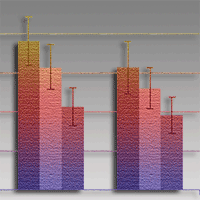
The effects of salicylic acid, oxalic acid and chitosan on damping-off control and growth in Scots pine in a forest nursery
iForest - Biogeosciences and Forestry, Volume 13, Issue 5, Pages 441-446 (2020)
doi: https://doi.org/10.3832/ifor3244-013
Published: Sep 24, 2020 - Copyright © 2020 SISEF
Short Communications
Abstract
Modern forestry in the European Union and in Poland is in constant search of environment-friendly technological solutions. These also relate to nursery production, in which attempts are made to apply non-chemical plant-protection products. The objective of this study was to assess the effects of salicylic acid, oxalic acid and chitosan (applied in the form of Beta-chikol®) in controlling damping-off and promoting the growth of Scots pine seedlings under nursery conditions. All the substances were used in seed treatment and in the form of foliar spray, 4 times during the growing season, in the following concentrations: salicylic acid 1% and 2%, oxalic acid 0.5% and 1%, and chitosan 2%. Seedlings were inventoried three times: 3 and 6 weeks after seed sowing, and at the end of the growing season. All seedlings were counted in 1-metre segments of individual rows of the seedbed. At the end of the growing season, parameters of seedling growth like shoot length, root-collar diameter, root length and the dry mass of above-ground parts were determined. The growth of pine seedlings was found to be stimulated by both chitosan and oxalic acid, while salicylic acid proved inhibitory to growth when present at 2% concentration, and showed no detectable influence on biometric parameters at 1% concentration. Numbers of seedlings germinating per 1-metre segment were significantly greater than in the (unprotected) control, where chitosan was applied. Likewise, oxalic acid applied at both concentrations was associated with greater numbers of germinating pine seedlings than in the control, albeit the statistical significance of this difference was achieved only 6 weeks after seed sowing, and only with the 0.5% concentration. Numbers of seedlings per metre-long segment were significantly lower in response to both concentrations of salicylic acid applied. Both chitosan (applied as Beta-chikol®) and 0.5% oxalic acid resulted in seedling protection against damping-off and enhanced growth, whereas the applied concentrations of salicylic acid were presumably excessive, hence the negative impact on both germination and growth.
Keywords
Plant Biostimulants, Induced Resistance, Pinus sylvestris, Growth Stimulation, Disease Control
Introduction
Damping-off of seedlings is a fungal disease capable of generating major losses in forest nurseries. It is caused by pathogens belonging to a range of different systematic groups, including genera Rhizoctonia, Fusarium, Cylindrocarpon, and Phytophthora ([10], [3]). This heterogeneity of origin combines with the emergence of resistance to fungicides to make this disease very hard to combat ([12]).
Modern forestry in the European Union and in Poland is in constant search of environment-friendly technological solutions. This is also true for nursery production, in which alternatives to agrochemicals are being looked for. Induced resistance has emerged as a potential alternative or complementary strategy for the control of plant diseases ([16], [24]).
Chitosan (CH) is a naturally-occurring polysaccharide which is a deacetylated derivative of chitin. It serves as an exogenous elicitor of plant defence responses. It induces local and systemic acquired resistance, as reflected in the activation of reactive oxygen species, synthesis of salicylic acid, phytoalexins, polyphenolics, terpenes, flavonoids and pathogenesis-related proteins (chitinase, β-1,3-glucanase, peroxidase, polyphenoloxidase), the lignification of cell walls, and callose synthesis ([35], [9], [38]). Chitosan affects plant defences in two ways: it does not only activate genes responsible for the initiation of resistance mechanisms in plants, but also has properties proved to be antiviral ([29]), antibacterial ([30]) and antifungal ([20], [40]). Moreover, it has been domonstrated that CH stimulates plant growth and development ([18]).
Salicylic acid (SA) is a phenolic compound that is a derivative of benzoic acid commonly found in plants at low concentrations (below 1 mg kg-1 fresh weight - [33]). However, in infected plants its concentration can increase 20-fold, activating the genes responsible for synthesizing defence-related proteins ([22]). Both endogenous and exogenous SA induce local resistance, given its role of signal molecule for the development of systemic acquired resistance ([34]). Moreover, SA is an endogenous regulator of plant growth and development ([14], [36]).
Oxalic acid (OA) is an organic acid widely distributed in plants, fungi and animals, and plays different roles in different living organisms ([43]). It is a virulence factor in several phytopathogenic fungi, including the model species Sclerotinia sclerotium ([23]). In plants, it can play two distinct roles, depending on the concentration. While a high concentration of OA induces programmed cell death and contributes to the progression of fungi, a low concentration gives rise to plant resistance to fungi ([21]).
Research on CH, SA and OA as plant protection products and growth stimulants has so far concerned various herbaceous crop plants ([43], [9], [36]), while only few studies have focused on woody plants, including forest trees ([35], [11], [1], [39]). The aim of this work was thus to assess the effects of these three natural substances on the control of damping-off and growth among Scots pines at a bare-root nursery. Our null hypotheses were that: (1) all these substances limit seedling infection from damping-off; and (2) they have a favorable effect on pine growth.
Materials and methods
Study site
Field research was conducted in the bare-root forest nursery of Spychowo Forest District, located about 150 km north-east of Warsaw (53° 36′ N, 21° 20′ E - WGS 84), in Poland. The nursery was established on former agricultural land in 1976. The soil in the study area was classified as typical rusty. Earlier work on pine seedlings and the soil at this trial site revealed the presence of Rhizoctonia solani, Fusarium oxysporum and Alternaria sp. The annual mean temperature in the study area is 7.6 °C. The warmest month is July (18.3 °C) and the coldest is January (3.1 °C). The vegetation period with an average daily temperature higher than 5 °C is 207 days ([5]).
Field experiment
The experiment compared: pine seedlings treated with either CH (2%), SA (1% and 2%) or OA (0.5% and 1%) and unprotected seedlings (control). The experiment was organised into a randomised-block design with four replicates. Within-block variants comprised 5 sown rows (seed tapes) over a length of 2 m. The soil was prepared by full ploughing. A nursery marker adapted to five-row tapes was used to prepare furrows where the seeds were sown. Pine seeds of local origin from commercial stands were sown at 6.5 g of seed per metre of tape (i.e., 5 sown rows); this denotes 1250 seeds (about 250 seeds / 1 m seed-row, 1250 seeds × 4 blocks = 5000 seeds per treatment). Seeds were covered by hand, under about 0.8 cm of soil. The experiment lasted 148 days, from June 6 to October 31.
Applications of chitosan, salicylic acid and oxalic acid
Chitosan was applied in the form of the commercial product called Beta-chikol® (Poli-Farm, Lowicz, Poland) as an organic plant-growth stimulant. Beta-chikol® (2%) was used according to manufacturer instructions. OA and SA were purchased from Biomus sp. z o.o. (Lublin, Poland), and were used as aqueous solutions.
All the substances were used in seed treatment. Seed were soaked in solutions for 6 hours (having not been prepared before treatment). Seeds from control variant were soaked in water. The substances were then further applied by foliar spraying 4 times during the growing season. The first application was made at the time of germination, and three subsequent ones at ten-day intervals. The concentrations were SA 1% and 2%, OA 0.5% and 1%, and Beta-chikol® 2%.
Seedling inventory and growth measurement
Seedlings were inventoried three times during the growing season: 3 and 6 weeks after seed sowing, and at the season’s end. All seedlings were counted in 1-metre segments of individual rows of the seedbed. There were 5 such segments in each treatment in a block. In late October, 40 seedlings in each treatment were collected and shoot and root length, root-collar diameter and dry mass of above-ground parts were measured.
Statistical analyses
A one-factor experiment was carried out for each type of tested substance. Additionally, for CH two-factor levels was used (CH vs. control) whereas for OA and SA 3- factor levels were used (OA: 0.5%, 1% and control; SA: 1%, 2% and control). We tested relationships among biometric parameters and numbers of seedlings germinating per 1-metre segment using a one-way Analysis of Variance (ANOVA) for complete randomised block design. The Tukey HSD test was used as post-hoc test in pairwise comparisons between different foliar sprays. Before analysis, the normality of the data distribution was verified using the Shapiro-Wilk test, while the equality of variances was assessed using the Levene test. All the studied factors presented a normal distribution and the variances were homogeneous. The statistical analysis was performed using R version 3.5.1.
Results
The growth of pine seedlings in the experiment was found to be stimulated by CH. All biometric parameters except root length, were significantly higher than in the unprotected control. Oxalic acid at 1% concentration stimulated the growth of shoot length only. Better results were obtained where OA was present at 0.5%, with stimulation of all growth parameters except dry mass of above-ground parts. Salicylic acid proved inhibitory to growth (only shoot length) where present at a 2% concentration, though no significant influence on biometric parameters was observed where the applied solution was 1% (Tab. 1).
Tab. 1 - Biometric parameters (with means and standard errors in parenthesis) for seedlings of Scots pine (Pinus sylvestris) protected with chitosan, salicylic acid and oxalic acid, and for those in the unprotected control. Means marked with different letters differ significantly (p < 0.05, Tukey HSD test). Results of ANOVAs (F and p).
| Treatment (n=40) |
Length of shoot (cm) |
Dry mass of above-ground parts (g) |
Root-collar diameter (mm) |
Total root length (cm) |
|---|---|---|---|---|
| Chitosan | 6.1 ± 0.119 b | 0.0925 ± 0.0062 b | 0.85 ± 0.0228 b | 12.5 ± 0.404 a |
| Control | 5.1 ± 0.113 a | 0.0675 ± 0.0050 a | 0.74 ± 0.0215 a | 12.1 ± 0.406 a |
| F | 33.17 | 9.58 | 14.29 | 0.54 |
| p-value | <0.0001 | 0.0031 | 0.0004 | 0.2051 |
| Oxalic acid 0.5% | 6.1 ± 0.127 b | 0.0599 ± 0.0027 a | 0.83 ± 0.0288 b | 13.2 ± 0.278 b |
| Oxalic acid 1% | 6.2 ± 0.128 b | 0.0711 ± 0.0046 a | 0.79 ± 0.0226 ab | 12.0 ± 0.321 a |
| Control | 5.2 ± 0.114 a | 0.0680 ± 0.0051 a | 0.73 ± 0.0216 a | 12.0 ± 0.400 a |
| F | 23.61 | 1.88 | 3.53 | 4.62 |
| p-value | <0.0001 | 0.1593 | 0.0338 | 0.0125 |
| Salicylic acid 1% | 5.2 ± 0.117 b | 0.0652 ± 0.0043 a | 0.69 ± 0.0170 a | 12.2 ± 0.164 a |
| Salicylic acid 2% | 4.6 ± 0.113 a | 0.0596 ± 0.0033 a | 0.77 ± 0.0105 b | 11.7 ± 0.232 a |
| Control | 5.1 ± 0.114 b | 0.0674 ± 0.0050 a | 0.72 ± 0.0205 ab | 21.1 ± 0.401 a |
| F | 9.05 | 1.00 | 6.74 | 1.02 |
| p-value | 0.0003 | 0.3712 | 0.0019 | 0.3649 |
Numbers of seedlings germinating per 1-m segment of seed row after CH application were significantly greater than in the control variant (Fig. 1a). Likewise, OA applied at both concentrations was associated with higher numbers of germinating pine seedlings than in the control, albeit the statistical significance to these differences was achieved only 6 weeks after sowing the seeds, and only at a 0.5% concentration OA (Fig. 1b). Numbers of seedlings per metre-long segment were significantly lower in response to both concentrations of SA applied (Fig. 1c). Seedling emergence was affected most strongly by parasitic damping-off, as confirmed by specific symptoms (the narrowing into a root collar and blackening of stem bases causing seedling droop; and the blackening and death of roots) that were observed in all variants of the experiment.
Fig. 1 - Mean numbers of Scots pine seedlings in 1-metre segments of the seed row 3 and 6 weeks after seed sowing and at the end of the growing season, in treatments with chitosan (A), oxalic acid (B), salicylic acid (C) or no protection (control). Means marked with different letters differ significantly (p < 0.05, Tukey HSD test). Error bars denote standard errors.
Discussion
In some forest nurseries, the risk of parasitic damping-off (caused by different species of fungi or oomycetes) is high ([10], [3]), as confirmed by the results of our experiment, where major differences in the number of germinating pine seedlings among different experimental variants were observed. The main reason for the lack of seedling emergence or their death was damping-off, whose symptoms were clearly observed. The three substances applied in the experiment - CH, OA and SA - are known for their capacity to induce plants’ resistance reaction to unfavourable biotic and abiotic factors, as well as to stimulate growth ([22], [35], [21]). Nevertheless, as reported by many authors, the efficacy of these substances is dependent on various factors such as dose and concentration, the plant species involved and their stages of development, and the species of pathogen involved ([7], [36]).
Our experiment revealed differentiated impacts of the analyzed substances on health state and growth of Scots pine seedlings. Both of our starting hypotheses were confirmed only in the case of CH. The properties of CH as both a fungicide and growth stimulator have been rather well-studied in many species, including woody plants ([35], [11], [1], [39]). However, the results of such studies sometimes failed to offer unambiguous confirmation of the agent’s effectiveness ([7], [39]). Unlike CH, SA and OA are not well known as plant protection agents, as well as in terms of their effectiveness and mode of action ([17], [16]).
The result of our work offered undoubted confirmation of the effectiveness of the action of CH in protecting pine seedlings against damping-off, suggesting a multi-aspect pesticidal activity of CH ([9], [38]). The efficacy of CH in protecting seedlings may reflect a direct action of Beta-chikol® on fungi and oomycetes causing damping-off, due to its inhibitory effect against mycelial growth and the germination of spores ([20], [40]). A further mechanism may involve the capacity of chitosan molecules to bind the mycotoxins emitted by the facultative pathogens (i.e., the fungi causing damping-off), as these chemicals are known to be released to facilitate the colonisation of plant tissues ([4]).
Chitosan applied in the form of Beta-chikol® also determined a stimulated seedling growth. This applied to all of the parameters studied except root length. Similar effects have been observed in many other studies ([27], [6], [1]). However, the lack of impact on root growth was rather a surprise, given that CH can stimulate root-cell division by activating plant hormones such as auxin and cytokinin ([18]).
A full identification of the mechanism underpinning plant growth stimulation due to CH treatment has not been achieved so far ([6]). The effect may involve a direct uptake of chitosan through roots, which could be utilized as an additional nutrient by plants ([27]). Indeed, chitosan is a rich source of nitrogen, calcium and other microelements such as copper, zinc and iron ([4], [32]). Enhanced plant nutrition might also be due to the chelating properties of chitosan ([19]), which could favor a greater abundance of plant-growth promoting rhizobacteria and fungi, which in turn could favor plant growth ([31], [13]).
Oxalic acid treatment showed a higher rate of seedling emergence and growth compared with untreated seedlings. However, better results were obtained with the lower concentration (0.5%). The few studies on OA in the literature were focused on safeguarding rice against Rhizoctonia solani ([16]), tomatoes against Fusarium oxysporum ([2]) and Norway spruce against blue-stain fungus Ceratocystis polonica ([17]). In our study, the effectiveness of OA in protecting pine seedlings from damping-off was relatively limited and at a far lower level compared to CH. However, a greater efficacy could be achieved at lower concentrations than those used in this study. Indeed, the levels of OA referred to as helpful in the literature vary greatly, from 3 mM in the case of Arabidopsis thaliana against Sclerotium rolfsii, to 20 mM in the case of tomatoes and Fusarium oxysporum ([2], [21]).
Similar to SA, the protective effect to OA may depend both on the species to protect and the plant pathogen. The protective mechanisms are also very little-known, though (unlike CH and SA) OA has no antifungal properties ([2]). In contrast, Lehner et al. ([21]) were able to demonstrate the induction of defence-related gene expression due to OA.
Thus far, research relating OA and plant growth has been confined to herbaceous plants, pointing out the lack of any negative effects as a result of its application ([21]). In contrast, a stimulation of seedling growth was observed in our study, especially at 0.5% OA concentration. Wang et al. ([43]) showed that exogenous OA could delay fruit senescence by reducing ethylene production. Ethylene is known to serve as a plant hormone that inter alia produces inhibition of stem and root elongation ([8]). Based on our results, we may hypothesize that the inhibition of ethylene synthesis in cells following treatment with OA could lead to a stimulatory effect on seedling growth.
When SA was applied at either 1% or 2%, it did not prove possible to sustain any of the research hypotheses put forward. Such treatments were associated with considerably smaller numbers of pine seedlings than in the control, suggesting either the lack of any protective effect of SA or even a toxic influence. This could be due to an excessive concentration of SA being applied in the experiment. However, as relevant research done hitherto was entirely confined to herbaceous plants, it is hard to suggest a concentration of exogenous SA that might be applied to ensure the effective safeguarding of woody plants against disease, and even the stimulation of their growth. This is all the more the case given that SA is known to have basal levels widely differing among species (up to 100-fold - [33]). What is more, disparities of this kind have been reported in species belonging to the same plant family ([36]).
The efficacy of SA in protecting seedlings from damping-off disease may depend on which species of fungal pathogen is causing the disease. Such a dependent relationship has been noted in the case of pathogens of genus Fusarium ([15]). Differential fungitoxicity due to SA may account for the limited effectiveness against damping-off, as this disease is caused by pathogens belonging to a wide range of systematic groups.
Several studies previously reported a delaying or inhibitory effect on seed germination due to SA ([36]). This effect could explain the limited germination of pine seeds observed in this study, especially at higher concentrations.
The effect of exogenous SA on growth depends on the plant species, developmental stage and the SA concentrations tested ([36]). Mechanisms by which SA influences plant growth have only been accounted for to a very limited degree so far. Shakirova et al. ([37]) suggested that the growth-promoting effects of SA could be related to changes in hormonal status, while Stevens et al. ([41]) invoked enhanced photosynthesis, transpiration and stomatal conductance. In this study, the lack of a stimulating effect seems to be related to the use of an excessive concentration of SA. High (1-5 mM) SA concentration curbs photosynthetic rate and RuBisCO activity ([28]), as well as reduces chlorophyll content ([25]) and changes the leaf ultrastructure, particularly regarding chloroplasts ([42]). Similar (1-5 mM) concentration of SA is seen to inhibit respiration ([26]), while the concentrations applied in this study were still higher than those given above (1% and 2%). Treatment of herbaceous plants with concentrations of SA below 1 mM induced a growth stimulation ([36]). Thus, the lack of the expected growth stimulation in pine seedlings observed in this work most probably reflects the high concentrations of SA applied, as well as the absence of any favourable influence of SA on photosynthesic process.
Conclusion
We tested the effect on Scots pine seedlings of three substances (CH, applied as Beta-chikol®, OA, and SA), believed to have resistance-inducing and growth-stimulating properties in plants. The best result was obtained with CH, which protected pine seedlings from damping-off and stimulated their growth. OA protected seedlings from damping-off to only a limited degree, though it was found to stimulate growth. However, better results were obtained when the lower concentration of OA (5%) was applied. The application of SA did not result in seedlings protection from the fungal disease under consideration, nor did it significantly affect seedling growth. This most likely reflected the use of an excessive concentration of SA. In general, CH applied as Beta-chikol® may be recommended for use in forest nurseries as an alternative to fungicides in the protection of Scots pine seedlings from damping-off disease. In contrast, the effectiveness of SA and OA must be evaluated in further studies using lower concentrations of these substances.
Acknowledgments
AS and GZ carried out the field experiment and lab measurements; MS performed the statistical analysis; MAT conceived the study and wrote the manuscript.
The authors extend their heartfelt thanks to Krzysztof Krasula, Forest Inspector of Spychowo Forest District, as well as to his deputy Maciej Ligocki for consenting to carry out the research work at the Piasutno Forest Nursery.
This work was supported by the Rector of Warsaw University of Life Sciences (SGGW) within the framework of research project no. 505-10-030400.
The editorial help of James R.A. Richards is also gratefully acknowledged.
List of abbreviations
CH: chitosan; OA: oxalic acid; SA: salicylic acid.
References
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Grzegorz Zawadzki 0000-0001-8494-1470
Marta Aleksandrowicz-Trzcinska 0000-0002-1397-4475
Department of Forest Protection, Institute of Forest Sciences, Warsaw University of Life Sciences, Nowoursynowska 159, 02-776 Warsaw (Poland)
Department of Biometry, Institute of Agriculture, Warsaw University of Life Sciences, Nowoursynowska 159, 02-776 Warsaw (Poland)
Corresponding author
Paper Info
Citation
Soltys A, Studnicki M, Zawadzki G, Aleksandrowicz-Trzcinska M (2020). The effects of salicylic acid, oxalic acid and chitosan on damping-off control and growth in Scots pine in a forest nursery. iForest 13: 441-446. - doi: 10.3832/ifor3244-013
Academic Editor
Alberto Santini
Paper history
Received: Sep 20, 2019
Accepted: Jul 24, 2020
First online: Sep 24, 2020
Publication Date: Oct 31, 2020
Publication Time: 2.07 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 39442
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 33457
Abstract Page Views: 2889
PDF Downloads: 2444
Citation/Reference Downloads: 2
XML Downloads: 650
Web Metrics
Days since publication: 1969
Overall contacts: 39442
Avg. contacts per week: 140.22
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 4
Average cites per year: 0.67
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Conservation of Betula oycoviensis, an endangered rare taxon, using vegetative propagation methods
vol. 13, pp. 107-113 (online: 23 March 2020)
Research Articles
The effect of silver and copper nanoparticles on the growth and mycorrhizal colonisation of Scots pine (Pinus sylvestris L.) in a container nursery experiment
vol. 11, pp. 690-697 (online: 23 October 2018)
Research Articles
Forest litter as the mulch improving growth and ectomycorrhizal diversity of bare-root Scots pine (Pinus sylvestris) seedlings
vol. 8, pp. 394-400 (online: 20 August 2014)
Research Articles
Controlled-release fertilizers combined with Pseudomonas fluorescens rhizobacteria inoculum improve growth in Pinus halepensis seedlings
vol. 8, pp. 12-18 (online: 12 May 2014)
Research Articles
The effectiveness of short-term microwave irradiation on the process of seed extraction from Scots pine cones (Pinus sylvestris L.)
vol. 13, pp. 73-79 (online: 13 February 2020)
Research Articles
Use of overburden waste for London plane (Platanus × acerifolia) growth: the role of plant growth promoting microbial consortia
vol. 10, pp. 692-699 (online: 17 July 2017)
Research Articles
Seedling quality and short-term field performance of three Amazonian forest species as affected by site conditions
vol. 17, pp. 80-89 (online: 21 March 2024)
Research Articles
Substrates and nutrient addition rates affect morphology and physiology of Pinus leiophylla seedlings in the nursery stage
vol. 10, pp. 115-120 (online: 02 October 2016)
Technical Reports
Effects of different mechanical treatments on Quercus variabilis, Q. wutaishanica and Q. robur acorn germination
vol. 8, pp. 728-734 (online: 05 May 2015)
Research Articles
The combined effects of Pseudomonas fluorescens CECT 844 and the black truffle co-inoculation on Pinus nigra seedlings
vol. 8, pp. 624-630 (online: 08 January 2015)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword










