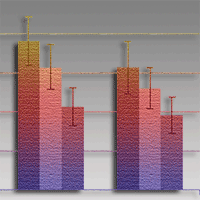Modern forestry in the European Union and in Poland is in constant search of environment-friendly technological solutions. These also relate to nursery production, in which attempts are made to apply non-chemical plant-protection products. The objective of this study was to assess the effects of salicylic acid, oxalic acid and chitosan (applied in the form of Beta-chikol®) in controlling damping-off and promoting the growth of Scots pine seedlings under nursery conditions. All the substances were used in seed treatment and in the form of foliar spray, 4 times during the growing season, in the following concentrations: salicylic acid 1% and 2%, oxalic acid 0.5% and 1%, and chitosan 2%. Seedlings were inventoried three times: 3 and 6 weeks after seed sowing, and at the end of the growing season. All seedlings were counted in 1-metre segments of individual rows of the seedbed. At the end of the growing season, parameters of seedling growth like shoot length, root-collar diameter, root length and the dry mass of above-ground parts were determined. The growth of pine seedlings was found to be stimulated by both chitosan and oxalic acid, while salicylic acid proved inhibitory to growth when present at 2% concentration, and showed no detectable influence on biometric parameters at 1% concentration. Numbers of seedlings germinating per 1-metre segment were significantly greater than in the (unprotected) control, where chitosan was applied. Likewise, oxalic acid applied at both concentrations was associated with greater numbers of germinating pine seedlings than in the control, albeit the statistical significance of this difference was achieved only 6 weeks after seed sowing, and only with the 0.5% concentration. Numbers of seedlings per metre-long segment were significantly lower in response to both concentrations of salicylic acid applied. Both chitosan (applied as Beta-chikol®) and 0.5% oxalic acid resulted in seedling protection against damping-off and enhanced growth, whereas the applied concentrations of salicylic acid were presumably excessive, hence the negative impact on both germination and growth.
Keywords
, , , ,
Citation
Soltys A, Studnicki M, Zawadzki G, Aleksandrowicz-Trzcinska M (2020). The effects of salicylic acid, oxalic acid and chitosan on damping-off control and growth in Scots pine in a forest nursery. iForest 13: 441-446. - doi: 10.3832/ifor3244-013
Academic Editor
Alberto Santini
Paper history
Received: Sep 20, 2019
Accepted: Jul 24, 2020
First online: Sep 24, 2020
Publication Date: Oct 31, 2020
Publication Time: 2.07 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 39613
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 33543
Abstract Page Views: 2932
PDF Downloads: 2478
Citation/Reference Downloads: 2
XML Downloads: 658
Web Metrics
Days since publication: 1986
Overall contacts: 39613
Avg. contacts per week: 139.62
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 4
Average cites per year: 0.67
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Aleksandrowicz-Trzcinska M, Bogusiewicz A, Szkop M, Drozdowski S (2015)Effect of chitosan on disease control and growth of Scots pine (
Pinus sylvestris L.) in forest nursery. Forests 6 (9): 3165-3176.
CrossRef |
Gscholar
(2)
Attitalla IH, Brishammar S (2002)Oxalic-acid elicited resistance to
Fusarium wilt in
Lycopersicon esculentum Mill. Plant Protection Science 38 (1): 128-131.
CrossRef |
Gscholar
(3)
Beyer-Ericson I, Damm E, Unestam T (1991)An overview of root dieback and its causes in Swedish forest nursery. European Journal of Forest Pathology 21: 439-443.
CrossRef |
Gscholar
(4)
Bornet A, Teissedre PL (2008)Chitosan, chitin-glucan and chitin effects on minerals (iron, lead, cadmium) and organic (ochratoxin A) contaminants in wines. European Food Research and Technology 226: 681-689.
CrossRef |
Gscholar
(5)
Bureau for Forest Management and Geodesy (2013)Management plan for Spychowo forest district for the years 2013-2022. Stand description data. Internal document for Spychowo Forest District, Bureau for Forest Management and Geodesy, Olsztyn, Poland.
Gscholar
(6)
Cho MH, No HK, Prinyawiwatkul W (2008)Chitosan treatments affect growth and selected quality of sunflower sprouts. Journal of Food Science 73 (1): 70-77.
CrossRef |
Gscholar
(7)
Duda B, Oszako T, Piwnicki J (2003)Possibilities of chitosan use in forestry. Bulletin of the Polish Academy of Sciences, Biological Sciences 51: 213-220.
Gscholar
(8)
Ecker JR (1995)The ethylene signal transduction pathway in plants. Science 268: 667-675.
CrossRef |
Gscholar
(9)
El Hadrami A, Adam LR, El Hadrami I, Daayf F (2010)Chitosan in plant protection. Marine Drugs 8: 968-987.
CrossRef |
Gscholar
(10)
Enebak SA, Palmer MA, Blanchette RA (1990)Managing soilborne pathogens of white pine in forest nursery. Plant Disease 74: 195-198.
CrossRef |
Gscholar
(11)
Fitza KNE, Payn KG, Steenkamp ET, Myburg AA, Naidoo S (2013)Chitosan application improves resistance to
Fusarium circinatum in
Pinus patula. South African Journal of Botany 85: 70-78.
CrossRef |
Gscholar
(12)
Goffeau A (2008)Drug resistance: the fight against fungi. Nature 452: 541-542.
CrossRef |
Gscholar
(13)
Harman GE (2011)Multifunctional fungal plant symbionts: new tools to enhance plant growth and productivity. New Phytologist 189: 647-649.
CrossRef |
Gscholar
(14)
Hayat Q, Hayat S, Irfan M, Ahmad A (2009)Effect of exogenous salicylic acid under changing environment: a review. Environmental and Experimental Botany 68 (1): 14-25.
CrossRef |
Gscholar
(15)
Jankiewicz U, Golab D, Frak M (2013)Wplyw kwasu salicylowego syntetyzowanego przez bakterie
Pseudomonas fluorescens i
P. chlororapgis na fitopatogeniczne grzyby z rodzaju
Fusarium [The effect of salicylic acid, synthesized by the bacteria
Pseudomonas fluorescens and
P. chlororapgis on phytopathogenic fungi
Fusarium]. Polish Journal of Agronomy 15: 65-68. [in Polish]
Gscholar
(16)
Jayaraj J, Bhuvaneswari R, Rabindran R, Muthukrishnan S, Velazhahan R (2010)Oxalic acid-induced resistance to
Rhizoctonia solani in rice is associated with induction of phenolics, peroxidase and pathogenesis-related proteins. Journal of Plant Interactions 5 (2): 147-157.
CrossRef |
Gscholar
(17)
Krokene P, Nagy NE, Solheim H (2008)Methyl jasmonate and oxalic acid treatment of Norway spruce: anatomically based defense responses and increased resistance against fungal infection. Tree Physiology 28: 29-35.
CrossRef |
Gscholar
(18)
Kumaraswamy RV, Kumari S, Choudhary RC, Pal A, Raliya R, Biswas P, Saharan V (2018)Engineered chitosan based nanomaterials: bioactivities, mechanisms and perspectives in plant protection and growth. International Journal of Biological Macromolecules 113: 494-506.
CrossRef |
Gscholar
(19)
Kumar MNVR (2000)A review of chitin and chitosan applications. Reactive and Functional Polymers 46: 1-27.
CrossRef |
Gscholar
(20)
Laflamme P, Benhamou N, Bussières G, Dessureault M (1999)Differential effects of chitosan on root rot fungal pathogens in forest nurseries. Canadian Journal of Botany 77: 1460-1468.
CrossRef |
Gscholar
(21)
Lehner A, Meimoun P, Errakhi R, Madiona K, Barakate M, Bouteau F (2008)Toxic and signaling effects of oxalic acid: natural born killer or natural born protector? Plant Signaling and Behavior 3: 746-748.
CrossRef |
Gscholar
(22)
Malamy J, Carr JP, Klessig DF, Raskin I (1990)Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250: 1002-1004.
CrossRef |
Gscholar
(23)
Marciano P, Dilenna P, Magro P (1983)Oxalic acid, cell wall degrading enzymes and pH in pathogenesis and their significance in the virulence of two
Sclerotinia sclerotiorum isolates on sunflower. Physiological Plant Pathology 22: 339-345.
CrossRef |
Gscholar
(24)
Martín-García J, Zas R, Solla A, Woodward S, Hantula J, Vainio EJ, Mullett M, Morales-Rodríguez C, Vannini A, Martínez-Álvarez P, Pinto G, Alves A, Amaral J, Wingfield MJ, Fourie G, Steenkamp ET, Ahumada R, Šerá B, Sanz-Ros AV, Raposo R, Elvira-Recuenco M, Iturritxa E, Gordon TR, Diez JJ (2019)Environmentally friendly methods for controlling pine pitch canker. Plant Pathology 68: 843-860.
CrossRef |
Gscholar
(25)
Moharekar ST, Lokhande SD, Hara T, Tanaka R, Tanaka A, Chavan PD (2003)Effects of salicylic acid on chlorophyll and carotenoid contents on wheat and moong seedlings. Photosynthetica 41: 315-317.
CrossRef |
Gscholar
(26)
Norman C, Howell KA, Millar H, Whelan JM, Day DA (2004)Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiology 134: 492-501.
CrossRef |
Gscholar
(27)
Ohta K, Morishita S, Suda K, Kobayashi N, Hosoki T (2004)Effects of chitosan soil mixture treatment in the seedling stage on the growth and flowering of several ornamental plants. Journal of the Japanese Society for Horticultural Science 73 (1): 66-68.
CrossRef |
Gscholar
(28)
Pancheva TV, Popova LP, Uzunova AN (1996)Effects of salicylic acid on growth and photosynthesis in barley plants. Journal of Plant Physiology 149: 57-63.
CrossRef |
Gscholar
(29)
Pospieszny H, Chirkov S, Atabekov J (1991)Induction of antiviral resistance in plants by chitosan. Plant Science 79: 63-68.
CrossRef |
Gscholar
(30)
Raafat D, Sahl H-G (2009)Chitosan and its antimicrobial potential - a critical literature survey. Microbial Biotechnology 2: 186-201.
CrossRef |
Gscholar
(31)
Ramamoorthy V, Viswanathan R, Raguchander T, Prakasam V, Samiyappan R (2001)Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Protection 20: 1-11.
CrossRef |
Gscholar
(32)
Ramírez MA, Rodriguez AT, Alfonso L, Peniche C (2010)Chitin and its derivatives as biopolymers with potential agricultural applications. Biotecnologia Aplicada 27: 270-276.
Gscholar
(33)
Raskin I, Skubatz H, Tang W, Meeuse BJD (1990)Salicylic acid levels in thermogenic and non-thermogenic plants. Annals of Botany 66: 369-373.
CrossRef |
Gscholar
(34)
Raskin I (1992)Role of salicylic acid in plants. Annual Review of Plant Physiology and Plant Molecular Biology 43: 439-463.
CrossRef |
Gscholar
(35)
Reglinski T, Taylor JT, Dick MA (2004)Chitosan induces resistance to pitch canker in
Pinus radiata. New Zealand Journal of Forestry Science 34: 49-58.
Online |
Gscholar
(36)
Rivas-San Vicente M, Plasencia J (2011)Salicylic acid beyond defence: its role in plant growth and development. Journal of Experimental Botany 62 (10): 3321-3338.
CrossRef |
Gscholar
(37)
Shakirova FM, Sakhabutdinova AR, Bezrukova V, Fatkhutdinova RA, Fatkhutdinova DR (2003)Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Science 164: 317-322.
CrossRef |
Gscholar
(38)
Sharp RG (2013)A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 3: 757-793.
CrossRef |
Gscholar
(39)
Silva-Castro I, Diez JJ, Martín-Ramos P, Pinto G, Alves A, Martín-Gil J, Martín-García J (2018a)Application of bioactive coatings based on chitosan and propolis for
Pinus spp. protection against
Fusarium circinatum. Forests 9: 685.
CrossRef |
Gscholar
(40)
Silva-Castro I, Martín-García J, Diez JJ, Flores-Pacheco JA, Martín-Gil J, Martín-Ramos P (2018b)Potential control of forest diseases by solutions of chitosan oligomers, propolis and nanosilver. European Journal of Plant Pathology 150: 401-11.
CrossRef |
Gscholar
(41)
Stevens J, Senaratna T, Sivasithamparam K (2006)Salicylic acid induces salinity tolerance in tomato (
Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regulation 49: 77-83.
CrossRef |
Gscholar
(42)
Uzunova AN, Popova LP (2000)Effect of salicylic acid on leaf anatomy and chloroplast ultrastructure of barley plants. Photosynthetica 38: 243-250.
CrossRef |
Gscholar
(43)
Wang Q, Lai T, Qin G, Tian S (2009)Response of jujube fruits to exogenous oxalic acid treatment based on proteomic analysis. Plant Cell Physiology 50 (2): 230-242.
CrossRef |
Gscholar