Conservation of Betula oycoviensis, an endangered rare taxon, using vegetative propagation methods
Jan Vítámvás (1) , Ivan Kuneš (1), Iva Viehmannová (2), Rostislav Linda (1), Martin Baláš (1)
iForest - Biogeosciences and Forestry, Volume 13, Issue 2, Pages 107-113 (2020)
doi: https://doi.org/10.3832/ifor3243-013
Published: Mar 23, 2020 - Copyright © 2020 SISEF
Research Articles
Abstract
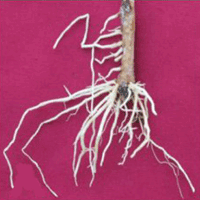
Ojcow birch (Betula oycoviensis Besser) is a rare Central European tree taxon, micro-populations of which are found in only several localities. With a view to maintaining the B. oycoviensis gene pool, this study tested the species’ potential for micropropagation, grafting, and propagation by cuttings. Plant material for vegetative propagation was collected from ten genotypes in the Czech Republic. In vitro culture was established from axillary buds surfaces sterilized with 0.1% HgCl2 and cultivated on woody plant (WP) medium supplemented with 1 mg l-1 6-benzylaminopurine (BAP). Two genotypes of the species were successfully multiplied by in vitro propagation using WP medium supplemented with 0-2 mg l-1 BAP. The BAP concentration of 1 mg l-1 proved to be optimal, yielding 2.5 new shoots per explant in genotype 516 and 3.5 shoots per explant in genotype 545. The shoots were rooted on half-strength Murashige and Skoog (MS) medium supplemented with various concentrations of α-naphthylacetic acid (NAA) and indole-3-butyric acid (IBA). The highest rooting percentages (72.5% and 77.5% for genotypes 516 and 545, respectively) were achieved on the medium with the combination of both auxins at concentrations of 0.3 mg l-1. The rooted plants were transferred ex vitro in substrate composed of sand, peat, and perlite (1:1:1) and acclimated in the greenhouse. After 4 weeks, more than 90% of plants survived. Grafting was carried out in spring using Betula pendula as rootstock. The efficiency of this technique ranged from 0% to 50% across genotypes, and 4 out of 10 genotypes were successfully propagated by grafting. The cuttings were treated with commercial root stimulators Stimulax I and Stimulator AS-1, planted in a mixture of peat and sand (1:1) in the greenhouse, and watered regularly. This technique resulted in 0% rooting, however, and no cutting survived until the end of the vegetation period. The results of this study show that protocols for in vitro propagation and grafting can be employed for effective mass propagation of B. oycoviensis, although these processes show genotype-dependent responses.
Keywords
Authors’ Info
Authors’ address
Ivan Kuneš 0000-0002-1875-384X
Rostislav Linda 0000-0002-9602-7915
Martin Baláš
Czech University of Life Sciences Prague, Faculty of Forestry and Wood Sciences, Kamýcká 1176, Praha 6 Suchdol (Czech Republic)
Czech University of Life Sciences, Faculty of Tropical AgriSciences, Kamýcká 129, Praha 6 Suchdol (Czech Republic)
Corresponding author
Paper Info
Citation
Vítámvás J, Kuneš I, Viehmannová I, Linda R, Baláš M (2020). Conservation of Betula oycoviensis, an endangered rare taxon, using vegetative propagation methods. iForest 13: 107-113. - doi: 10.3832/ifor3243-013
Academic Editor
Werther Guidi Nissim
Paper history
Received: Sep 18, 2019
Accepted: Jan 21, 2020
First online: Mar 23, 2020
Publication Date: Apr 30, 2020
Publication Time: 2.07 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 43624
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 36100
Abstract Page Views: 3546
PDF Downloads: 3149
Citation/Reference Downloads: 2
XML Downloads: 827
Web Metrics
Days since publication: 2151
Overall contacts: 43624
Avg. contacts per week: 141.97
Citation Metrics
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 2
Average cites per year: 0.33
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
References
In vitro sterilization protocol for micropropagation of Solanum tuberosum cv. “Kufri Himalini”. Academia Arena 2 (4): 24-27.
Gscholar
Metodická príručka k určování domácích druhu bríz. Certifikovaná metodika [Methodological manual for native birch species determination]. Lesnický pruvodce 3/2014. Výzkumný ústav lesního hospodárství a myslivosti, v. v. i., pp. 40. [in Czech]
Gscholar
In vitro regeneration of curly birch, Betula pendula var. carelica. Thaiszia - Journal of Botany 1: 119-124.
Gscholar
In vitro propagation of Paradox walnut Juglans hindsii × Juglans regia rootstock. HortScience 19: 507-509.
Gscholar
Plant propagation, principles and practices (7th edn). Prentice-Hall, Englewood Cliffs, NJ, USA, pp. 880.
Gscholar
Plant propagation, principles and practices (3rd edn). Prentice-Hall, Englewood Cliffs, NJ, USA, pp. 662.
Gscholar
Micropropagation of mature trees of birch (Betula pendula Roth.) and aspen (Populus tremula L.). Lesnictví 35: 983-993.
Gscholar
Razmnozhenie berezy karel’skoj metodom privivki [Propagation of Karelian birch by the grafting method]. Lesnaya Genetika, Selekcia i Semenovodstvo, Petrozavodsk, pp. 282-293. [in Russian]
Gscholar
Klíč ke kvetene Ceské republiky [Key to the flora of the Czech Republic]. Revised and updated edition, Academia, Prague, Czech Republic, pp. 1172. [in Czech]
Gscholar
Rozmieszczenie geograficzne brzozy ojcowskiej (Betula oycoviensis Bess.) [Geographic distribution of Oyców birch (Betula oycoviensis Bess.)]. Ochrona Przyrody 32: 133-170. [in Polish]
Gscholar
New locality of Betula oycoviensis Bess. in Poland. Fragmenta Floristica et Geobotanica 14: 155-156.
Gscholar
Betula L. - bríza: Kvetena Ceské republiky, část II [Betula L. - birch: Flora of the Czech Republic, Part II] (Hejný S, Slavík B eds). Academia, Prague, Czech Republic, pp. 35-46. [in Czech]
Gscholar
Klíč ke kvetene Ceské Republiky [Key to the Flora of the Czech Republic]. Academia, Prague, Czech Republic, pp. 928. [in Czech]
Gscholar
Selekciya i razvedenie karel’skoj berezy [Selection and breeding of Karelian birch]. Lesnaya Promyslennost, Moskva, Russia, pp. 123. [in Russian]
Gscholar
Mikropropagace brízy trpasličí [Micropropagation of Betula nana]. Zprávy lesnického výzkumu 57: 202-206. [in Czech]
Gscholar
Rooting softwood cuttings of mature Betula papyrifera. Combined proceedings, International Plant Propagators’ Society 35: 519-525.
Gscholar
Non parametric statistics for the behavioural sciences. MacGraw Hill Int., New York, USA, pp. 399.
Gscholar
“Betula × oycoviensis” Besser in the environs of Kraków (S. Poland). Veröffentlichungen des Geobotanischen Institutes der Eidgenössische Technische Hochschule, Stiftung Rübel, Zürich 107: 94-97.
Gscholar
Vegetative propagation of birch. New Zealand Journal of Forestry Science 4 (2): 237-241.
Gscholar