. occidentalis to develop from one egg stage to the next, including embryonic-postembryonic development and adult maturation (533 degree-days, DDs, according to [4]). Furthermore, the life history stages at which development was supposed to cease when the temperature is below the LDT were calculated based on the DD calculations of Barta ([4]) for each developmental stage (DDs for the development of egg, five nymphal instars, and adult maturation are 123, 35, 47, 56, 111, 76, and 85 DDs, respectively). CDDs and possible number of L. occidentalis generations per year were also calculated for four increased average temperature scenarios by adding 0.5, 1.0, 1.5, and 2.0 °C (the range of temperature increase expected to occur by 2040 - [11]) to the daily average temperatures and repeating the same procedure given above.
Finally, the diapause induction in L. occidentalis was hypothesized to occur when a certain critical day-length threshold is passed as shown in several coreids ([33], [17]). To estimate voltinism under this hypothesis, four different critical day-lengths (11, 12, 13, and 14 h), after which reproduction ceases, were selected. Possible number of generations until each critical day-length threshold is reached were calculated in each locality for the current temperature conditions. An online day-length calculator ([41]) was used to determine the dates corresponding to each critical day-lengths for each locality.
Results
Dryocosmus kuriphilus
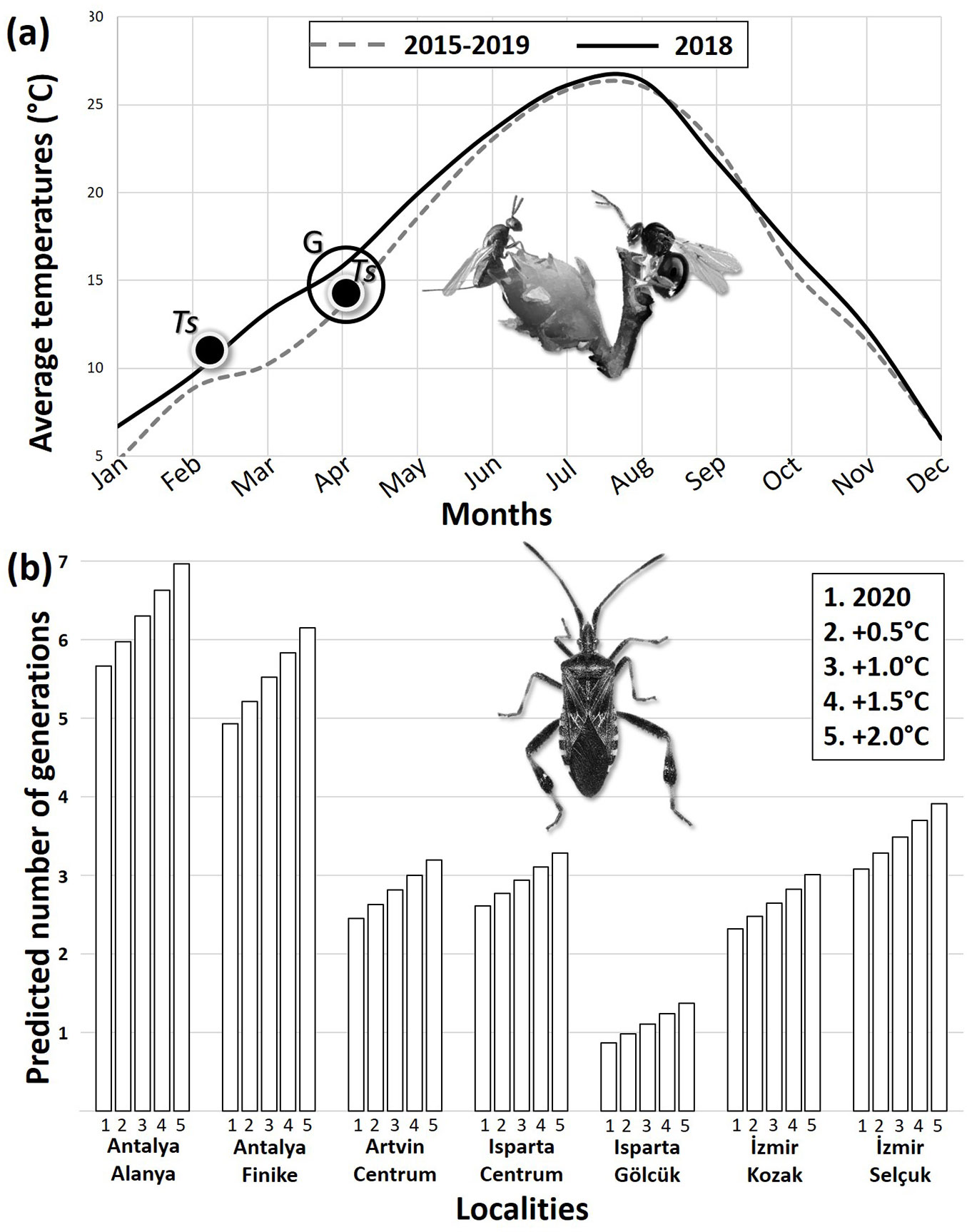
Galls of D. kuriphilus started to appear almost with the same timing each year, in early April, between 2015 and 2019, except in 2018, the year when the first gall induction was recorded earlier (in late March) than in other study years. Adult emergence of T. sinensis was even earlier in 2018. Although, T. sinensis adult emergence started concurrently with gall induction in all other study years, 71.6% (2327 out of 3250) of T. sinensis adults emerged between early February and early March in 2018, much before the gall induction in nature (late March - Tab. 2, Fig. 2a). Almost all (98%) of these early emerging adults died until the first galls formed in nature. Although, no statistically significant difference was found between mean annual temperatures among 2018 and other years (p > 0.05), plotting the average annual temperatures against months showed that the winter of 2018 was significantly warmer than the winters of the other study years (Fig. 2a). While average temperatures in January, February and March were 0, 8.7, and 10 °C, respectively, for the 2015-2019 period (except 2018), they were 7, 9.8, and 13.4 °C, respectively, in 2018. In 2019, T. sinensis adults in the outdoor booth started to emerge on March 27, while those in the climate chamber started to emerge on April 3. Both emergences were concurrent with the budburst in nature. Consequently, the gall induction occurred at the same time as the budburst of the chestnut tree which did not fluctuate significantly from year to year, whereas T. sinensis emergence showed an independent pattern from the timing of the budburst, and T. sinensis seems to respond to increasing temperature by maturation before the budburst and gall induction (Tab. 2).
Fig. 2 - (a) Comparison of average monthly temperatures between 2015-2017 and 2019 periods along with recorded timing of Torymus sinensis emergence (Ts) and Dryocosmus kuriphilus gall induction (G) in Yalova Province, Turkey. (b) Estimated number of Leptoglossus occidentalis generations to be produced according to cumulative degree-day accumulations in 2020 and under four different average temperature increase scenarios in seven different localities from south to north in Turkey.
Leptoglossus occidentalis
The highest CDD for L. occidentalis among the study localities was in Antalya-Alanya (elevation: 15 m a.s.l.; 3020.6 DD), and the lowest was in Isparta-Gölcük (1410 m a.s.l.; 460.5 DD - Tab. 3). Average temperatures did not decrease below the LDT at the end of 2020 in Antalya (15-515 m). This suggested a continuous development in which case the greatest number of generations (five) among the study localities might be reached in Antalya (Fig. 2b, Tab. 3). The lowest number of generations (~1) occurred in Isparta-Gölcük (Fig. 2b, Tab. 3). The CDD in the northern- and easternmost locality of the present study (Artvin-Centrum, 610 m) was 1304.2 DD, which corresponded to two generations. The life history stages at which development was supposed to cease, based on the fact that the development ceases below the LDT, were presented in Tab. 3. According to this calculation, in 2020, development was expected to cease at the third nymphal instar of the third generation in Artvin-Centrum, fourth nymphal instar of the third generation in Isparta-Centrum (1000 m a.s.l.), immature adult stage of the first generation in Isparta-Gölcük, second nymphal instar of the third generation in Izmir-Kozak (505 m), and egg stage of the fourth generation in Izmir-Selçuk (5 m). Regarding to the two localities in Antalya (Alanya and Finike), the development was not expected to cease, and at the end of the year, L. occidentalis was expected to be at the fifth nymphal instar of the sixth generation and immature adult stage of the fifth generation, respectively.
Tab. 3 - Cumulative degree-day (CDD) calculations for Leptoglossus occidentalis in different localities in Turkey, and corresponding numbers of generations that were estimated to be produced in 2020 and that were predicted to be produced under different temperature and photoperiod scenarios. (1): Dates when the air temperature recorded in the corresponding locality drops below the lower developmental threshold (LDT); (2): air temperature never decrease below the LDT; (3) stages in the life history where development is expected to cease as a result of air temperature below the LDT; (4): development does not cease in autumn; (Ai): immature adult; (E): egg; (N1-N5): nymphal instars.
| Scenario | Parameter | Antalya- Alanya | Antalya- Finike | Artvin | Isparta- Centrum | Isparta- Gölcük National Park | Izmir- Selçuk | Izmir- Kozak |
|---|---|---|---|---|---|---|---|---|
| 2020 | CDDs | 3020.6 | 2626.7 | 1304.2 | 1390.2 | 460.5 | 1643.6 | 1236.3 |
| Voltines | 5.7 | 4.9 | 2.4 | 2.6 | 0.9 | 3.1 | 2.3 | |
| Date (1) | - (2) | - | Nov 6, 2020 | Oct 31, 2020 | Oct 19, 2020 | Nov 15, 2020 | Oct 30, 2020 | |
| Stage (3) | - (4) | - | N3 | N4 | Ai | E | N2 | |
| Different Temperature Scenarios |
CDDs (+0.5°C) | 3186.0 | 2781.2 | 1401.3 | 1475.8 | 524.7 | 1749.1 | 1321.8 |
| Voltines (+0.5°C) | 6.0 | 5.2 | 2.6 | 2.8 | 1.0 | 3.3 | 2.5 | |
| CDDs (+1.0°C) | 3358.2 | 2942.0 | 1500.1 | 1564.3 | 590.9 | 1857.6 | 1411.4 | |
| Voltines (+1.0°C) | 6.3 | 5.5 | 2.8 | 2.9 | 1.1 | 3.5 | 2.6 | |
| CDDs (+1.5°C) | 3534.6 | 3108.2 | 1601.1 | 1656.0 | 659.2 | 1970.0 | 1504.8 | |
| Voltines (+1.5°C) | 6.6 | 5.8 | 3.0 | 3.1 | 1.2 | 3.7 | 2.8 | |
| CDDs (+2.0°C) | 3713.2 | 3277.5 | 1704.0 | 1750.7 | 730.1 | 2085.5 | 1601.6 | |
| Voltines (+2.0°C) | 7.0 | 6.1 | 3.2 | 3.3 | 1.4 | 3.9 | 3.0 | |
| Different Photoperiod Scenarios |
CDDs (11 h) | 2628.3 | 2354.2 | 1238.0 | 1368.7 | 460.5 | 1447.6 | 1219.3 |
| Voltines (11 h) | 4.9 | 4.4 | 2.3 | 2.6 | 0.9 | 2.7 | 2.3 | |
| CDDs (12 h) | 2292.3 | 2072.6 | 1087.3 | 1245.0 | 438.4 | 1387.6 | 1120.8 | |
| Voltines (12 h) | 4.3 | 3.9 | 2.0 | 2.3 | 0.8 | 2.6 | 2.1 | |
| CDDs (13 h) | 1851.1 | 1667.6 | 899.7 | 988.5 | 346.0 | 1168.4 | 906.6 | |
| Voltines (13 h) | 3.5 | 3.1 | 1.7 | 1.9 | 0.6 | 2.2 | 1.7 | |
| CDDs (14 h) | 1355.8 | 1170.8 | 714.0 | 670.7 | 231.8 | 809.7 | 636.5 | |
| Voltines (14 h) | 2.5 | 2.2 | 1.3 | 1.3 | 0.4 | 1.5 | 1.2 |
CDDs and the potential number of generations under increased temperature scenarios were also calculated. As expected, rising temperatures resulted in increased CDDs and thus, increased voltinism in all localities except Isparta-Gölcük, where the number of generations could not exceed one (1.4 - Fig. 2b). Even an increase of 0.5 °C in daily average temperatures in Antalya-Alanya resulted in the completion of the fifth generation in a single year in this locality. In all localities except Isparta-Gölcük, an increase of 2 °C resulted in a minimum of three generations. The greatest number of generations was seven and it was reached in Antalya-Alanya after 2 °C increase.
Finally, when the photoperiod was included in the estimation procedure, as a possible factor determining diapause induction, significantly reduced the possible number of generations: from five to two in Antalya, two to one in Artvin-Centrum, Isparta-Centrum and Izmir-Kozak, three to one in Izmir-Selçuk (Fig. S1 in Supplementary material).
Discussion
The theoretical framework set in this study along with the field records and observations suggested that warmer forests in the future may be under the pressure of increased damage caused by the two considered forest pest insects in Turkey. Although the results of the present study were largely obtained in a theoretical framework, being therefore far from conclusive and investigating only a small fraction of possible scenarios, D. kuriphilus case can exemplify failure in biocontrol as a result of the disruption of host-parasitoid synchrony, and L. occidentalis case can exemplify increasing voltinism, which are two of the commonly expected effects of climate change on forest insects ([19], [26]). On the other hand, for example, parasitism alone, which seems to be positively affected from increasing temperature, can potentially reverse this situation, and pave the way for a decrease in population sizes of the forest pest insects in general ([37]).
Asian chestnut gall wasp, D. kuriphilus, and its parasitoid, T. sinensis, seem to respond differently to rising temperature. Gall induction and budburst of the chestnut in nature fluctuated concurrently, whereas T. sinensis emergence was asynchronous in 2018 when the winter temperatures were higher than the other study years; actually, the winter of 2018 was the warmest winter ever after 2011 ([31]). This result suggests that the gall wasp is more dependent on the phenology of chestnut trees than other factors; and chestnut phenology does not seem to fluctuate significantly from year to year as T. sinensis phenology does (Tab. 2), likely because plant phenology does not respond to temperature fluctuation as strong as insect phenology ([35]). Gall induction, which is a function of physiology of vegetative growth to be triggered in spring, occurred also earlier in 2018 than in the other years; however, it was not as early as T. sinensis emergence, which was completely asynchronous (occurring in early February) with the gall induction (late March) in 2018. This suggests that T. sinensis is directly affected from the ambient temperature unlike its host. The parasitoid stays dormant in the dry gall during the winter until the next spring, whereas its host seems to follow the chestnut budburst to develop. A similar pattern has also been observed in a recent study ([16]). Phenological asynchrony between parasitoids and their hosts or predators and preys is one of the predicted outcomes of climate change ([21]). Increasing temperatures may affect parasitoids and hosts differently as a result of their different developmental rates ([19]), and in some cases these differences can work against the parasitoid population ([2]). For example, if the developmental temperature of a parasitoid population is lower than that of the host population, increasing winter-spring temperatures can cause the parasitoid population to emerge earlier than its hosts, and this can drive the parasitoid population to extinction and biocontrol programs to failure ([19]). In order to keep T. sinensis emergence at the laboratory synchronous with the gall induction in nature after the asynchrony incidence in 2018, a climate chamber was established at the laboratory where dry chestnut galls hosting T. sinensis larvae were stored at a constant low temperature until the first gall induction commenced in nature. Although this method provided a synchronous T. sinensis emergence, it is not a stable strategy for the long-term control of the pest in a continuously warming environment because classical biocontrol against D. kuriphilus aims to establish a persistent T. sinensis population in nature to provide a constant control. Early T. sinensis emergence due to increasing winter temperatures may lead to three different outcomes for this system: (i) extinction of T. sinensis as a result of early emerged adults failing to find the host (just like the case in 2018) and biocontrol of D. kuriphilus fails; (ii) host-shift in T. sinensis (adapting to oak gall wasps - [8]), and biocontrol of D. kuriphilus fails; (iii) adaptive penological shift in T. sinensis populations to delay emergence to get re-synchronized with the gall induction as a result of negative selection on early emerging individuals ([46]), and biocontrol of D. kuriphilus is sustained; or (iv) both of the latter two alternatives, which may end up two different T. sinensis strains, one phenologically re-synchronized with D. kuriphilus and the other displaying host-shift. In any case, this system necessitates a long-term monitoring because understanding the consequences of climate change on host-parasitoid dynamics is of fundamental and applied importance ([20]).
Western conifer seed bug, L. occidentalis, was estimated to be able to produce one to five generations per year in the studied localities in Turkey according to DD calculations in the present study. Barta ([4]) estimated four L. occidentalis generations in Nicosia, Cyprus. This result is consistent with the estimation for Antalya (five generations), the closest locality to Nicosia among the localities sampled in the present study. The difference of one generation between Antalya and Nicosia may be due to the difference in altitude between the meteorological stations (16 m a.s.l. for Antalya, 160 m for Nicosia) or to the increased average temperatures from 1998-2000, when the data of Barta ([4]) was recorded, to 2020, when the data used in the present study was recorded. On the other hand, the methods used do not seem to cause the difference, as repeating the calculation for the dataset of Barta ([4]) with the method used in the present study yielded the same results as in Barta ([4]). L. occidentalis overwinters mostly as adult ([43]), therefore, to make inferences about the number of generations and population size, it is important to know when the development ceases. If it is at an early nymphal stage, it would increase the overwintering mortality and decrease the number of adults starting the next generation after the winter ([43]). The CDD calculations in the present study suggested that development ceased at early nymphal instars in Artvin, Isparta, and Izmir, a result consistent with the observation of low population sizes in these localities in spring (M. Avci and Y. Aksu, pers. comm.). On the other hand, increasing temperature is likely to change this picture. Possible number of generations were predicted to increase significantly with increasing temperature in the study localities. Voltinism in some forest insect species can depend upon temperature ([38]). Therefore, increasing the number of generations per year in forest pest populations as a result of increasing metabolism and shortening development is one of the expected symptoms of a global warming ([27]). Some forest pests, such as the coffee berry borer, Hypothenemus hampei Ferrari (Coleoptera) or European spruce bark beetle, Ips typographus L. (Coleoptera), have already started to produce more generations annually ([22], [25]). Regarding to the future temperature increase scenarios, the present study predicted that L. occidentalis can produce up to three generations per year in case of 2 °C increase in the average temperatures in Izmir-Kozak, which is one of the two greatest Turkish hot spots for pine nut production, yielding 80% of the annual pine nut production of the country; and pine nut in this region has a geographic indication certificate registered in 2008. All the other localities studied have important conifer seed orchards and stands along with gene conservation sites which are frequently utilized by the OGM for afforestation practices in the country. Thus, increasing the number of L. occidentalis generations may threaten pine nut production, natural regeneration, and afforestation of conifer forests in Turkey. Moreover, overwintering mortality rate for most of the herbivorous insects can also decrease, and as a result, their outbreak frequency and damage can multiply ([5]). When the increasing amount of damage by L. occidentalis is added to the expected contraction in gymnosperm range in southern Turkey due to climate change ([42]) and forest fires, along with anthropogenic activities threatening the biodiversity in Turkey ([40]), it is plausible to conclude that L. occidentalis may contribute to the decrease in population sizes of conifer species in Turkey by the middle of the century. Options such as moving southern (e.g., Antalya) seed orchards, which are extensively used for afforestation purposes, to higher elevations in the south or northern latitudes, where significantly less number of generations of L. occidentalis is predicted, should be considered. On the other hand, further studies are necessary to make precise predictions. For example, voltinism is a direct effect of the length of diapause, and temperature may not be the only factor affecting it. Diapause induction or termination, or both, are often under the control of photoperiod ([29]), and sometimes its effect is even stronger than that of temperature ([32]). However, photoperiod-diapause relationship in L. occidentalis remains unknown. In the present study, analyses with different possible photoperiod thresholds (which are commonly observed in other coreids -[33], [17]) for the induction of diapause in L. occidentalis showed that their effect on the number of generations could be significant. Similarly, upper developmental threshold and diapause obligation in L. occidentalis, which can directly affect the number of generations, are also unknown. The number of generations that was estimated in the present study omitting the effect of photoperiod, therefore, could be overestimated. This can only be clarified through further studies on the developmental physiology of L. occidentalis.
As well-settled communities composed of coevolved networks would probably be affected from the climate change in a complex way, the fate of individual species in such communities could be more complicated to predict. However, this may not apply to new alien invasive species such as D. kuriphilus and L. occidentalis, which are not involved yet in community level complex and settled networks. Therefore, it may be relatively safe to treat these species individually. Finally, the two species that were considered individually in the present study, their hosts and natural enemies may potentially coevolve to adapt new climatic norms which may invalidate some of the predictions of the present study. We will probably only be able to learn this through long-term ecological monitoring.
Conclusions
This study, based on two invasive alien insect pests, Asian chestnut gall wasp and Western conifer seed bug, suggested that climate change can boost some of the pest problems in forests of Turkey in the near future. Rising temperature can result in disrupted biocontrol and increased voltinism in the forest pests discussed here. As a warmer climate can generally favor insects ([18]), predictions of the present study may be a common trend in the Turkish forests; however, there does not seem to be a single pattern regarding the impact of climate change on forest insects in general ([19], [6], [30]). Unless the theoretical framework drawn especially for L. occidentalis in the present study is tested with laboratory and field observations, it will remain largely as speculation. Consequently, specific studies integrating insect ecology-physiology and climate science are highly needed for a better resolution for the impacts of climate change on the individual species of forest pest insects focused in this study.
Acknowledgements
I thank to Arife Memis and Fatih Memis for their devoted efforts during early biocontrol practices against the Asian gall wasp in Yalova, Turkey, to Akin Emin, Özgür Toprak and Prof. Mustafa Avci for their support in collecting the meteorological data, and to Prof. Andrea Battisti, Prof. Mustafa Avci, Celal Tonbul, and Özden Açici for their comments and ideas. I also thank the Izmir Metropolitan Municipality for the permission to access the data of the meteorological station in Izmir-Kozak.
References
CrossRef | Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Kirsehir Ahi Evran University, Faculty of Agriculture, Department of Plant Protection, Bagbasi, 40100, Kirsehir (Turkey)
University of Padova, Department of Environmental Agronomy - Entomology, Via Università 16a, 35020, Legnaro (Italy)
Corresponding author
Paper Info
Citation
Ipekdal K (2022). Estimating the potential threat of increasing temperature to the forests of Turkey: a focus on two invasive alien insect pests. iForest 15: 444-450. - doi: 10.3832/ifor3960-015
Academic Editor
Anna Loy
Paper history
Received: Sep 01, 2021
Accepted: Aug 24, 2022
First online: Nov 03, 2022
Publication Date: Dec 31, 2022
Publication Time: 2.37 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2022
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 27569
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 21957
Abstract Page Views: 2639
PDF Downloads: 2527
Citation/Reference Downloads: 0
XML Downloads: 446
Web Metrics
Days since publication: 1183
Overall contacts: 27569
Avg. contacts per week: 163.13
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Case study of a new method for the classification and analysis of Dryocosmus kuriphilus Yasumatsu damage to young chestnut sprouts
vol. 5, pp. 50-59 (online: 10 April 2012)
Research Articles
The complexity of mycobiota associated with chestnut galls induced by Dryocosmus kuriphilus in Galicia (Northwestern Spain)
vol. 17, pp. 378-385 (online: 14 December 2024)
Research Articles
Decline in commercial pine nut and kernel yield in Mediterranean stone pine (Pinus pinea L.) in Spain
vol. 13, pp. 251-260 (online: 03 July 2020)
Research Articles
Gnomoniopsis castaneae associated with Dryocosmus kuriphilus galls in chestnut stands in Sardinia (Italy)
vol. 10, pp. 440-445 (online: 24 March 2017)
Research Articles
Fungal community of necrotic and healthy galls in chestnut trees colonized by Dryocosmus kuriphilus (Hymenoptera, Cynipidae)
vol. 12, pp. 411-417 (online: 13 August 2019)
Review Papers
Forest health under climate change: impact of insect pests
vol. 17, pp. 295-299 (online: 30 September 2024)
Short Communications
Local spread of an exotic invader: using remote sensing and spatial analysis to document proliferation of the invasive Asian chestnut gall wasp
vol. 5, pp. 255-261 (online: 24 October 2012)
Research Articles
Nonlinear mixed model approaches to estimating merchantable bole volume for Pinus occidentalis
vol. 5, pp. 247-254 (online: 24 October 2012)
Research Articles
Seeing, believing, acting: climate change attitudes and adaptation of Hungarian forest managers
vol. 15, pp. 509-518 (online: 14 December 2022)
Research Articles
Potential impacts of regional climate change on site productivity of Larix olgensis plantations in northeast China
vol. 8, pp. 642-651 (online: 02 March 2015)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword