Effect of restoration methods on natural regeneration in the Brazilian Atlantic Forest
iForest - Biogeosciences and Forestry, Volume 18, Issue 1, Pages 23-29 (2025)
doi: https://doi.org/10.3832/ifor4598-017
Published: Feb 15, 2025 - Copyright © 2025 SISEF
Research Articles
Abstract
Climate change and ecosystem degradation have achieved unprecedented levels nowadays. In this context, assessing restoration projects to recover native vegetation and ecosystem services is fundamental to understanding the success of these initiatives. This study evaluated the effects of different restoration techniques on the basal area, diversity, and composition of regenerating and planted species in a restoration area in the Atlantic Forest, Southeast Brazil. The experiment consisted of three treatments: row planting (ROW, 2 × 2 m spacing); nucleation (NUC, 13 individuals/nucleus, 5 × 5 m spacing); and control with passive restoration (PAS). The active restoration techniques had different planting densities at project implementation (NUC: 5.200 seedlings ha-1; ROW: 2.177 seedlings ha-1). A floristic survey was carried out within 8 sampling units (15 × 15 m) per treatment 10 years after implementation of the project, including all planted and regenerating individuals with a diameter at ground level > 2.5 cm. The abundance, basal area, and species richness of the planted and regenerated communities were estimated. The active restoration techniques (ROW and NUC) resulted in higher values of basal area, abundance and species richness compared to passive restoration (PAS). A higher abundance of natural regeneration was observed in ROW than in NUC. Additionally, the rarefaction curves suggested a trend toward higher diversity indices in ROW. Regarding the successional trajectory, greater variation in species composition was observed in ROW after 10 years of planting. The species composition was similar in ROW and NUC and differed from the composition in PAS. The species identified as indicator species in each treatment were Annona glabra L. in ROW, Trema micranthum (L.) Blume in NUC, and Vernonanthura polyanthes (Sprengel) Vega & Dematteis in PAS. Our study revealed that active restoration techniques, such as seedling planting in rows and nuclei, were more effective in restoring ecological indicators than passive restoration. These results emphasize the importance of restoration techniques in enhancing plant community diversity and structure in the Brazilian Atlantic Forest.
Keywords
Restoration Ecology, Tree Planting, Nucleation, Ecological Indicators, Biodiversity
Introduction
Ecological restoration has become a global strategy to cope with climate change, ecosystem degradation, and biodiversity loss ([12], [6]). It is, therefore, part of the international agenda of nature-based solutions ([18]). In tropical regions, ecological restoration is a tool that can promote the recovery of biodiversity and the reestablishment of ecosystem services, such as carbon sequestration ([21]). In Brazil, there has been a significant increase in ecological restoration initiatives and research in the last 30 years, especially concentrated in the Brazilian Atlantic Forest ([30], [24]). The history of environmental degradation, habitat loss and fragmentation and the high number of endemic species make the Atlantic Forest a priority hotspot for biodiversity conservation ([30]) and restoration ([50]). Furthermore, approximately 70% of the Brazilian population is concentrated within the area of this biome, further stressing the need for ecological restoration, because the maintenance of human well-being depends on the provision of ecosystem services ([10]).
One of the major challenges for ecological restoration projects is the assessment of the restoration success ([39], [48], [36]). Forest structure, species diversity, and species composition are fundamental indicators for assessing success ([36]) and the combination of different indicators has been pointed out as an effective approach to evaluate the successional trajectories and restoration success ([48], [31]). It is also important to evaluate the effect of different restoration techniques on the success of restoration, since studies have demonstrated that different techniques can lead to divergent results ([2], [33], [13], [29]). This knowledge is crucial for facilitating decision-making, enhancing project effectiveness, and guiding the development of ecological restoration strategies at various scales ([48]).
The present study evaluated the effect of different ecological restoration techniques on the basal area, species diversity and species composition of an area of Brazilian Atlantic Forest 10 years after the implementation of the project, considering the planted individuals and natural regeneration. Two different methods of planting native tree seedlings (rows and nucleation) were analyzed, along with passive restoration. Active restoration strategies tend to accelerate the recovery of vegetation structure ([49], [27]), while the good performance of passive restoration depends on favorable environmental conditions and the landscape context (e.g., proximity to forest fragments, low soil degradation, absence of fires - [14], [32]). Thus, the specific objectives of this study were: (i) to test the effect of different ecological restoration techniques on the basal area, species diversity and species composition 10 years after planting; (ii) to test the effect of these different techniques on natural regeneration (abundance, richness and composition); (iii) to evaluate the successional trajectory by the comparison of the floristic composition at the initial phase (project implementation) and 10 years after planting. We believe that our study makes relevant contributions to understanding the role of restoration techniques and the importance of monitoring for assessing the success of ecological restoration projects.
Material and methods
Study area
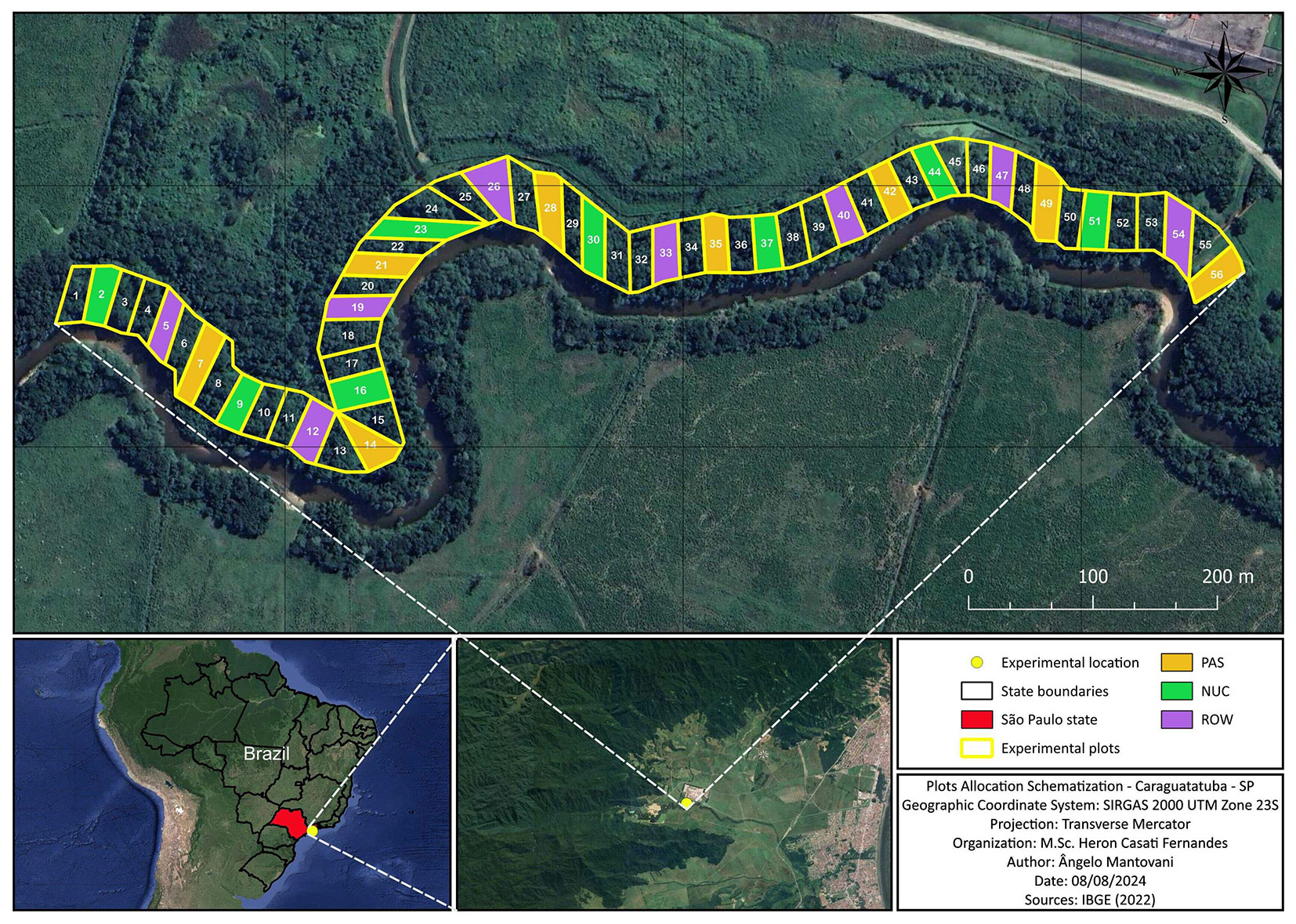
The study was conducted in an experimental planting area within a section of the permanent preservation area of the Monteiro Lobato Gas Treatment Unit (UTGCA/Petrobras). The site is located at coordinates 23° 39′ 27.70″ S and 45° 30′ 17.49″ W on the southeastern coast of Brazil, between the Serra do Mar mountain range and the ocean, in the municipality of Caraguatatuba, São Paulo (Fig. 1). The experimental area spans 6.5 ha in a strip of land 50 m wide and 1.3 Km long, serving as a restoration site for the riparian forest of the Juqueriquerê River. According to the Köppen classification ([1]), the region’s climate is classified as Af (Humid Tropical) with an average annual temperature of 22.79 °C, characterized by the absence of a defined dry season and heavy rainfall during the summer. The soil composition varies, with some areas containing slightly more clay due to past landslides, though sandy soils typical of coastal plains predominate. According to vegetation maps from IBGE ([47]), the area is part of the Brazilian Atlantic Forest, situated in a microregion that transitions between restinga forest (sandy coastal plains) and Lowland Tropical Forest.
Fig. 1 - Experimental area at the Monteiro Lobato Gas Treatment Unit (UTGCA/Petrobras) in Caraguatatuba, São Paulo, Brazil.
Experimental design
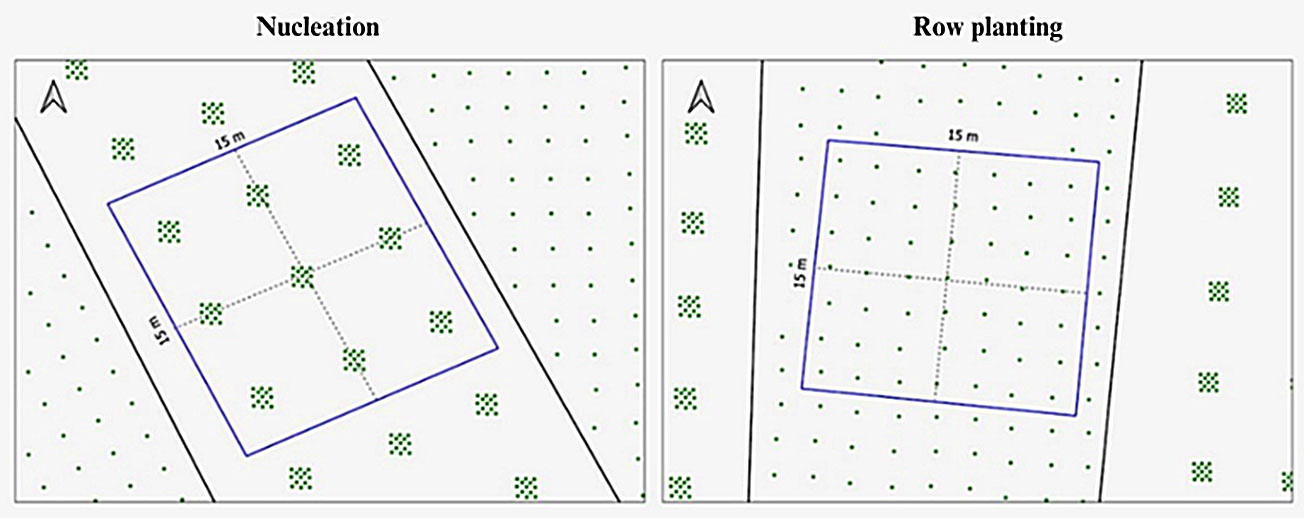
The experiment was implemented in 2012 through a partnership between UTGCA/Petrobras and the Laboratory of Tropical Forest Ecology (Lab-Trop - IB/USP). The experimental design consisted of eight blocks of three 20 × 50 m plots (Fig. 1 and Fig. 2), each plot containing one treatment. The three treatment types were systematically arranged in the same order within each block. The treatments consisted of three restoration methods: a control with passive restoration (PAS) and two active planting systems, namely, row planting (ROW) and nucleus planting (NUC). Row planting (ROW) was conducted with a 2 × 2 m spacing, and nucleus planting (NUC) with a 5 × 5 m spacing between nuclei, using 13 species per nucleus (five pioneer and eight non-pioneer species), with a 30 cm spacing between seedlings (Fig. 1 and Fig. 2). The restoration project implemented in the permanent preservation area of the Monteiro Lobato Gas Treatment Unit included other plots established within each block destined for fertilization experiments, which are not the focus of our present study. For collection of floristic data, we systematically established one subplot measuring 15 × 15 m (0.54 ha) in the center of each plot, thus far from the edges of the plot for minimizing the influence of the adjacent treatments (Fig. 2). Data collection was carried out in 2022, 10 years after the project implementation. We collected data on abundance, basal area, species richness, and species composition based on all individuals with diameter at soil level (DSL) ≥ 2.5 cm within each subplot and analyzed them according to the origin of the plants in each treatment: planted community or natural regeneration. This analysis was possible through the identification of individuals planted in 2012. Thus, our study allowed us to analyze the relationship between the planted and the natural regeneration communities. The subplots demarcated within the plots included 49 planted individuals in the ROW treatment and 117 planted individuals in the NUC treatment (Fig. 2), which is a reflection of the different planting densities employed in the active restoration techniques at the moment of implementation of the project (NUC: 5.200 seedlings ha-1; ROW: 2.177 seedlings ha-1). Control of invasive exotic grasses, such as Urochloa sp. and Melinis minutiflora P. Beauv., was achieved through mechanical weeding between the rows of planted areas until 2018, which was the sixth year of restoration. In contrast, the control the growth of exotic grasses in the PAS treatment involved only selective mechanical weeding, avoiding the cutting of woody plants, over a period of 10 years.
Fig. 2 - Schematic representation of the subplots (15 × 15 m) within the plots where the nucleation (NUC, left) and row planting (ROW, right) treatments were established in an experimental area at the Monteiro Lobato Gas Treatment Unit (UTGCA/Petrobras), Caraguatatuba, São Paulo - Brazil. On the left, the NUC treatment with 9 nuclei (5 × 5 m) in the subplot (N = 117 / 225 m2). On the right, the ROW treatment (2 × 2 m) with 49 pits in the subplot (N = 49 / 225 m2).
Data analysis
The data analysis initially included the entire community observed in each plot, comprising both planted and regenerating individuals in active restoration treatments (ROW and NUC) and only regenerating individuals in passive restoration (PAS), since no planting was conducted in the later treatment. Linear and negative binomial generalized linear models were fitted with and without blocks as a random effect to evaluate abundance and species richness data, and linear models with or without blocks as a random effect were fitted to evaluate basal area data. Models were compared using Akaike (AIC) and Bayesian (BIC) information criteria. We also performed a likelihood-ratio test to compare the goodness of fit of models with and without blocks as a random effect. When no difference was observed, we chose the simpler model. For all three variables, linear models without block as a random effect showed the best results. Thus, an ANOVA was conducted and the residual normality and homoscedasticity assumptions were checked graphically. When the ANOVA F-test indicated a difference, the Tukey’s multiple comparison test was used to compare treatment means. All these analyses considered a significance level of 5%.
For the active restoration treatments, we conducted further analysis to evaluate the interaction between restoration methods (ROW and NUC) and the origin of the plants (planted community or natural regeneration). For abundance and species richness, linear, Poisson, and negative binomial GLMs were fitted with or without blocks as a random effect. For basal area, linear models with or without blocks as a random effect were fitted. Models were compared using the AIC and BIC, and a likelihood-ratio test was conducted to compare the goodness of fit of models with or without blocks as a random effect. A Poisson GLM without blocks as a random effect was used to assess abundance and richness, and a linear model without blocks as a random effect was used for the basal area. The models’ assumptions were evaluated through graphical analysis. The significance of the coefficients was tested using the Wald test for the Poisson GLM and the t-test for the linear model.
The analyses were performed using the R statistical software version 4.4.1 ([37]), its base functions and packages, and functions of the “ExpDes.pt” (version 1.2.2, 2021), “lme4” (original version, 2015), and “emmeans’ (version 1.10.1, 2014) packages. Boxplots were made with the “ggplot2” (original version, 2016) package. Differences in species richness were also evaluated through rarefaction curves based on three Hill numbers, where the argument q refers to the Hill-number family: 0 = species richness, 1 = Shannon diversity, and 2 = Simpson diversity. The analyses and graphs were created using the “iNEXT” and “ggplot2” packages in the R software.
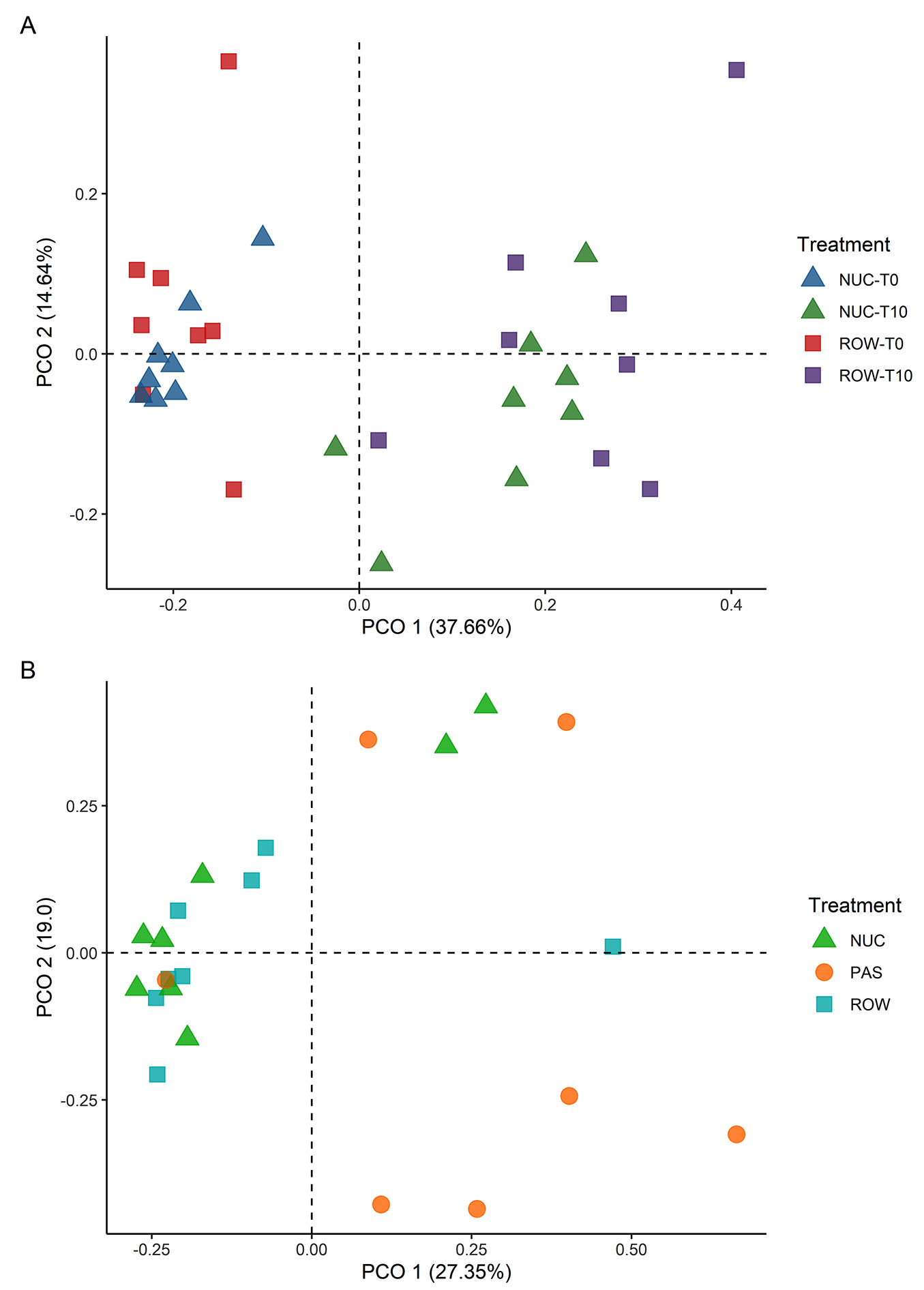
A principal coordinate analysis (PCoA) was used to visualize the differences in the successional trajectory in terms of species composition and abundance, using Bray-Curtis distance matrices for ordination of the composition ([16]) in the R software, using the “ggplot2” package to create the graphs. To evaluate the differences in the successional trajectory between ROW and NUC, we compared the species composition in these treatments at the beginning of planting (T0) and 10 years after planting (T10). T0 represents the species composition formed by the planted seedlings. In contrast, T10 represents the changes in species composition influenced by the mortality of planted individuals and the recruitment of individuals during natural regeneration. Species can serve as true symmetric indicators, providing valuable insights into the groups and characteristics of a given area ([16]). We employed the Indicator Value Index (IndVal) to identify indicator species within each restoration technique. This index combines the relative frequency and relative abundance of the species to calculate an Indicator Value, which is tested for statistical significance using Monte Carlo randomization ([16]). IndVal calculations were performed using the “indicspecies” package ([17]) in the R software, with the significance threshold set at p < 0.10.
Results
Planted and naturally regenerating communities
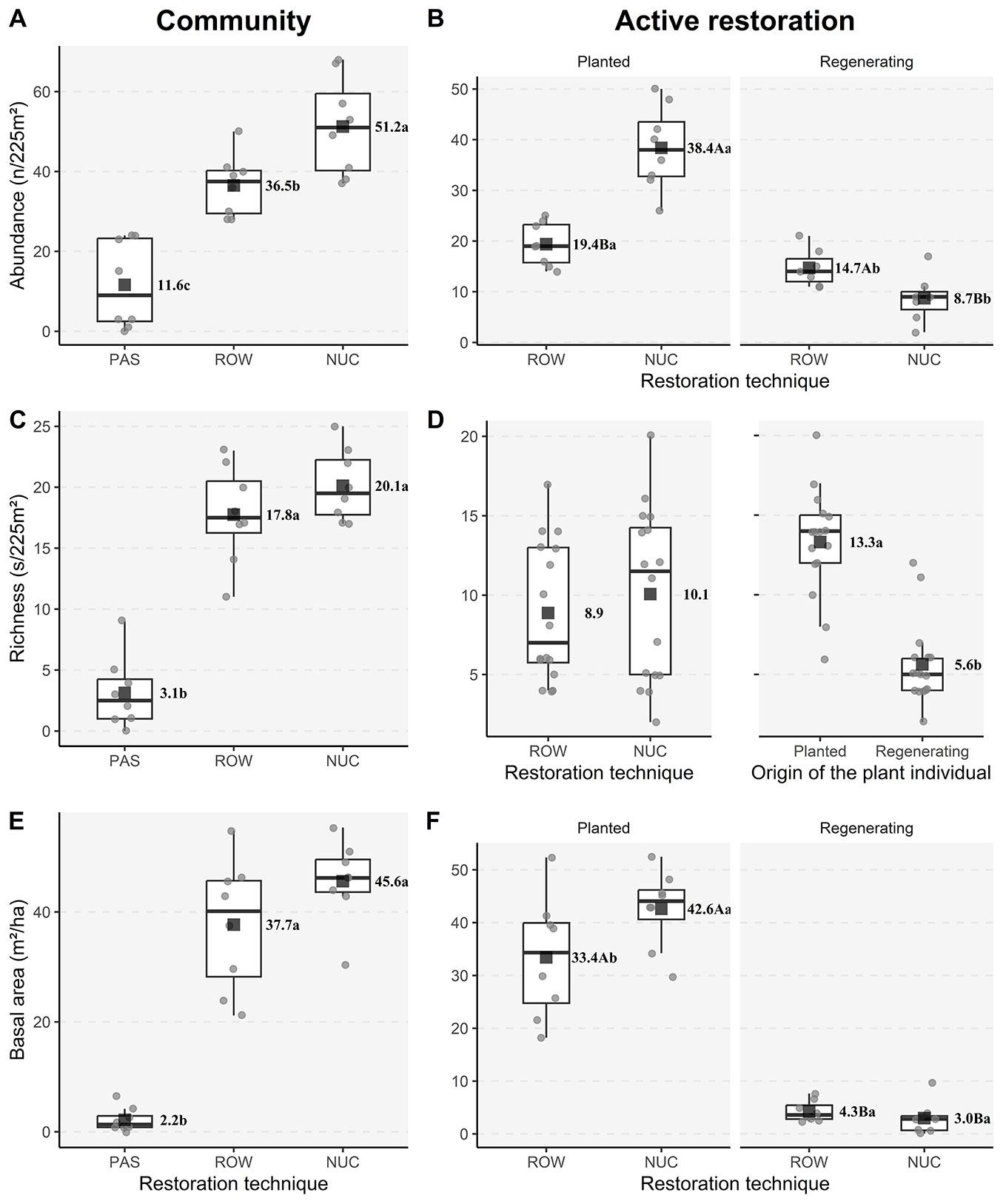
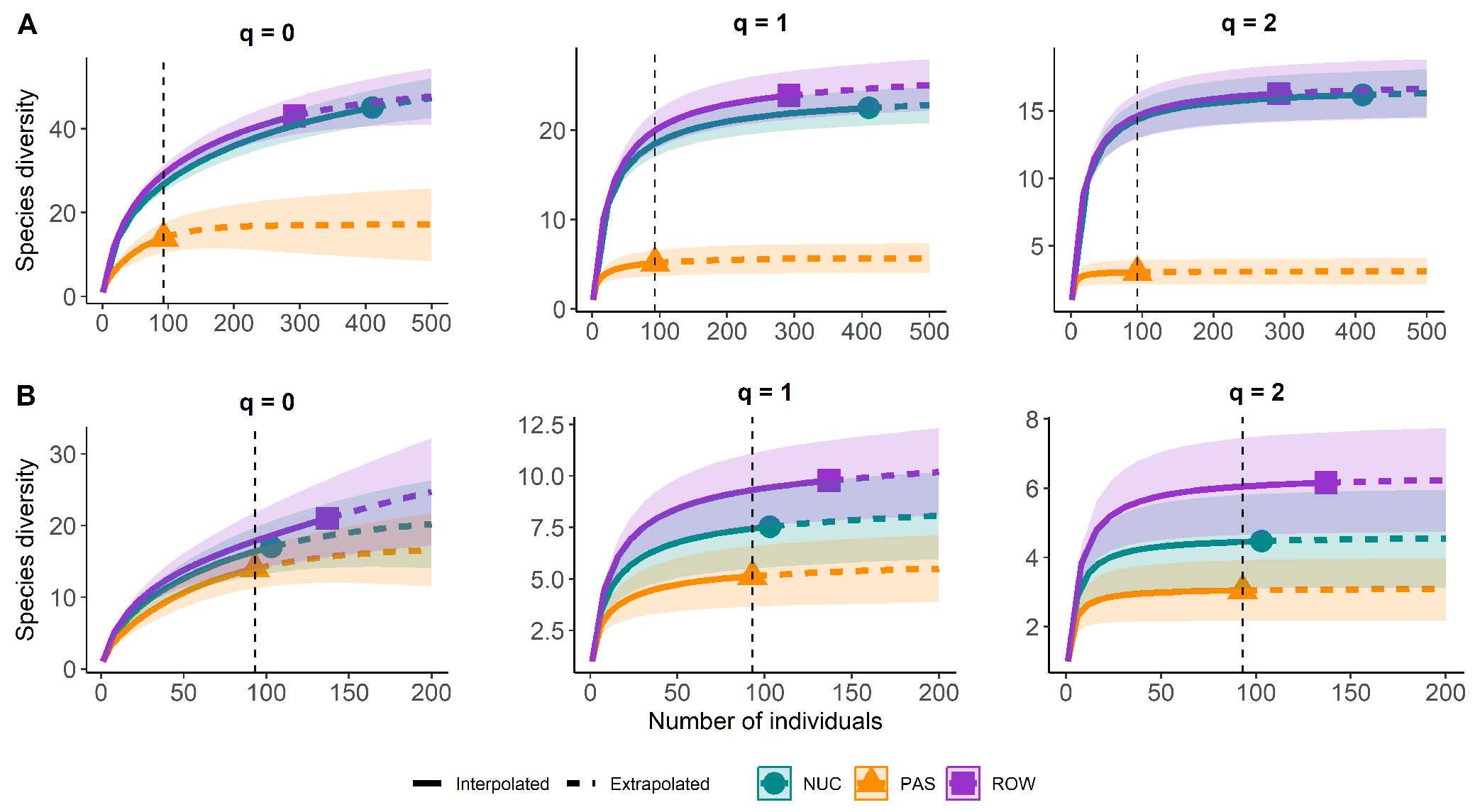
The analysis of the communities considering planted and regenerating individuals showed significantly higher abundance in NUC (51.25 ± 12.27) followed by ROW (36.5 ± 7.63) and PAS (11.62 ± 10.98) (Fig. 3A). There were no significant differences in basal area and species richness between ROW and NUC, but both exhibited higher values than PAS (Fig. 3C and Fig. 3E). In NUC and ROW, we observed an interaction between the treatment and plant origin (planted or regenerated) for abundance and basal area (see Fig. 3B and Fig. 3F), but not for richness (see Fig. 3D). Abundance and basal area of planted individuals were greater in NUC than in ROW. A reversed pattern was seen when only the natural regeneration was evaluated: abundance and basal area were higher in ROW than in NUC. The rarefaction curves showed that active restoration (ROW and NUC) resulted in higher values of all diversity indices - species richness, Shannon, and Simpson - compared to passive restoration (Fig. 4A). However, when considering only the natural regeneration, the rarefaction curve revealed no significant difference among the active (ROW and NUC) and passive (PAS) restoration techniques. PAS and NUC had similar Shannon and Simpson index values, while PAS differed from ROW in these diversity measures (Fig. 4B).
Fig. 3 - Abundance, richness, and basal area of the community (A, C, and E) in different restoration techniques, specifically for the active restoration (B, D, and F) considering the interaction between restoration techniques and the origin of the plants in an area under forest restoration for ten years in Caraguatatuba, São Paulo,Brazil. For community (A, C, and E), means followed by different letters differ from each other by the Tukey test (p < 0.05). For abundance (B) and basal area (F) in active restoration, means followed by a different uppercase letter inside a panel and by a different lowercase letter between panels differ from each other by the Wald and F test (p < 0.05), respectively. For richness in active restoration (D) the origin of the individual means followed by different letters differ from each other by the Wald test (p < 0.05).
Fig. 4 - Individual-based rarefaction curves of the whole community (planted + natural regeneration) (A) and considering only the natural regeneration (B), showing the three Hill numbers (q = 0 for species richness, q = 1 for Shannon diversity, and q = 2 for Simpson diversity) for the row planting (ROW), nucleation (NUC), and passive restoration (PAS) treatments in an experimental area at the Monteiro Lobato Gas Treatment Unit (UTGCA/Petrobras), Caraguatatuba, São Paulo, Brazil.
Successional trajectory of the species composition
A total of 795 individuals, including regenerating and planted individuals, were recorded, representing 51 species from 25 botanical families (see Tab. S1 in Supplementary material). NUC had the highest number of species (45 species), followed by ROW (43 species), and PAS (14 species). When considering only the natural regeneration, the differences in the number of species among treatments were marginal (ROW: 21; NUC: 17; PAS: 14 - Tab. S1 in Supplementary material). The analysis of the successional trajectory over 10 years after establishment of the experiment revealed a high floristic similarity between the two active restoration treatments at the time of planting (T0) and a trend of dispersion over time (T10 - Fig. 5a). However, considering the differences in seedling density of each technique (NUC: 5.200 seedlings ha-1; ROW: 2.177 seedlings ha-1), higher mortality in absolute numbers was observed in NUC (NUC: 3.475 ha-1; ROW: 1.372 ha-1 - Tab. S2 in Supplementary material). The floristic composition analysis considering only the natural regeneration community showed a difference between active planting (ROW and NUC) and passive restoration (PAS) after 10 years of implementation of the experiment (Fig. 5b). The analysis of indicator species assigned one species to each treatment: Annona glabra L. in ROW, Trema micranthum (L.) Blume in NUC, and Vernonanthura polyanthes (Sprengel) Vega & Dematteis in PAS.
Fig. 5 - Principal Coordinate Analysis (PCoA) of the species composition, based on abundance data, in the active planting treatments (NUC: nucleation; ROW: row planting) at the implementation (T0) and 10 years after implementation (T10) of the experiment (panel A); and PCoA of the species composition of the natural regeneration in the active planting (ROW and NUC) and passive restoration (PAS) treatments (panel B) in the experimental area at the Monteiro Lobato Gas Treatment Unit (UTGCA/Petrobras), Caraguatatuba, São Paulo, Brazil.
Discussion
We found that active restoration techniques promoted an enhancement of vegetation structure and species richness compared to passive restoration. These results are in contrast with previous findings in tropical forests, where similar ([3]) or better results ([14], [33]) were obtained in passive regeneration compared to active restoration techniques. The higher values of basal area and diversity indices for active planting in the present study may be associated with the legacy of the planted community and the positive effect on natural regeneration ([41], [31]). On the other hand, it is known that the presence of invasive exotic grasses in abandoned pastures poses barriers to natural regeneration ([42]), and this may have contributed to the results we found in PAS. Furthermore, there was a difference in the abundance of regenerating individuals between the two active planting techniques. This result suggests that planting density (which was higher in nuclei) may hinder natural regeneration. In fact, previous studies describe the negative effect of high planting density on seedling growth and natural regeneration ([35], [5]). Another explanation for the lower abundance of regeneration in NUC may be associated with the spacing between nuclei in this design (5 m between nuclei). The open spaces between plant nuclei may have facilitated the growth of invasive exotic grasses, leading to competition with natural regeneration ([28]). Additionally, the high density of seedlings planted in the nuclei (13 individuals with 0.3 m spacing between them) may have created a significant barrier to natural regeneration due to increased competition for resources ([43], [31]).
The convergence observed between row planting and nucleation in our study suggests a strong influence of the legacy of the initial species composition of the planted seedlings, as described by other authors ([28], [31]). This indicates that under certain environmental conditions and landscape contexts, different restoration techniques may lead to convergent results during the early stages. Although the general pattern indicates a convergence between planting techniques, the recruitment of new individuals through natural regeneration appeared to be favored in the ROW treatment. It is important to note that several studies have highlighted the role of different ecological restoration techniques in catalyzing ecological succession ([25], [31]) and recovering ecological processes ([46], [8]).
Among regenerating individuals, greater similarity in species composition was observed between the active planting treatments than between active and passive restoration. This pattern may be explained by the changes in environmental conditions (soil and radiation) caused by planting ([34], [19]), as well as by biotic interactions (e.g., priority effect - [38]). Some studies also highlight that successional trajectories are affected not only by the restoration strategy and local conditions ([26], [45]), but also by the time elapsed after planting and the proximity to forest remnants ([26], [4]). Thus, recovering species composition to a level similar to that of tropical forest remnants is a long process that may take more than 50 years ([22], [44]). This reinforces how species composition can be an unpredictable indicator ([44]) and points to the importance of monitoring the early stages of restoration so as to contribute to adaptive management ([23]). Therefore, it may be premature to draw conclusions from evaluating the species composition of an ecosystem only ten years after project implementation, and continuous studies over longer periods are recommended.
Although the species composition was similar among treatments, we identified specific indicator species for each restoration technique. The species V. polyanthes, A. glabra, and T. micranthum have distinct ecological characteristics, which may indicate that the treatments had different environmental conditions. V. polyanthes, the indicator species found in PAS, is a pioneering shrub with anemochorous dispersion, frequently associated with the early stages of ecological succession ([9]). The predominance of V. polyanthes in PAS confirms the characteristics observed in the field: an open area with a high presence of herbaceous and shrubby species. On the other hand, A. glabra, found in ROW, has zoochorous dispersion ([40]) and has been associated with sites presenting high soil moisture, such as swamps and riverbanks, which were observed in some sections of the study area with more persistent flooding points ([40]). As a non-pioneer species, A. glabra tends to establish in more advanced stages of succession ([40]). Additionally, the fact that A. glabra was among the planted species suggests the possibility of self-recruitment, reinforcing the already discussed effects of the initial planting composition. In turn, T. micranthum, an indicator species found in NUC, is classified as a pioneer, shade-intolerant species with zoochorous dispersal and is frequently found in degraded areas ([20]). The occurrence of T. micranthum in NUC may have been favored by the open areas between the nuclei, probably with a higher light incidence promoting its establishment.
Practical implications for ecological restoration
The choice of restoration techniques is an essential factor directly influencing the associated costs and the desired ecological metrics in restoration projects ([5], [7]). The search for efficient and economically viable strategies is vital to ensure the long-term success of such projects ([6]). Despite the lower costs of passive restoration ([11], [6], [3]), the results in terms of community basal area and species richness of the control treatment in our study were inferior compared to active restoration. Despite the presence of forest fragments in the region, the experimental areas are situated 800 to 1000 meters away from forest remnants. According to the study by Crouzeilles et al. ([15]) on the potential for natural regeneration in the Atlantic Forest, 90% of the areas undergoing regeneration are found less than 200 meters from forest remnants. Their results and our present findings indicate that even when there are forest fragments in the landscape, the distance between them and the restoration area may hinder the natural regeneration later. Therefore, it is important to consider not only the immediate costs, but also the landscape context and the desired ecological results, especially in the long term ([25]). The results obtained in this study with the active planting strategies, row planting and nucleation, proved more promising. The nucleation technique applied is generally associated with lower planting density about traditional row planting, and has intermediate costs ([49], [27], [3]). However, in the present study, the arrangement employed in nucleation involved a high density of seedlings per nuclei, that increased the logistical challenges and the implementation and maintenance costs ([6], [3]).
Conclusions
Active restoration techniques through seedling planting in rows and nuclei proved to be more effective to recover ecological indicators compared to passive restoration. Despite the significant differences in planting density between the active techniques at the implementation of the project (NUC: 5.200 seedlings ha-1; ROW: 2.177 seedlings ha-1), no difference was found in basal area, species richness and composition between these treatments. However, when the analysis focused only on natural regeneration, higher abundance and richness values were observed in row planting. Considering the relevance of natural regeneration in the restoration process, row planting can be pointed out as the appropriate technique for the local context. However, it is important to emphasize that the findings we obtained are specific to our study location and timeframe analyzed. Finally, we stress that ongoing monitoring is fundamental to evaluate the results and develop potential strategies for intervention and management support in the region.
Acknowledgments
This study was funded by PETROBRAS (#5900.0110930.19.9) through a grant to DM and partly financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES), Finance Code 001; JBBS is supported by a PQ-2 grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Jerônimo BB Sansevero 0000-0002-3389-2581
Universidade Federal Rural do Rio de Janeiro - UFRRJ, Programa de Pós-Graduação em Ciências Ambientais e Florestais - PPGCAF, Seropédica, RJ (Brazil)
Dulce Mantuano 0000-0002-8049-6936
Mariana Machado Saavedra 0000-0001-9063-1858
Moab T Andrade 0000-0002-0318-0815
Universidade Federal do Rio de Janeiro - UFRJ, Departamento de Botânica, Rio de Janeiro, RJ (Brazil)
Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA - Solos), Rio de Janeiro (Brazil)
Universidade de São Paulo - USP, Departamento de Ecologia, São Paulo (Brazil)
Corresponding author
Paper Info
Citation
Casati Fernandes H, Manhães AP, Alonso JM, Mantuano D, Martini AMZ, Saavedra MM, Andrade MT, Sansevero JBB (2025). Effect of restoration methods on natural regeneration in the Brazilian Atlantic Forest. iForest 18: 23-29. - doi: 10.3832/ifor4598-017
Academic Editor
Michele Carbognani
Paper history
Received: Mar 06, 2024
Accepted: Nov 05, 2024
First online: Feb 15, 2025
Publication Date: Feb 28, 2025
Publication Time: 3.40 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2025
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 10144
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 3703
Abstract Page Views: 3342
PDF Downloads: 2828
Citation/Reference Downloads: 3
XML Downloads: 268
Web Metrics
Days since publication: 359
Overall contacts: 10144
Avg. contacts per week: 197.79
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Can the dynamics of forest restoration reduce landscape fragmentation in the Atlantic forest?
vol. 18, pp. 61-68 (online: 04 April 2025)
Research Articles
Rewilding beech-dominated temperate forest ecosystems: effects on carbon stocks and biodiversity indicators
vol. 18, pp. 1-9 (online: 02 February 2025)
Review Papers
Shaping the multifunctional tree: the use of Salicaceae in environmental restoration
vol. 6, pp. 37-47 (online: 21 January 2013)
Research Articles
Three prescribed fire regimes on the restoration of flooded savannah grasslands under encroachment of Vochysia divergens Pohl, Pantanal, Brazil
vol. 17, pp. 165-171 (online: 17 June 2024)
Research Articles
Outplanting performance of three provenances of Quillaja saponaria Mol. established in a Mediterranean drought-prone site and grown in different container size
vol. 13, pp. 33-40 (online: 21 January 2020)
Research Articles
Approaches to classifying and restoring degraded tropical forests for the anticipated REDD+ climate change mitigation mechanism
vol. 4, pp. 1-6 (online: 27 January 2011)
Research Articles
Strong relationships between soil and vegetation in reference ecosystems of a riparian Atlantic rainforest in the upper Doce River watershed, southeastern Brazil
vol. 16, pp. 226-233 (online: 17 August 2023)
Research Articles
Tropical seedling performance under drought: a functional trait approach for species selection in restoration
vol. 19, pp. 9-17 (online: 10 January 2026)
Research Articles
Post-fire recovery of the plant community in Pinus brutia forests: active vs. indirect restoration techniques after salvage logging
vol. 11, pp. 635-642 (online: 04 October 2018)
Research Articles
Managing invasive tree stumps in the restoration of legacy chestnut orchards
vol. 18, pp. 350-356 (online: 30 November 2025)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword