Performances of an expanding insect under elevated CO2 and snow cover in the Alps
iForest - Biogeosciences and Forestry, Volume 1, Issue 4, Pages 126-131 (2008)
doi: https://doi.org/10.3832/ifor0466-0010126
Published: Aug 27, 2008 - Copyright © 2008 SISEF
Research Articles
Abstract
Variations of phenology and distribution have been recently highlighted in numerous insect species and attributed to climate change, particularly the increase of temperature and atmospheric CO2. Both have been shown to have direct and indirect effects on insect species of various ecosystems, though the responses are often species-specific. The pine processionary moth, Thaumetopoea pityocampa (Lepidoptera, Notodontidae) is an important pest of conifers in the Mediterranean region, and has been recently shown to expand its altitudinal range in the Alps, including the mountain pine Pinus mugo as a novel host. We had the opportunity to transplant colonies of the pine processionary moth to a high elevation site well outside of the current range of the insect (Stillberg, Davos, Switzerland, 2180 m), where trees of the mountain pine have been grown for five years under ambient and elevated CO2 concentrations (ca. 570 ppm). The aim of the study was to evaluate the response of first instar larvae to extreme conditions of temperature and to an altered performance induced by the change of host metabolism under elevated CO2. Larval mortality and relative growth rate did not differ between host trees grown in ambient or elevated CO2. As extended snow cover may be an important mortality factor of larval colonies on the dwarf trees of mountain pine, we tested the survival of colonies transplanted at two extreme sites of Eastern Alps. The snow cover extended over more than one month proved to be an important mortality factor of larval colonies on mountain pine. We concluded that the first instar larvae of the pine processionary moth are not concerned by unusually low temperature and CO2 increase whereas they can be later strongly affected by snow accumulation. The decrease of snow cover observed in the last decades, however, may reduce such a risk.
Keywords
Climate change, Thaumetopoea pityocampa, Range expansion, Pinus
Introduction
Numerous studies have shown that it is possible to detect the effects of a changing climate on ecological systems ([36], [37]), taking into account all components of food webs ([40]). However, the study of climate change effects on ecosystems is complex because of many potentially interacting factors, e.g., species, genotype. A principal driver of climate change is the increase in atmospheric CO2 concentration. It has increased by 31% since the pre-industrial time, from 280 ppm to more than 370 ppm ([29]) and is expected to increase in the next 100 years to 500-900 ppm, depending on models of projection ([43]). This change has a significant indirect effect on herbivorous insect by altering quantity and quality of food supply. The survival and performance of insect could be affected by the variation in tissue C/N ratio, water content, leaf toughness and carbon-based defence compounds occurring in their host plant ([27], [41]). However, the responses of different plant species show enormous interspecific variation, as do herbivores feeding on them ([23]). Recent studies have shown that herbivorous insects grown in elevated CO2 may respond with a lower development and higher mortality ([11], [32]); other studies showed no variation of performance but a higher fecundity in female and increasing of lipid concentration in males ([20]), and again no effect on growth rate, larval instar duration and pupal weight ([30]). However, Zvereva & Kozlov ([55]) pointed out in their review that insect’s fitness is reduced under elevated CO2, although the negative effects can be mitigated by a corresponding temperature increase. In addition, responses of different host species to increased CO2 may differentially affect the performance of oligophagous/polyphagous herbivores ([1], [41]). A possible effect is the change of food preference with potential consequences for population dynamics of the plants ([18], [2], [1]), although there are studies where no preference shifts were observed ([15], [34]). The first experiments of CO2 enrichment were carried out under controlled conditions on detached plant material or directly on plants grown in greenhouse, and the artificiality of these experiments may mask processes of ecological relevance and lead to detection of effects not occurring in the field ([27], [20], [31], [32]). However, data are now available from FACE (Free Air CO2 Enrichment) facilities set up in the field, even in the tree canopy (e.g., [25]), as well as for sites located at the alpine treeline ([3], [4]).
The pine processionary moth (Thaumetopoea pityocampa - [14]) is an insect pest of pines that is expanding its range as a consequence of climate change ([5]), including novel hosts that may become readily exploited, as it was observed for the mountain pine in the Alps. The mountain pine group includes two major forms known as upright (Pinus uncinata Ramond) and dwarf or creeping (Pinus mugo Turra), differing mainly in their growth habit but showing often intermediate forms ([50]). As the stands of mountain pine are extensively distributed beyond the historical range of T. pityocampa, it is likely that new problems will arise if the current trend of expansion will be maintained. However, the expansion could be limited by the accumulation of snow on the branches during the winter, when the larvae need to leave the nest for feeding ([7], [17]).
The main objective of the study is two-fold, first to assess survival and performance of young larvae of processionary moth in high-elevation FACE facility on mountain pine trees during the summer. We tested the hypothesis that a change in food quality, due to the increase in CO2, affects insect performance in an environment where the larvae cope with extremely low temperature. The second aim is to evaluate whether the insect winter survival in stands near to the border of the range is affected by the snow-pack. The creeping habit of mountain pine furthers the cover of the nests for extended periods of time, and this could create a risk of colony mortality. We have also analysed the snow cover data of a possible expansion area, to predict insect performance.
Materials and methods
FACE experiment
The experiment was carried out at the treeline FACE site established in 2001 at Stillberg (Davos) in the Swiss Central Alps (see [26] and [22] for details about CO2-enrichment system and experimental set-up). The site is located on a slope oriented towards North-East at 2180 m elevation. The long-term annual precipitation is 1050 mm, the average temperature is -5.8 °C in January and 9.4 °C in July ([42]). The soil is classified as a Ranker (U.S. system: Lythic Haplumbrept) with a 10-cm-deep organic soil underlain by siliceous bedrock (Paregneis - [42]). The experimental site is located in an afforestation area planted in 1975 with three tree species (Larix decidua L., Pinus cembra L., Pinus uncinata Ramond). In 2001, an area of approximately 2500 m2 situated at 2180 m of elevation was selected to provide an experimental setup for a CO2-enrichment study at tree line ([26], [22]). At the time of the experiment, there were 9 trees of mountain pine (P. uncinata upright form) that were grown for the previous 5 years under an elevated CO2 atmosphere (ca. 570 ppm) from June to September and 10 trees grown under ambient CO2 (ca 370 ppm - see [26] and [22] for details about CO2-enrichment system and experimental set-up). The leaf chemistry of needles of the enrichment experiment was shown in previous studies ([26], [22]).
We used sets of insects for the experiment consisting of artificially created groups of 50 first instar larvae. At the end of July 2005, 30 egg batches were collected from several trees in Venosta/Vinschgau valley, Italian Alps and transferred to laboratory. After hatching, groups of 50 larvae were formed by picking individuals from different colonies. The larvae were provided with needles of mountain pine in Petri dishes. The food was renewed every 2-3 days and the dead larvae replaced with new ones. On August 8th we transferred and exposed the larvae groups on ten trees under ambient and nine trees under elevated CO2 conditions, all belonging to the enrichment experiment; we also added ten naturally established trees of mountain pine (P. mugo semi-prostrate form) growing on the same slope at slightly lower elevation (2030 m) to control for a putative temperature effect. The trees were of a size similar to those used in the enrichment experiment. On each tree, we selected one lateral branch that was protected with a 0.1 mm mesh net sleeve, about 40 cm long and including all the needle age classes occurring on that branch. On every branch, a group of 50 first instar larvae was gently transferred from the Petri dish to the needles inside the sleeve. On September 9, the sleeves were opened and their content (faeces, dead larvae, fallen needles) transferred into a vial. Then the larvae were taken from the twig with a brush and put into a Petri dish. Later the amount of faecal pellets was measured by volume assessed in a micropipette, larvae were counted and weighed, and the larval instar was assessed. After freezing at -20°C, the dry weight was obtained after 24 h of exposure at 60°C. The relative growth rate (RGR mg-1 mg-1 day) was calculated for the groups of 50 larvae using both dry and fresh weight and using the initial weight from three groups of 15 larvae. At each site, temperature was recorded hourly by data loggers (Hobo, Onset Computer Corporation, Pocasset, MA). At the end of the experiment we took a sample of three needle pairs for each tree to measure needle toughness, by determining the force needed to penetrate the needles using a calibrated penetrometer constructed for this purpose. Each pine needle was fixed between two metal plates, and the probe (Bohemia insect pin®, diameter 0.55 mm) of the penetrometer was inserted through the central part of the upper, rounded surface. Three readings were taken per needle, avoiding the distal parts.
Transplant experiment
The transplant experiment was carried out in the two sites in South-Eastern Alps. The first (Resia) is located in an area of recent colonization by the insect, on a north-facing slope at an altitude of 500 m. The second site (Tanamea) is situated at 850 m on a northwest facing slope, in an area where the insect is not occurring yet. Both sites have natural stands dominated by mountain pine but with presence of Pinus nigra at lower frequency, especially in the second site. At the end of July 2002, 90 egg batches were collected in pine stands close to the experimental area. The egg batches were exposed in the field, before hatching. At Resia site 30 egg batches were exposed on each of the two host plants, whereas at Tanamea we exposed 30 egg batches only on P. mugo, because of the scarcity of P. nigra. Each egg batch was tied to the branch of a separated tree, and the larval development was checked every two weeks, noting the larval development stage (based on the size of faeces visible though the silk), nest size and recent feeding. After moulting to the fifth instar (end of March), the nests were collected and placed individually in a pot. The pot was closed with a net to avoid the larvae escape. The larvae were regularly fed; on the bottom of the pot we positioned sand to consent the pupation of the larvae, that was observed in May. During the adult emergence period (July) the pots were checked every four days and all present adults were collected and subsequently the sand winnowed to find diapausing cocoons.
As snow resulted to be an important limiting factor to insect survival, we analysed daily data of the snow in addition to temperature in the period December - April. Four sites close to the experimental area were chosen in the elevation range of 540-900 m; we selected the mean height of snow as variable, which was analysed for the period 1972-2007.
Statistical analyses
The larval performance (% survival and RGR) and needle toughness on the three different tree conditions were analysed with a one-way ANOVA. In all cases in which ANOVA was employed, the basic assumption of homogeneity of variance was met, and variables were tested without any transformation. Tukey’s test was used for pairwise comparison of means. The larvae distribution in different stages was analysed by a χ2 test on the three tree conditions, comparing observed frequencies with those expected of the trees. The number of faecal pellet was analysed by ANCOVA using the number of larvae as a covariate. The temperatures in different sites were analysed by a Student’s t-test. The survival in the transplant experiment was analysed by a χ2 test, comparing observed frequencies at different sites and host plant species with those expected calculated on the base of medium values. The data of mean height of snow was analysed with discontinuity analysis that allowed to identify the period and the year with the highest probability of a breakpoint in the data set. All the analyses were done with Statistica software ([45]), except for the snow data, for which we used the open source package Strucchange written in R language ([53]).
Results
CO2 experiment
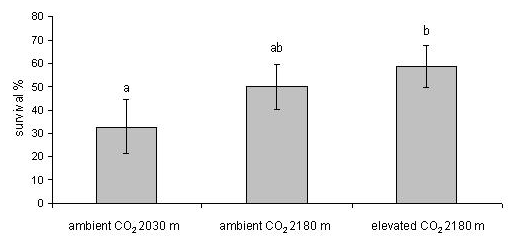
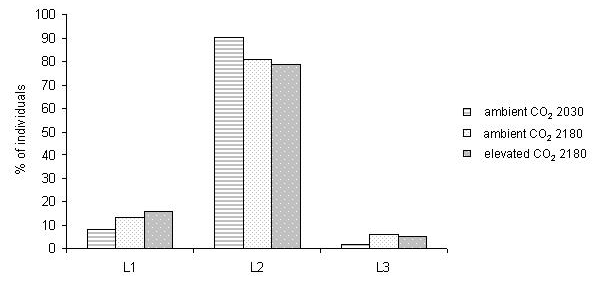
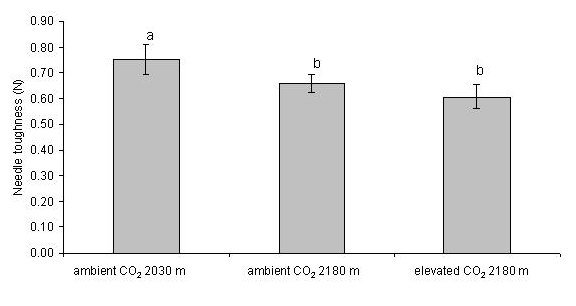
The larvae did not show any difference in mortality between ambient and elevated CO2 at 2180 m, whereas those feeding at 2030 m on naturally established trees under ambient CO2 conditions showed a higher mortality (F2.25 = 4.895, P < 0.01 - Fig. 1). The larval stage was more advanced on native trees under ambient CO2 (χ2 = 10.69, d.f. = 4, p = 0.03 - Fig. 2). The RGR did not differ among treatments using either dry (F2.25 = 0.678, p = 0.317; mean = 0.036, SE = 0.0044) or fresh weight (F2.25 = 0.706, p = 0.502; mean = 0.038, SE = 0.0045). During the experiment, the mean minimum daily temperature was 4.9 °C, with several nights with temperature below or around 0 °C, and the maximum daily temperature was 19 °C at FACE site (2180 m), whereas at lower site (2030 m) the values were 0.61 °C and 2.63 °C higher, respectively (t-test, minimum temperature: t = 5.27, d.f. = 62, p < 0.01; maximum temperature: t = 7.00, d.f. = 62, p < 0.01). The volume of faecal pellets did not differ among treatments (F2.24 = 1.316, p = 0.286). The needles of native trees under ambient CO2 were significantly tougher than those of trees grown at higher elevation under both elevated and ambient CO2 (F2.25 = 24.41, p < 0.01 - Fig. 3).
Transplant experiment
Some egg batches did not hatch and thereby we utilized 27 colonies on P. nigra and P. mugo at Resia and 21 colonies on P. mugo at Tanamea. There was no difference in the colony survival on P. mugo and P. nigra located at Resia before (χ2 = 3.64, d.f. = 1, p = 0.056) and after (χ2 = 1.02, d.f. = 1, p = 0.311) the cold period. When comparing the two sites on P. mugo, the survival was similar before the cold period (χ2 = 1.763, d.f. = 1, p = 0.184) but differed dramatically after (χ2 = 6.373, d.f. = 1, p = 0.0115), as total mortality was observed in Tanamea, where colonies were buried in snow for more than one month (Tab. 1).
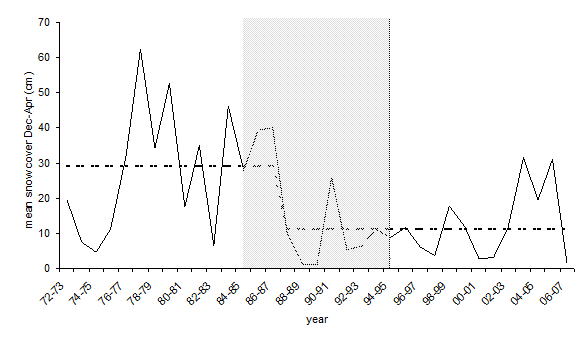
The mean height of snow cover showed a decreasing trend in the period 1972-2006 (Fig. 4), although there was strong variation among years. We individuated the period 1984-1994 as the more likely time when a change has occurred (at 90% confidence level), and the year 1986-87 as the more likely breakpoint in the data set.
Discussion
CO2 enrichment had no effect on the herbivorous insect, Thaumetopoea pityocampa, although there was significant variation at the plant level, consisting in an increase of non-structural carbohydrates and a reduction of specific leaf area ([22]). Both survival and performance of larvae did not differ between individuals feeding on plants of mountain pine exposed to ambient and elevated CO2. However, the larvae exposed on naturally established mountain pines growing at slightly lower altitude showed higher mortality than those on plants at the upper site, but a significant difference was observed only when contrasted to survival on elevated CO2 trees. As expected, the larval stage reached by the larvae at lower site was more advanced compared to the upper site due to temperature-dependent growth. In spite of the generally low temperature for the development of first instar larvae, about 10 °C lower than that observed at cold sites at the edge of the range (unpublished data), we observed a considerable survival also on a novel host, the mountain pine, that opens the question about the possibility of a successful development of the insect at range’s edge. Final survival of transplanted colonies at two extreme sites on mountain pine showed that snow cover is preventing larval activity and establishment, whereas low temperature is not the major constraint factor. However, as snow cover is decreasing in the last decades, we may expect that range expansion will further occur in T. pityocampa.
Mountain pine responded to CO2 enrichment with an increase of non-structural carbohydrates ([26]), as it has been pointed for other conifers ([21], [39]). The increase was due to variation in starch fraction, whereas the concentration of soluble sugars was unchanged ([22]). The increase of starch may positively affect herbivore insects, improving the digestion efficiency and increase fat reserves ([19], [3]). However, it may involve a lower N concentration per unit mass and an increase of C/N ratio, that indicates a lower food quality for herbivorous insects ([35]). The studies carried out under similar situations have shown contrasting responses on larval performance. Several highlighted a negative response ([24], [10]), whereas others showed no variations ([20], [52]) or a positive effect ([19]). Asshoff & Hättenschwiler ([4]), in an experiment carried out in the same FACE site, showed that alteration in needle chemistry had a reduced effect on larch bud moth performance. The response of the pine processionary moth could be due to opposite effects, such as the dilution in N content and the increase of starch and sugars that may be phagostimulatory to herbivores ([46]). Moreover, the same quantity of faeces produced in the two treatments does not support the hypothesis of compensatory feeding, that has been associated with increase of C/N ratio and lower tissue quality ([51]), although some recent studies have suggested that a differential post-ingestive adsorption may compensate for diminished food quality ([6]). In the study of Stastny et al. ([44]), the performance of the processionary moth larvae did not differ between three host-plant species with different nitrogen content, showing a high plasticity. This may display such a post-ingestive compensatory behaviour. Leaf toughness, however, can be a serious constrain to larval development especially in early instars ([47], [54]). It is common that plant phenotype may show different resistance to herbivore attack ([38]). In our experiment there were two phenotypes of mountain pine (naturally established vs. introduced) which differed in needles toughness, a physical hurdle to larval feeding, that may explain the higher larval mortality on native mountain pine grown at lower altitude. Although leaf toughness may be an important determinant of larval survival on some hosts, it rarely results in total mortality as the insect shows a high adaptive potential to tough needles ([54]).
Extreme temperature and snow cover appear to be the most important abiotic factors for assessing the impact of climate change on alpine ecosystem ([28], [48]). The snow cover of the nest was a major constraint factor for survival during winter in our transplant experiment. The creeping habit of P. mugo, a species most widespread at the insect range edge, makes it possible that colonies become buried by snow when the snow is as high as 20 cm (unpublished data). The mean snow depth, the duration of continuous snow cover and the number of snowfall days in the Swiss Alps all have showed very similar trends in the period 1931-99: a gradual increase until the early 1980s followed by a statistically significant decrease towards the end of the century, mainly at low and mid altitudes ([8], [33]). Our data from the north-eastern Alps showed a similar trend to the Swiss Alps. There is no universal agreement, however, on snow cover in the past century, as Brown ([9]) did not detect evidence of a significant long-term decrease of spring snow in the northern hemisphere.
There are few studies that consider the effect of the change in snow cover on ecosystems. Wipf et al. ([56]) showed shifts in vegetation communities as a results of experiments of snow removal, furthermore in a study about growth of Norway spruce saplings the decrease in snow cover showed two opposite effects, a positive one due to decrease of attack by the black snow mold (Herpotrichia nigra) and to longer growing season, and a negative response of saplings to exposure to low temperatures ([13]). Vanbergen et al. ([49]) suggested that the reduction of snow cover in the Scottish moorland, conceivable with climate change, had increased the probability of winter moth (Operophthera brumata) outbreaks on Calluna vulgaris, through improvement of adult emergence and higher breeding success. Finally, Atalopedes campestris is a lepidopteran that cannot survive for a longer period under snow as larvae, and its future expansion could be favoured by the decrease of snow ([12]). If snow cover reduction may relax the conifer hosts from the attack of snow-associated pathogens like the black snow mold, it may contribute to worse the attack of organisms that are normally limited by snow, such as the winter moth and the pine processionary moth. In the latter case, it can be easily predicted that the expansion on mountain pine will continue as long as temperature increase will be associated with a decrease of snow cover and the insect will not be limited by higher CO2. Interestingly, the temperature and the land-use change have been considered as predictors of range expansion of mountain pine in the alpine region ([16]), likely leading to an altitudinal shift of plant and insect communities in mountain habitats.
Acknowledgements
We thank Roman Asshoff, Emiliano Buffo, Andrei Gourov for their help in CO2 experiments and Alessandro De Bellis in transplant experiments. The “Servizio Territorio montano e manuntezioni” of Friuli Venezia Giulia district for providing snow-data and Alessandro Chiaudani for the statistical analysis. The Swiss National Foundation for permission to work at the Stillberg and the team involved in Stillberg FACE project for availability and technical support. A special thanks to Roman Asshoff, Tanya Handa, Stig Larsson, and two anonymous reviewers for their useful comments on earlier versions of the manuscript.
| Site | Host | No. colonies |
% survival November |
% survival February |
|---|---|---|---|---|
| Resia (recent expansion) |
P. nigra | 27 | 40.7 | 14.8 |
| Resia (recent expansion) |
P. mugo | 27 | 66.7 | 25.9 |
| Tanamea (outside the range) |
P. mugo | 21 | 46.6 | 0 |
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Andrea Battisti
Department of Environmental Agronomy - Entomology, University of Padova, v. Università 16a, I-35020 Legnaro, PD (Italy)
Corresponding author
Paper Info
Citation
Petrucco-Toffolo E, Battisti A (2008). Performances of an expanding insect under elevated CO2 and snow cover in the Alps. iForest 1: 126-131. - doi: 10.3832/ifor0466-0010126
Paper history
Received: Apr 22, 2008
Accepted: Jul 21, 2008
First online: Aug 27, 2008
Publication Date: Aug 27, 2008
Publication Time: 1.23 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2008
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 50922
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41402
Abstract Page Views: 3863
PDF Downloads: 4505
Citation/Reference Downloads: 60
XML Downloads: 1092
Web Metrics
Days since publication: 6390
Overall contacts: 50922
Avg. contacts per week: 55.78
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2008): 4
Average cites per year: 0.22
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Abundance and impact of egg parasitoids on the pine processionary moth (Thaumetopoea pityocampa) in Bulgaria
vol. 14, pp. 456-464 (online: 02 October 2021)
Research Articles
Effects of defoliation by the pine processionary moth Thaumetopoea pityocampa on biomass growth of young stands of Pinus pinaster in northern Portugal
vol. 3, pp. 159-162 (online: 15 November 2010)
Research Articles
The impact of post-defoliation foliage of Pinus halepensis Mill. on the larval performance of Thaumetopoea pityocampa and its relationship with the tree-induced defense
vol. 18, pp. 186-193 (online: 01 July 2025)
Research Articles
Plant phenotype affects oviposition behaviour of pine processionary moth and egg survival at the southern edge of its range
vol. 11, pp. 572-576 (online: 01 September 2018)
Research Articles
Change Detection methods for forest expansion assessment in the last twenty years in the Mediterranean Basin
vol. 18, pp. 69-78 (online: 16 April 2025)
Research Articles
Landsat TM imagery and NDVI differencing to detect vegetation change: assessing natural forest expansion in Basilicata, southern Italy
vol. 7, pp. 75-84 (online: 18 December 2013)
Review Papers
Impacts of climate change on the establishment, distribution, growth and mortality of Swiss stone pine (Pinus cembra L.)
vol. 3, pp. 82-85 (online: 15 July 2010)
Review Papers
Forests and climate change - lessons from insects
vol. 1, pp. 1-5 (online: 28 February 2008)
Research Articles
Hemlock woolly adelgid niche models from the invasive eastern North American range with projections to native ranges and future climates
vol. 12, pp. 149-159 (online: 04 March 2019)
Research Articles
Is Pinus pinea growth affected by climate change in western Anatolia?
vol. 18, pp. 93-101 (online: 28 April 2025)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword