The impact of post-defoliation foliage of Pinus halepensis Mill. on the larval performance of Thaumetopoea pityocampa and its relationship with the tree-induced defense
iForest - Biogeosciences and Forestry, Volume 18, Issue 4, Pages 186-193 (2025)
doi: https://doi.org/10.3832/ifor4697-018
Published: Jul 01, 2025 - Copyright © 2025 SISEF
Research Articles
Abstract
Induced defense in trees refers to the increased production of specific substances in response to herbivore attacks, which negatively affects the herbivore. This type of resistance can contribute to the sustainable management of herbivorous insect problems in forest ecosystems. The Aleppo pine, a drought-resistant forest tree species, holds great ecological and socioeconomic importance. One of its predators is the Pine Processionary Moth (PPM) Thaumetopoea pityocampa. This study explores the impact of new foliage that emerges following severe defoliation caused by the PPM on the subsequent year’s larval performance. Moreover, we investigate whether there is a difference in the chemical composition of the foliage of defoliated and undefoliated trees in terms of terpenes (monoterpenes, sesquiterpenes, and diterpenes), total phenols, nitrogen (N), carbon (C), and the C/N ratio. The aim is to determine whether these components act as an induced defense mechanism. The results indicate that the performance of L1 larvae was negatively affected by new foliage after defoliation. Larvae feeding on new foliage experienced high mortality rates and were significantly shorter than those that fed on the foliage of undefoliated trees. The chemical composition analysis revealed that only the new foliage after defoliation contained bornyl acetate (monoterpene), and the concentration of p-cymene (another monoterpene) was higher in this new foliage compared to that of undefoliated trees. Furthermore, two diterpenes, methyl dehydroabietate and dehydroabietal, showed a stronger correlation with defoliation. These compounds have demonstrated insecticidal activity against other insects and could potentially serve as starting components for a bio-insecticide against the PPM. These findings suggest the presence of an induced defense in Aleppo pine, as the tree produces foliage containing specific terpenes that are unfavorable to larvae. This is one of the factors contributing to the reduction of the PPM population. However, due to the current climate change situation, further research is necessary to better understand how these conditions impact the susceptibility of trees to defoliation.
Keywords
Larval Performance, Induced Defense, Terpenes, Pinus halepensis, Thaumetopoea pityocampa
Introduction
Aleppo pine (Pinus halepensis Mill.), a resinous species of the Pinaceae family, is widely distributed in the Mediterranean region and is the most important forest tree species in North Africa in terms of area ([41]). Tolerant of drought and challenging edapho-climatic factors ([27]), Aleppo pine is recommended for reforestation operations in semi-arid regions ([32]). It has been planted in countries such as Argentina, Mexico, South Africa, and Australia ([39]). Aleppo pine holds undeniable ecological and socio-economic importance, providing ecosystem services such as habitat protection, biodiversity conservation, climate and water regulation, and carbon sequestration and storage, all of which help mitigate climate change. Additionally, it plays a crucial role in protecting land from degradation and desertification, while also provides resources for cultural services ([9], [35]). This species is vulnerable to pests such as the pine processionary moth, Thaumetopoea pityocampa (Denis & Schiff.) (Lepidoptera, Notodontidae), commonly referred to by the acronym PPM. This moth is considered the major defoliator of pine trees in the Mediterranean basin ([36]). Its defoliations cause significant economic and ecological damage ([4]), and its urticating hairs affect human health ([4]). Defoliation can result in a reduction in radial growth of up to 90% in cases of total defoliation ([24]). It also negatively impacts cone production in adult trees ([20]). Severe and successive defoliation can lead to tree mortality ([46]). Although various control techniques have been employed to combat this insect, their effectiveness remains questionable ([7]). Furthermore, global warming appears to be facilitating the insect’s expansion into new regions and affecting new species ([20], [3], [38]). Therefore, investigating insect-tree interactions is crucial in addressing this problem. Such investigations could provide information on the vulnerability and subsequent response of trees to defoliation ([25]). The induced resistance of trees is a response that can contribute to the sustainable management of insect herbivore problems in forest ecosystems ([11]). Induced defense refers to the increased production of specific substances by trees in response to insect attacks. This negatively affects the insects, either directly or indirectly, by attracting their antagonists ([46]). The chemical compounds present in foliage, including terpenes, phenols, and nutritional compounds, such as nitrogen (N), and carbon-to-nitrogen (C/N) ratio, can significantly impact the performance of early-stage PPM larvae ([21], [25]). These substances include essential defense molecules, such as diterpenes, monoterpenes, and sesquiterpenes, which are toxic to numerous invasive species ([34], [46]). In addition, the physical and chemical characteristics of pine needles affect the population dynamics of PPM ([25]). Several studies have shown that for various species of pine, such as Austrian pine (Pinus nigra - [2]), Scots pine (Pinus sylvestris - [21]), and Atlas cedar (Cedrus atlantica - [40]), the new foliage produced after defoliation negatively affects larval performance. These studies suggest that trees respond to defoliation by producing foliage that hinders larval development when they feed on it. This can be a factor in the decline of the PPM population, thereby protecting the trees against future attacks. In this regard, Zamoum ([48]) observed a population decline and larval growth retardation in heavily defoliated Aleppo pine areas one year before a decline was noted in lightly defoliated areas. As plant defenses can influence and regulate herbivore population dynamics ([29]), identifying the compounds responsible for this reaction is important and could contribute to enhance pest management. Despite the progress made in this field, many cases remain unknown. The study conducted by Hódar et al. ([22]) on pines did not provide convincing evidence for the existence of an induced defense against PPM in three pine species (Pinus pinaster, P. nigra, and P. sylvestris). A similar issue arises with Aleppo pine, as no previous research has specifically addressed this species. However, the response to PPM attacks varies between pine species in terms of chemical compounds ([12]). In this context, this study aims to enhance our understanding of defoliation in Aleppo pine due to PPM in an area typically located in the semi-arid stage of the Algerian Saharan Atlas Mountains. It focuses on the response of trees to PPM attacks, particularly the effect of post-defoliation foliage on PPM larval performance and its relationship with induced tree defense. The study involves monitoring the behavior of young larvae feeding on foliage from both defoliated and undefoliated trees, along with an analysis of the chemical composition of these foliages.
Materials and methods
Study area
This study was conducted in the Djelfa area, situated in the central Saharan Atlas Mountains of Algeria, approximately 300 km south of Algiers (Fig. 1). The climate is semi-arid, with an average annual rainfall of less than 310 mm. Average temperatures range from 26 °C in July to 5.4 °C in January. The soil is shallow and calcareous, and the vegetation is dominated by steppe formations, particularly Alfa steppe (Stipa tenacissima L.) and Armoise steppe (Artemisia herba-alba Asso). However, the tree formations in the study area consist solely of Aleppo pine, which creates relatively dense and even-aged stands. The undergrowth is sparse and consists mainly of Phoenician juniper (Juniperus phoenicea L.), oxycedra (Juniperus oxycedra L.), and alfa (Stipa tenacissima L.). It is worth mentioning that the extensive areas of artificial Aleppo pine tree formations are the result of reforestation efforts undertaken in the 1970s as part of the Green Dam project. Both natural and artificial stands are homogeneous and dense, with a cover of up to 70%. In the reforested stands, the trees have an average height of 2 to 4 m and are under heavy attack by PPM ([19]). Experiments and foliage sampling were conducted at two sites (E1 and E2), the first located in artificial stands (reforestation) and the second in a natural forest (Tab. 1). The two sites were chosen for their representativeness and accessibility. The two sites were selected to conduct a biological trial on the insect.
Tab. 1 - Characteristics of the study sites.
| Parameter | Station | |
|---|---|---|
| E1 | E2 | |
| Latitude N | 34°39′ 04.52″ | 34°43′ 28.16″ |
| Longitude E | 03°11′ 31.55″ | 03°14′ 38.60″ |
| Altitude | 1122 m | 1245 m |
| Stand type | Reforestation | Natural |
Methodological approach
The experiments focused on two aspects. (i) Performance of young larvae: This involved examining the performance of young larvae in relation to the type of foliage (from defoliated trees vs. undefoliated trees); young larvae were reared in the laboratory and newly hatched colonies were introduced onto both undefoliated trees and previously defoliated trees through assisted defoliation. The impact of the two types of foliage on the young larvae was assessed by estimating their survival and growth. (ii) Foliage biochemical composition: The second aspect involved comparing the biochemical composition of the two types of foliage used to feed the larvae.
Sampling and assisted defoliation
In November 2021, assisted defoliation was performed by infecting Aleppo pine trees with third-stage larval nests collected from neighboring trees to maintain similar stationary conditions. A total of 48 trees were subjected to assisted defoliation, of which 23 trees at site E1 and 25 trees at site E2. To achieve a 90% defoliation rate, 3 to 5 nests were used per tree, depending on the crown size ([22]). Alomg with the trees selected for assisted defoliation, 19 additional trees were designated as controls, of which 9 trees were located in site E1 and 10 in site E2. The control trees were chosen for their similarity to the defoliated trees in terms of size and social position. They were also chosen for the absence of any signs of PPM attack.
Laboratory rearing of young larvae L1
The rearing of young larvae at the L1 stage is crucial, as the first L1 instar of PPM heavily relies on needle food quality ([22]). Larvae were reared in August, September, and October 2022 at the research station of the Institut National de Recherche Forestière (INRF) in Djelfa, Algeria, under ambient conditions. At the beginning of August 2022, twelve egg batches were randomly collected and incubated in glass tubes in the laboratory. Each clutch was transferred to a petri dish upon hatching. Following hatching, the larvae were fed foliage collected from trees in E1 and E2, specifically three defoliated and three control trees from E1, and three defoliated and three control trees from E2. The foliage used to feed the larvae was renewed daily by carefully replacing the exhausted twigs with freshly harvested ones. Covering the base of the twig with cotton soaked in distilled water prevented the needles from drying out quickly. The survival rate was estimated by counting the larvae that reached the second larval instar compared to the initial number of hatchlings.
Monitoring of young colonies on trees
Monitoring was conducted by attaching egg batches to pine trees after ensuring that no other egg batches were present. In September 2022, a total of 195 egg batches were placed on 65 trees. This included 31 trees at site E1 and 34 trees at site E2, with three egg batches attached to each tree (for a similar procedure, see [22]). The trees were monitored daily to observe the emergence of young colonies on the crowns. Once the second larval stage (L2) was reached, two parameters were evaluated: the number of remaining pre-nests on the trees, and the estimated survival rates of the young colonies. These colonies were then transferred to the laboratory to measure the length of the larvae.
Larval length measurements
Larval length was measured in a sample of 200 larvae from 40 colonies, with 5 larvae per colony. Each colony was associated with a single tree, comprising 20 defoliated trees and 20 undefoliated trees. Measurements were taken using a graduated micrometer loupe at 10× magnification. After preservation in formalin for 24 hours, the larvae were spread on glass slides and fixed with transparent glue.
Chemical analysis of the foliage
The chemical analysis of the foliage enabled us to identify the compounds associated with the feeding behavior observed for the larvae. The analysis focused on terpenes, total phenols, nitrogen (N), and carbon (C). Terpene analysis was performed using GC-MS (Gas Chromatography-Mass Spectrometry - [31], [10]). Foliage samples were collected from the previously identified trees (see above) in early September 2022, a date corresponding to the onset of development of PPM L1 larvae ([48]). The needle twigs taken from different parts of the crown were carefully wrapped in aluminum foil, placed in sealed bags, and transported to the laboratory in ice coolers. The essential oils (EOs) were extracted using the hydrodistillation method ([10]). The GC-MS analysis of the essential oils was performed using the method described by Laoudi et al. ([30]). For total phenols, Nitrogen, and Carbon, samples were taken from the same trees in October. These samples were then cleaned, dried, and ground to a fine powder. After preparation of the aqueous extract, total phenolics were determined according to the method of Singleton et al. ([42]). Nitrogen analysis was performed using the Kjeldahl method, while the total weight of carbon in the plant material was estimated by multiplying the weight of the incinerated plant material by 0.72.
Statistical analysis
The survival rate of L1 larvae reared in the laboratory and larval length were compared using Student’s t-test. A two-factor ANOVA was employed to analyze the survival rate of L1 larvae and ascertain any differences in the length of larvae from colonies on defoliated and undefoliated trees in E1 and E2. Regarding foliage, a principal component analysis (PCA) was performed on all variables obtained from the chemical analysis, including 50 identified compounds, monoterpenes, sesquiterpenes, diterpenes, total phenols, Nitrogen (N), Carbon (C), and the C/N ratio. All identified compounds were included in the PCA analysis, as terpenes may occur in tree defense in significant amounts or as minor compounds ([47]). Additionally, a t-test was applied to variables that exhibited the strongest correlation with the PC1 axis. The data were tested for normality and homoscedasticity. All statistical tests and graphs were performed using R software.
Results
Larval survival in laboratory rearing
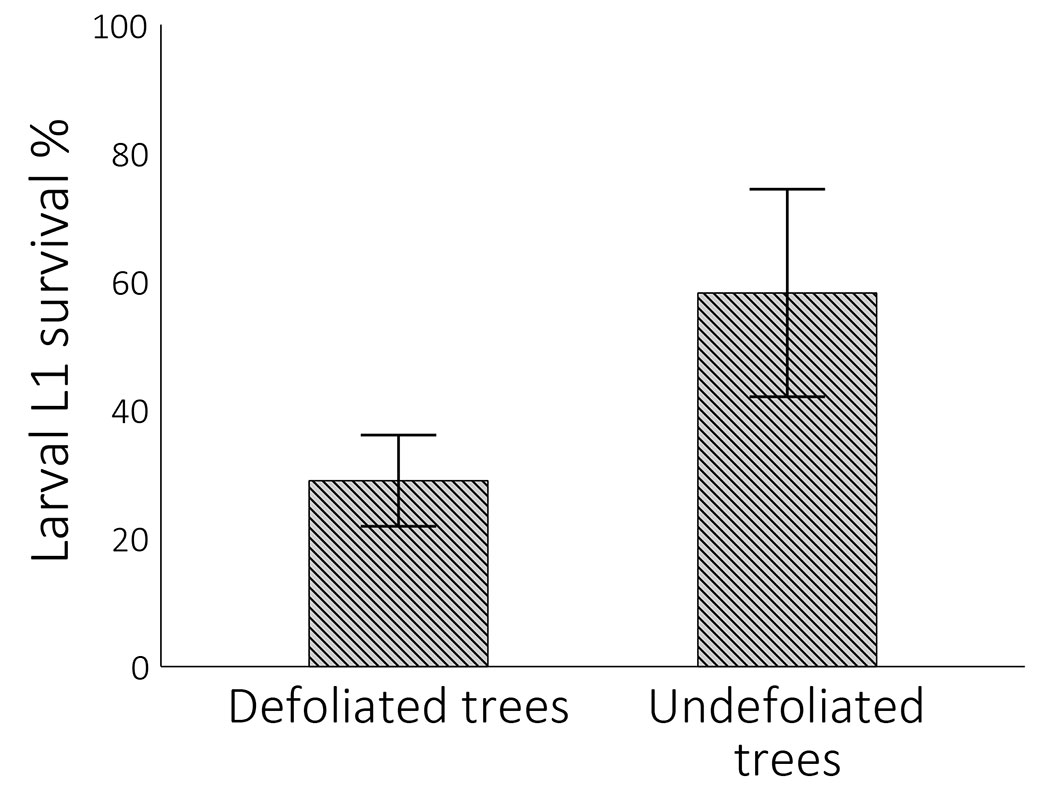
The survival rate of L1 larvae reared in the lab and fed on foliage taken from undefoliated trees was twice as high as that of larvae fed on new foliage taken from defoliated trees. The average survival rates were 58.2 ± 15.4% and 28.96 ± 6.77%, respectively (Fig. 2), corresponding to a reduction of 29.24%, which was statistically significant (t-test, t = -4.2577, df = 6.865, p-value = 0.003). Moreover, two-factor ANOVA analysis revealed that the survival rate of L1 larvae was dependent on the type of foliage, rather than on the harvest site (Tab. 2). This indicates that the foliage of undefoliated trees provides superior nutritional value for L1 larvae survival compared to the foliage of defoliated trees.
Fig. 2 - Mean (± SE) survival (%) of larvae L1 by foliage taken from defoliated and undefoliated trees from experimental sites E1 and E2.
Tab. 2 - Results of a two-factor ANOVA examining the survival rate of larvae L1 fed on foliage taken from undefoliated and defoliated trees of sites E1 and E2.
| Factor | df | SS | MS | F value | Pr(<F) |
|---|---|---|---|---|---|
| Site | 1 | 0.00028 | 0.00028 | 0.016 | 0.90 |
| Foliage | 1 | 0.25 | 0.256 | 14.88 | 0.005** |
| Site × Foliage | 1 | 0.00331 | 0.0033 | 0.192 | 0.672 |
| Residuals | 8 | 0.138 | 0.017 | - | - |
Survival of young colonies on trees
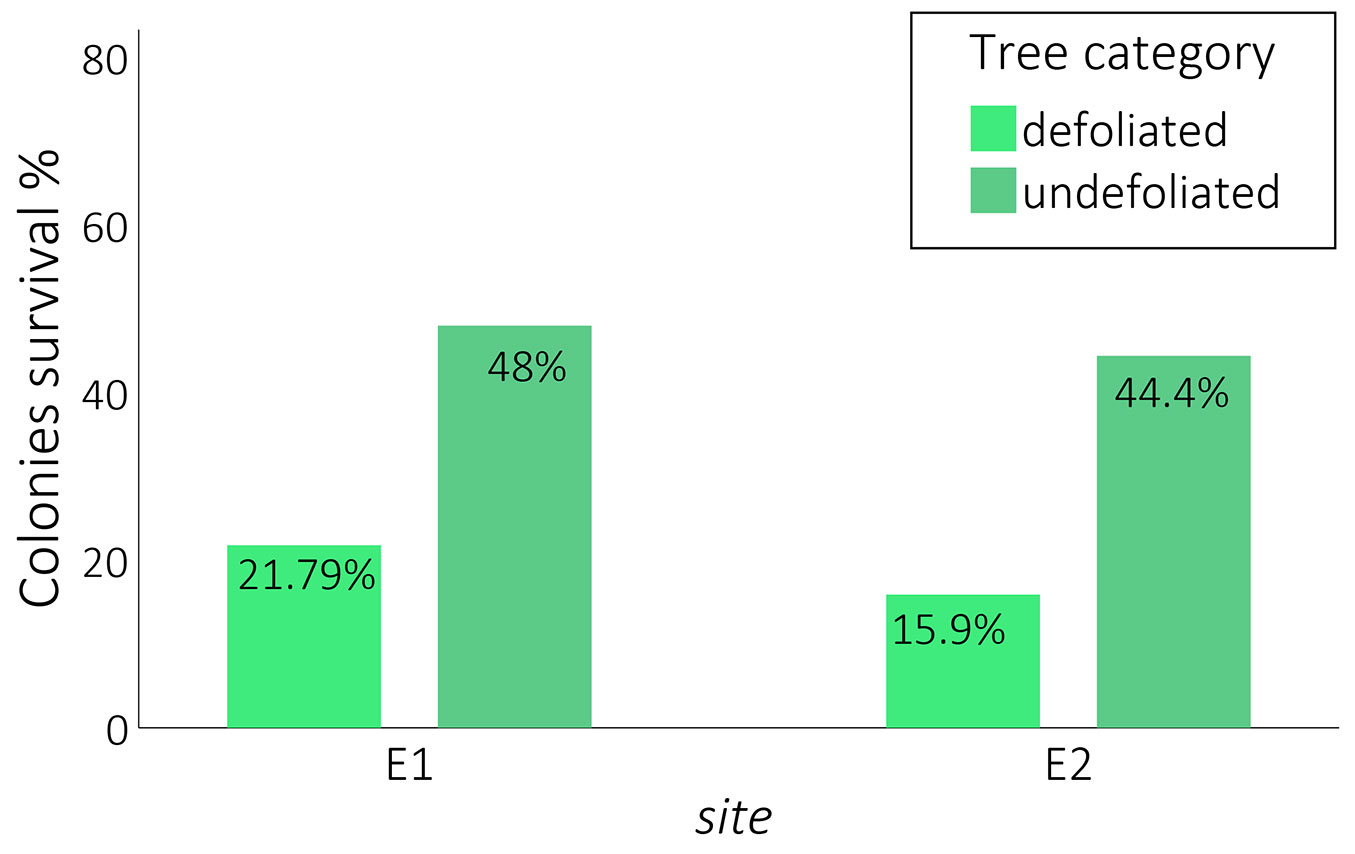
Monitoring colonies on trees provided critical data for understanding larval development following a year of heavy defoliation, thus facilitating the development of new control and prevention methods. We found that colony survival rates were significantly higher on undefoliated trees (48% for site E1 and 44.4% for site E2) compared to defoliated trees (21.79% for E1 and 15.9% for E2). The survival rate of colonies is more than twice as high on undefoliated trees than on defoliated trees, regardless the collecting site (Fig. 3).
Length of larvae from colonies on trees
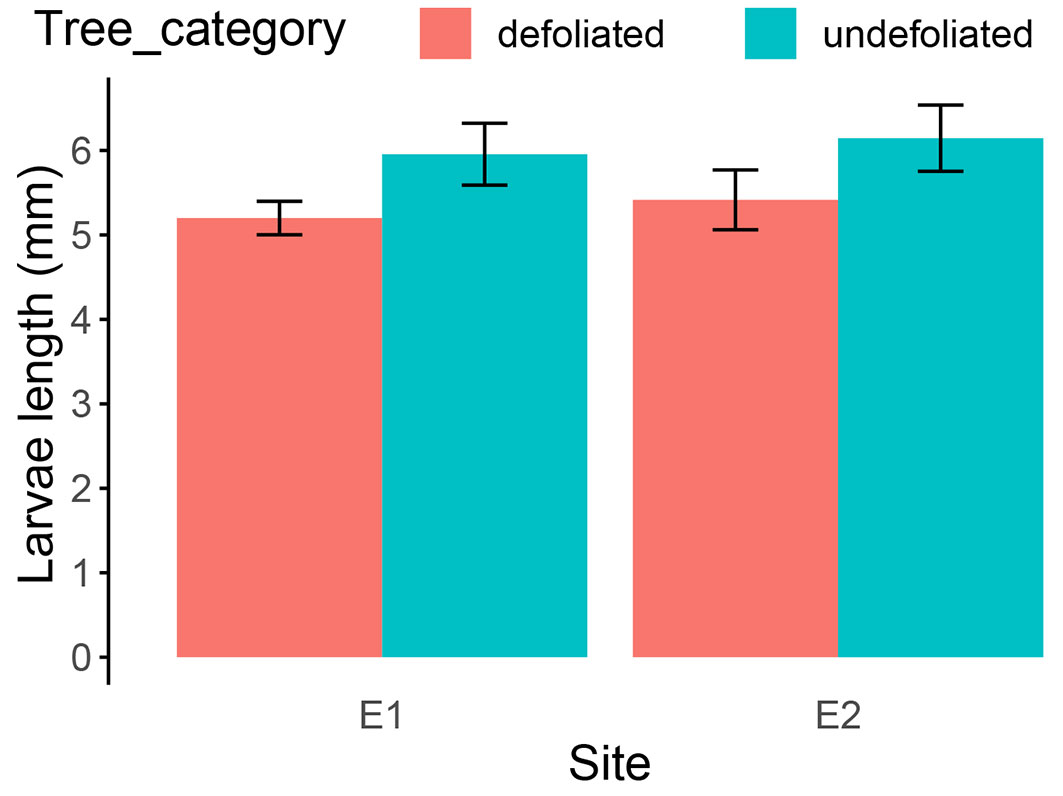
We measured the average length of the larvae from colonies on trees (Fig. 4). The results of the two-factor ANOVA (Tab. 3) indicate that the tree category (defoliated or undefoliated) had a significant impact on larval length. Meanwhile, the site (E1 or E2) does not significantly influence the length of larvae. On undefoliated trees, the average larval length was 5.96 ± 1.22 mm for site E1 and 6.14 ± 1.48 mm for site E2. In contrast, on defoliated trees, the average length of larvae was 5.20 ± 0.75 mm for E1 and 5.41 ± 1.22 mm for E2, i.e., an average reduction in growth of 0.76 mm in E1 (t = -3.5555, df = 65.811, p-value = 0.0007) and 0.73 mm in E2 (t = -2.6724, df = 97.519, p-value = 0.009).
Tab. 3 - Results of a two-factor ANOVA examining the length of the larvae. The factors included are the category of tree (defoliated or undefoliated) and the site (E1 or E2).
| Factor | df | SS | MS | F value | Pr(<F) |
|---|---|---|---|---|---|
| Site | 1 | 3.93 | 3.934 | 2.74 | 0.10 |
| Tree category | 1 | 27.14 | 27.135 | 18.90 | <0.001*** |
| Site × Tree category | 1 | 0.01 | 0.008 | 0.006 | 0.94 |
| Residuals | 195 | 279.98 | 1.436 | - | - |
Chemical composition of foliage
The GC-MS chemical analysis allowed the determination of the chemical composition of the essential oils (EOs), which led to the identification of 50 distinct chemical compounds. The sum of their concentrations corresponds to an average of 98.03 ± 1.33% (± SD) of the EOs, indicating a very high degree of precision in the identification of the EOs volatile compounds. The most abundant compounds were trans-caryophyllene (22.13 ± 7.20%), phenethyl isovalerate (18.52 ± 4.02%), α-pinene (8.06 ± 4.13%), β-myrcene (5.60 ± 1.37%), α-humulene (5.17 ± 1.46%). Nevertheless, the presence of bornyl acetate (0.25 ± 0.14%) only in the foliage samples from defoliated trees should be mentioned.
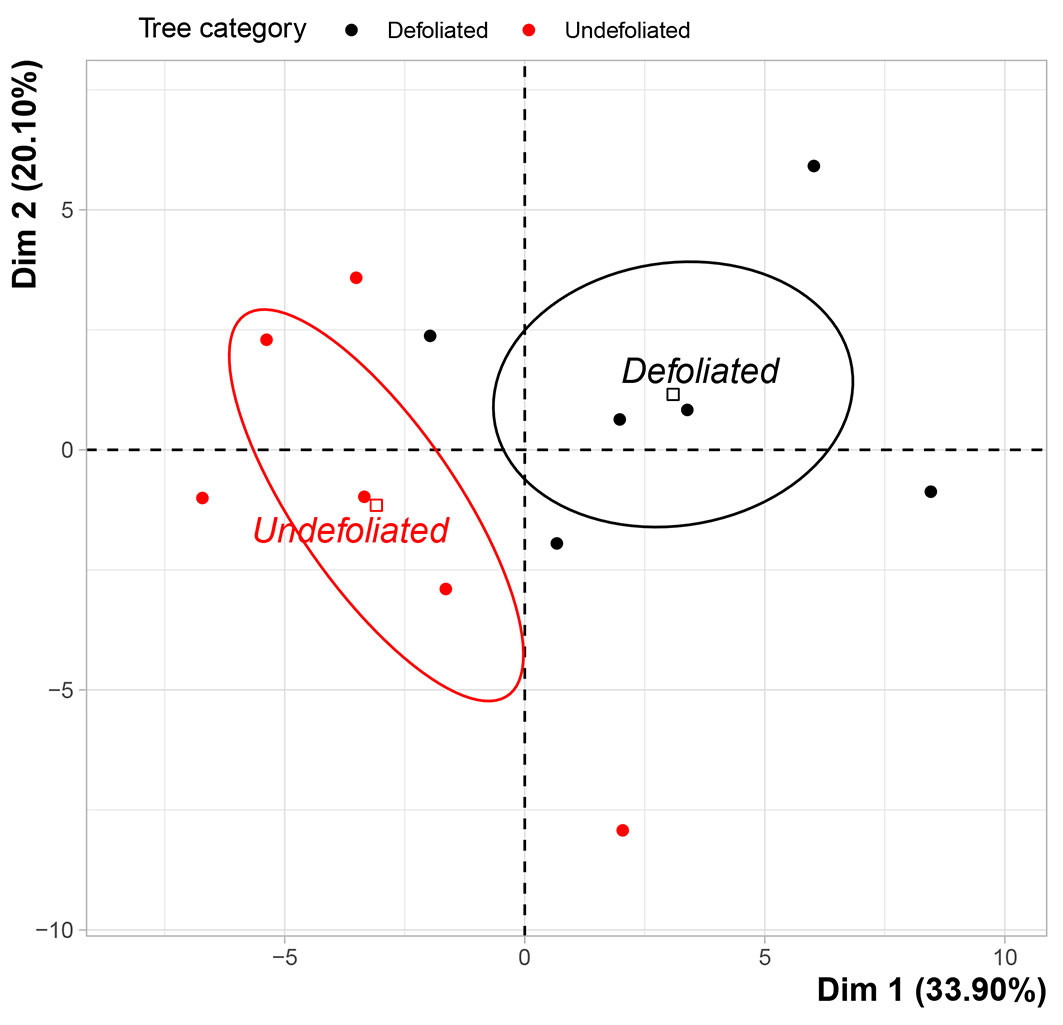
Principal component analysis (PCA) effectively distinguished between the two categories of foliage collected from the defoliated and undefoliated trees, while sites E1 and E2 showed no significant influence (no distinction). The first axis (PC1) accounted for 33.90% of the total variance (Fig. 5) and the second axis (PC2) for 20.10%, overall accounting for 54.01% of the total variance. The two foliage categories were significantly separated along the first axis (R2 = 0.49, p-value= 0.01).
Fig. 5 - Principal component analysis (including all 50 chemical compounds from GC-MS analysis) of the foliage from defoliated and undefoliated trees.
PC1 was found to be associated with 28 variables, with the most significant correlations observed for methyl dehydroabietate, α-humulene, trans-caryophyllene, dehydroabietal, and sesquiterpenes (Tab. 4). The terpenes that exhibited the most positive correlation with PC1 were methyl dehydroabietate, dehydroabietal, and p-cymene.
Tab. 4 - Variables most strongly correlated with PC1.
| Variables | Correlation with PC1 |
|---|---|
| Methyl dehydroabietate | 0.94 |
| Dehydroabietal | 0.85 |
| p-cymene | 0.79 |
| (3E)-Cembrene A | 0.77 |
| Cembrene | 0.77 |
| α-Ylangene | -0.81 |
| Germacrene D | -0.84 |
| sesquiterpenes | -0.84 |
| Trans-caryophyllene | -0.93 |
| α-Humulene | -0.94 |
The t-test showed a significant difference between defoliated and undefoliated tree foliage for the following compounds: p-cymene (t = 3.365, df = 9.991, p-value = 0.007), α-humulene (t = -3.279, df = 7.854, p-value = 0.01), and trans-caryophyllene (t = -3.85, df = 9.989, p-value = 0.003). Methyl dehydroabietate and dehydroabietal showed no significant difference between the two types of foliage.
The p-cymene concentration was 0.60 ± 0.13% in the foliage of defoliated trees, while it was 0.34 ± 0.13% in undefoliated trees. Conversely, the concentrations of trans-caryophyllene and α-humulene were higher in the foliage of undefoliated trees, with values of 27.46 ± 4.72% and 6.18 ± 0.74%, respectively, in comparison to defoliated trees, which had values of 16.80 ± 4.87% and 4.16 ± 1.32%.
The concentration of nitrogen (dry weight) was higher in the foliage of defoliated trees (1.13 ± 0.004%), while in undefoliated trees it was 1.12 ± 0.004% (t = 2.503, df = 9.9827, p-value = 0.03). On the other hand, sesquiterpene concentration was higher in the foliage of undefoliated trees, with a value of 59.39 ± 6.59%, while its value in defoliated trees was 46.96 ± 5.16%; these differences was significant (t = -3.6332, df = 9.453, p-value = 0.005). For total phenols, monoterpenes, diterpenes, C, and C/N ratio, the difference in concentrations between the two types of foliage was not significant.
Discussion
This study was conducted to determine the effect of defoliation of Aleppo pine (Pinus halepensis) by the pine processionary moth (PPM) on the performance of young PPM larvae. The methodology was based on the behavior of larvae feeding on new foliage that develops on trees after severe or total defoliation, and compared to those feeding on foliage of undefoliated trees. This study was prompted by previous observations indicating that years of heavy attack are usually followed by periods without attack, as well as by reference to previous work addressing similar issues in other pine species. The two types of foliage were also studied to determine their chemical composition and to assess any differences between them.
Larval survival
The results indicate that the new foliage appearing after defoliation negatively impacts the performance of PPM L1. High mortality was observed among larvae fed with foliage from defoliated trees in the laboratory, indicating that the new needles growing after defoliation are unfavorable for larval development. Modifications in foliar nutrients or other traits that enhance the response to defoliating insects could represent a form of induced resistance ([8]). A very similar finding was revealed for colonies attached to trees. Indeed, both for E1 reforestation and E2 natural forest sites, the survival rate among PPM colonies was higher on undefoliated trees than on defoliated trees. This finding supports the hypothesis that a direct relationship exists between the prior defoliation of Aleppo pine and the survival of PPM larvae ([49]). This study demonstrates that the type of foliage used to feed L1 larvae has a significant impact on their survival rate. This is also consistent with findings from previous studies on other pine species ([2], [21]) and Atlas cedar ([40]).
Larval length
Larvae were significantly longer on undefoliated trees than on defoliated trees, a trend observed at both study sites. This highlights the importance of mature foliage for optimal larval development. Similarly, Zamoum ([48]) noted a retardation in larval growth in heavily defoliated areas compared to lightly defoliated or undefoliated areas. Evidence exists on the impact of herbivore-induced plant responses on the performance and development of individual insect herbivores ([29]). Moreover, it has been demonstrated that defense chemicals, such as terpenes, can have toxic or inhibitory effects on several insect species ([47]).
GC-MS analysis
The GC-MS chemical analysis enabled us to determine the chemical composition of the essential oils (EOs), identifying 50 different compounds. The chemical composition of foliage was very similar to that reported in studies carried out in the same area ([10], [17]). A total of 19 monoterpenes, 15 sesquiterpenes, and 13 diterpenes were identified.
Monoterpenes and diterpenes
The presence of monoterpenes and high levels of diterpene resins in pine trees has a detrimental effect on defoliating insects ([34]). Our results reveal the presence of bornyl acetate (a monoterpene) only in the new foliage that resprouted after defoliation. Foti et al. ([15]) asserted that the induction of this compound constituted the most significant response to PPM attack in Calabrian pine (Pinus nigra subsp. laricio [Poiret] Maire). Furthermore, Cates et al. ([6]) demonstrated that bornyl acetate exerts a growth-inhibitory effect and affects the survival of the larvae of Choristoneura occidentalis, indicating that it may act as a toxin or a feeding inhibitor. Moreover, bornyl acetate has been shown to possess insecticidal activity against a wide range of insect species ([13], [14], [43]).
P-cymene is also a monoterpene that is positively correlated with defoliation, and its concentration is higher in defoliated tree foliage. P-cymene has demonstrated larvicidal activity against Musca domestica (Diptera: Muscidae - [16]). Additionally, p-cymene is a significant constituent of the essential oils (EOs) derived from the leaves and inflorescences of Chenopodium ambrosioides (L.), which has demonstrated toxicity against Tribolium confusum ([28]).
The two compounds most positively correlated with defoliation are methyl dehydroabietate and dehydroabietal, which are diterpenes. Suárez-Vidal et al. ([45]) also found the induction of diterpenes in Aleppo pine as a response to attack by the pine weevil Hylobius abietis, including abietic and dehydroabietic acids. According to Hao et al. ([18]), dehydroabietic acid and its derivatives possess a strong insecticidal activity. Hwang et al. ([23]) reported the presence of two compounds, methyl dehydroabietate and dehydroabietal, in the resin of Pinus rigida, a species known for its resistance to pinewood nematodes (PWN). In comparison, Pinus densiflora and Pinus koraiensis are considered to be less resistant to these pests. The authors noted that these compounds has a direct impact on the mobility and mortality of PWN.
Sesquiterpenes
Trans-caryophyllene (also known as β-caryophyllene) and α-humulene (also known as α-caryophyllene) are sesquiterpenes negatively correlated with defoliation. The high concentration of these two compounds in undefoliated foliage, compared to newly developing foliage after defoliation, is particularly revealing. This observation is consistent with findings reported by Achotegui-Castells et al. ([1]) in Scots pine (Pinus sylvestris), who suggested that the decrease in sesquiterpene concentration in the foliage following defoliation may result from a compensatory response among different terpenes in the tree after the attack. This assumption is supported, among other findings, by Hwang et al. ([23]), which indicates that sesquiterpene levels are lower in the resin of Pinus rigida Mill., a species that is more resistant to PWN.
Nitrogen, arbon, C/N, and total phenol
The production of low-nutritive-quality foliage after defoliation may serve as a defense strategy, as new plant tissues do not sufficiently meet the nutritional requirements of defoliators ([8]). In the present study, the nitrogen (N) rate is of particular significance with regard to the new foliage. On the other hand, no significant differences were observed in the carbon content and C/N ratios between the two types of foliage. The observed increase in larval mortality in the foliage after defoliation suggests that the nutritive quality did not affect the survival of PPM L1 larvae. It is also noteworthy that the nitrogen concentration in the new foliage increased after defoliation, as previously observed by Hódar et al. ([21], [22]). In response to defoliation, trees often undergo compensatory growth, which entails boosting photosynthesis and increasing leaf nitrogen ([8], [22]). This result contradicts the CNB hypothesis, which suggests that abundant nutrients, like nitrogen, enhance plant growth while reducing chemical defenses. In contrast, it supports the GDB hypothesis, which proposes a trade-off between growth and the production of defense compounds ([44], [46]).
The total phenol content showed no significant differences between the two types of foliage. In response to PPM defoliation, the increase in phenol content, which acts as a defense mechanism, was not consistently observed in several cases ([21], 2015).
Terpenes associated with induced defense against PPM
Unlike the findings of Hódar et al. ([22]) regarding Pinus pinaster, P. nigra and P. sylvestris, the principal component analysis (PCA) of the chemical compounds enabled us to clearly distinguish the foliage from defoliated and undefoliated trees. This differentiation may be due to the induced defense mechanisms against PPM in the Aleppo pine. Notably, chemical defenses against herbivores in Mediterranean pine species exhibit considerable diversity, even within populations ([46])
Several studies have investigated the relationship between terpenes and PPM defoliation. However, research on these terpenes and their contributions to defensive mechanisms against defoliators is still in its early stage ([12]). Furthermore, our study presents the initial observation of four terpenes that may play a role in the defense response of Aleppo Pine against one of its main herbivores. The compounds identified in this study, including bornyl acetate, p-cymene, methyl dehydroabietate, and dehydroabietal, may represent a potential starting point for the development of a bio-insecticide against PPM. Essential oils and their components, such as terpenes, can be synthesized and formulated into stable bioinsecticides for use in targeted pest management strategies ([37]). These formulations offer an environmental friendly alternative to synthetic chemicals, thereby reducing reliance on conventional insecticides ([37], [33]). However, these findings are still preliminary and require further validation. Additional studies utilizing a greater number of samples are necessary to substantiate these findings. Moreover, further investigation is needed to assess the effectiveness of these terpenes against the PPM.
Conclusion
The results of this study indicate the existence of an induced defense mechanism against PPM in Aleppo pine. Our findings suggest that severe defoliation in this pine species could be one of the factors contributing to the reduction of the PPM population. The tree seems to react by producing foliage containing specific terpenes with insecticidal properties that are detrimental to the larvae, thus preventing a second consecutive severe attack. This result must also be considered in the management of insect populations. It is important to note that the present study focused on a single population of Aleppo pine, thus not including possible additional variation from different provenances. Furthermore, due to the ongoing effects of climate change, additional research is needed to better understand how this impact affects trees’ susceptibility to defoliation, as it could positively influence the population of PPM ([5]). Moreover, it is possible that climate change could also impact the defensive mechanisms of trees ([26]).
Acknowledgements
The Authors thank all the teams at INRF Algeria (Institut National de la Recherche Forestière), especially at the Djelfa station, for their invaluable assistance during the fieldwork and sample preparation. We are indebted to the team at the Baïnem station in Algiers, particularly the Biochemistry Department, for their expertise in chemical analysis and essential oil preparation. We are also grateful to the staff of the National Higher School of Agronomy (ENSA) laboratories in Algiers for their assistance and permission to conduct some chemical analyses.
References
CrossRef | Gscholar
CrossRef | Gscholar
Online | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Mohamed Sbabdji 0000-0002-1961-4219
Conservation, Management and Improvement of Forest Ecosystems laboratory, National Higher Agronomic School, El Harrach, Algiers (Algeria)
National Institute of Forest Research (INRF), El Hammamet, Algiers (Algeria)
Agricultural Sciences Department, Faculty of Natural Sciences and Life, Ziane Achour University of Djelfa (Algeria)
Scientific and Technical Center of Research in Physical and Chemical Analysis CRAPC, BP384, Bou-Ismail,42004 Tipaza (Algeria)
Corresponding author
Paper Info
Citation
Guadguad M, Sbabdji M, Mouissa H, Chebrouk F (2025). The impact of post-defoliation foliage of Pinus halepensis Mill. on the larval performance of Thaumetopoea pityocampa and its relationship with the tree-induced defense. iForest 18: 186-193. - doi: 10.3832/ifor4697-018
Academic Editor
Matteo Marchioro
Paper history
Received: Jul 27, 2024
Accepted: Mar 19, 2025
First online: Jul 01, 2025
Publication Date: Aug 31, 2025
Publication Time: 3.47 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2025
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 6525
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 2444
Abstract Page Views: 2324
PDF Downloads: 1647
Citation/Reference Downloads: 1
XML Downloads: 109
Web Metrics
Days since publication: 238
Overall contacts: 6525
Avg. contacts per week: 191.91
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effects of defoliation by the pine processionary moth Thaumetopoea pityocampa on biomass growth of young stands of Pinus pinaster in northern Portugal
vol. 3, pp. 159-162 (online: 15 November 2010)
Research Articles
Abundance and impact of egg parasitoids on the pine processionary moth (Thaumetopoea pityocampa) in Bulgaria
vol. 14, pp. 456-464 (online: 02 October 2021)
Research Articles
Plant phenotype affects oviposition behaviour of pine processionary moth and egg survival at the southern edge of its range
vol. 11, pp. 572-576 (online: 01 September 2018)
Research Articles
Performances of an expanding insect under elevated CO2 and snow cover in the Alps
vol. 1, pp. 126-131 (online: 27 August 2008)
Research Articles
Spruce budworm biological and nutritional performance responses to varying levels of monoterpenes
vol. 6, pp. 310-314 (online: 16 July 2013)
Research Articles
Controlled-release fertilizers combined with Pseudomonas fluorescens rhizobacteria inoculum improve growth in Pinus halepensis seedlings
vol. 8, pp. 12-18 (online: 12 May 2014)
Research Articles
Pinus halepensis Mill. in the Mediterranean region: a review of ecological significance, growth patterns, and soil interactions
vol. 18, pp. 30-37 (online: 15 February 2025)
Research Articles
Comparison of alternative harvesting systems for selective thinning in a Mediterranean pine afforestation (Pinus halepensis Mill.) for bioenergy use
vol. 14, pp. 465-472 (online: 16 October 2021)
Research Articles
Seasonal variations in monoterpene profiles and ecophysiological traits in Mediterranean pine species of group “halepensis”
vol. 1, pp. 65-74 (online: 28 February 2008)
Research Articles
Carbon and nutrient contents in the miscellaneous fraction of litterfall under different thinning intensities in a semiarid Pinus halepensis afforestation
vol. 12, pp. 375-382 (online: 12 July 2019)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword