How environmental factors condition natural regeneration in the altitudinal gradient of a montane rainforest
iForest - Biogeosciences and Forestry, Volume 17, Issue 3, Pages 132-139 (2024)
doi: https://doi.org/10.3832/ifor4319-017
Published: May 04, 2024 - Copyright © 2024 SISEF
Research Articles
Abstract
The response of plant species to the variation in abiotic factors affects the regeneration capacity and, consequently, the structure of the forest community. This study aims to describe the structure of the regenerating stratum in a Brazilian montane rainforest and investigate its relationship with environmental and spatial variables along an altitudinal gradient. Data on the height and diameter at soil height of regenerating individuals and environmental variables were collected from 28 sample units, distributed in seven altitudinal sites. To understand the spatial influence on species distribution, spatial variables (MEMs - Moran’s Eigenvector Maps) were created based on geographic coordinates. Phytosociological parameters were calculated by species. Floristic similarity between the altitudinal quota was determined by the Bray-Curtis index (UPGMA), and the species that characterize each group were determined by the Indicator Species Analysis. Redundancy Analysis (RDA) was performed, and generalized linear models were adjusted to verify the influence of environmental and spatial factors on regenerating vegetation. The species Palicourea sessilis had the highest Importance Value in the regenerating community. Two floristic groups were formed: the highest sites (1420 to 1550 m a.s.l.) were floristically more similar to each other (Group 1) than to the sites in the 1112 to 1391 m elevation range (Group 2). Overall, 11 species were indicators of Group 1 and only one of Group 2. Finally, a pattern of species substitution was verified as a function of abiotic factors. The first two axes of the RDA explained 51.02% of the variation in the floristic composition of the regenerating community. Natural regeneration demonstrated environmental preferences, being influenced by luminosity, abundance in adult components, calcium and sodium contents, plant litter accumulation, altitude, and the spatial structure of the environment. Altitude did not seem to influence the pattern of abundance or richness of regenerating species.
Keywords
Environmental Variation, Species Distribution, Vegetation-Environment Relationship, Elevation, Understory
Introduction
The forest is a dynamic system in constant change. The reconstitution of forest cover is a natural and continuous process that occurs through the growth of young individuals in the understory, until they reach the upper stratum, gradually replacing the senescent trees in the canopy ([26]). This process is part of the forest maintenance cycle, which ranges from the initial stages of development to its recruitment and establishment ([11]).
The regeneration capacity and species composition in a plant community, besides being profoundly affected by the presence of progenitors, is directly associated with local environmental factors, such as light radiation, atmospheric temperature, rainfall, and soil characteristics ([21], [11]). These environmental factors can change intensely with altitude ([35], [38]). This environmental heterogeneity linked to altitude can generate distinct patterns of species distribution for each location ([48]). Moreover, geographical distance can act as a limiting factor for the dispersal of some species, affecting the composition of regeneration. Thus, the spatial component can also be crucial in understanding vegetation patterns in elevation gradients ([24]).
The environmental and compositional heterogeneity of montane tropical forests, characterized by high diversity and endemism, creates sites that can potentially serve as refuges for numerous species in the face of natural or anthropogenic disturbances ([45]). Therefore, forest cover is constantly changing as species migrate to more favorable environmental areas along small altitudinal gradients ([45]). Studies focused on natural regeneration help to predict the future forest behavior and development ([46]), as the dynamics of processes occurring in the regeneration stratum affects the characteristics of the adult vegetation, thus influencing the level of biodiversity and stability of the ecosystem ([3], [34]). However, knowledge on how environmental changes along altitudinal gradients affect natural regeneration is scarce, especially in montane ombrophilous forests, probably due to the limited number of protected areas and their challenging accessibility.
This study aims to understand how the structure of natural regeneration in a Dense Ombrophilous Forest is affected by environmental and spatial factors along an altitudinal gradient.
Material and methods
Study area
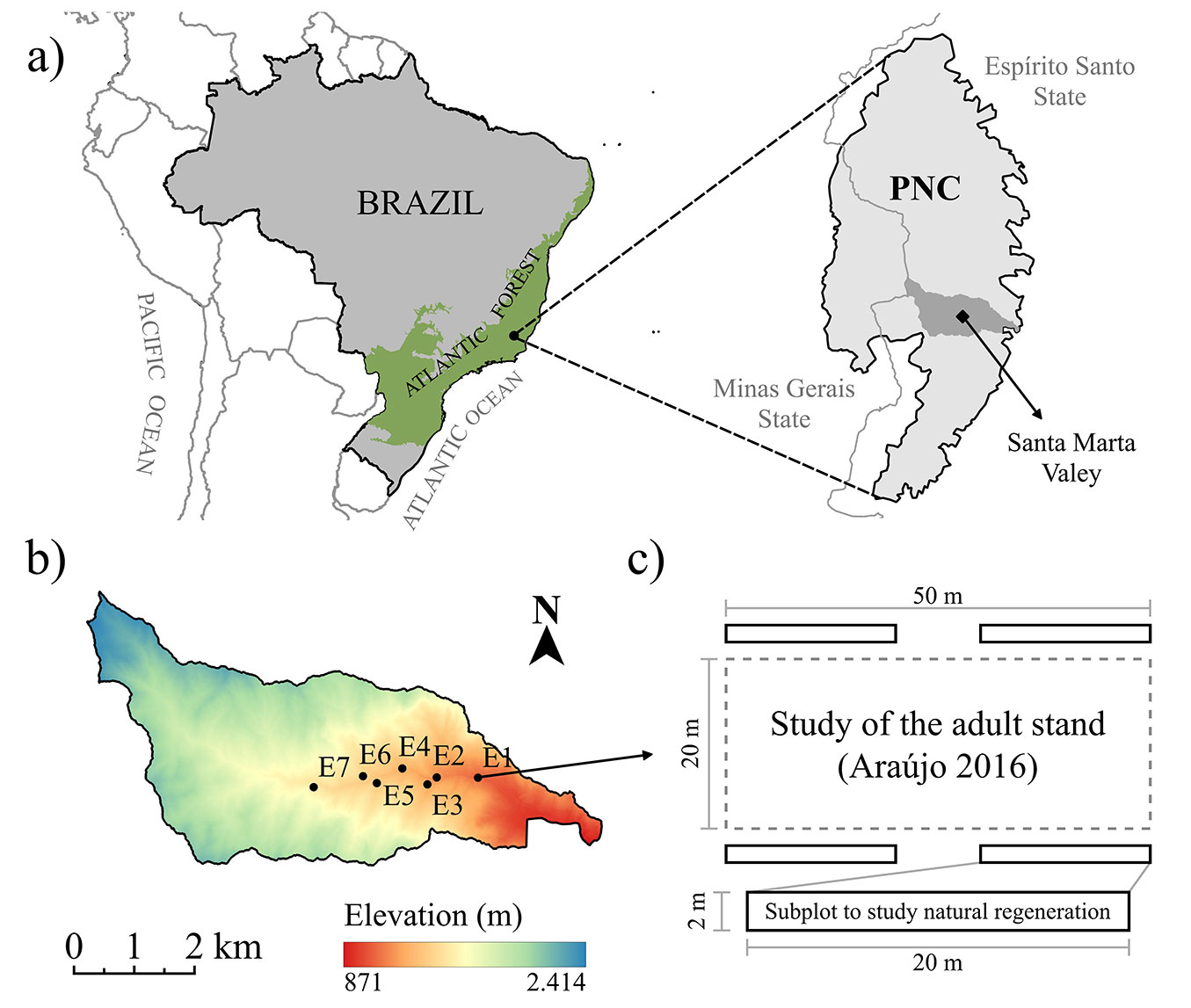
The study was carried out in a Dense Ombrophilous Forest in the Santa Marta valley, municipality of Ibitirama, state of Espírito Santo, Brazil. The valley is located in the southern portion of the Caparaó National Park (Parque Nacional do Caparaó, PNC), an area with 31.853 hectares of protected Atlantic Forest, situated on the border between the states of Espírito Santo and Minas Gerais, Brazil (20°37′ and 20°19′ S; 41°43′ and 41°45′ W - Fig. 1a). In 2012, Araújo ([6]) established seven plots (20 × 50 m) in this area to characterize the upper story vegetation (adult stand) at different elevations along two valley steep slopes (Fig. 1b) with different aspects (Tab. 1). Additionally, we systematically arranged four subplots measuring 2 × 20 m adjacent to each of the plots installed by Araújo ([6]) to understand how the structure of natural regeneration is influenced by environmental and spatial factors, resulting in a total of 28 subplots (Fig. 1c). The set of plots and subplots were considered sites in this study.
Fig. 1 - Location of the study region in the Caparaó National Park, Brazil (a); elevational gradient of the Santa Marta valley and the sample sites’ location (b); and sampling plot and subplot design (c).
Tab. 1 - Average slope and aspect of the seven sites along an altitudinal gradient in the Santa Marta valley, Caparaó National Park, Espírito Santo state, Brazil.
| Site | Elevation (m a.s.l.) |
Average slope (%) |
Aspect |
|---|---|---|---|
| E1 | 1112 | 69.55 | East/Northeast |
| E2 | 1219 | 58.29 | East/Southeast |
| E3 | 1302 | 75.00 | Northeast |
| E4 | 1319 | 75.00 | East |
| E5 | 1391 | 48.24 | North |
| E6 | 1420 | 73.39 | South/Southeast |
| E7 | 1550 | 58.00 | Northeast |
The Santa Marta valley ranges between 800 and 2300 m a.s.l. in altitude and is covered by ombrophilous forests with two partially distinct physiognomies: Dense Ombrophilous Montane Forest (altitude between 500 and 1500 m) and Upper Montane Forest (altitude above 1500 m - [28]). The soils in the valley are classified as Dystrophic Yellow Latosol, Dystrophic Red-Yellow Latosol, Dystrophic Humic Cambisol, and Folic Organosol ([18]). According to the Köppen climate classification for Brazil, the predominant climate in the region is subtropical highland (Cwb - [4]), with average temperatures from 19 to 22 °C, relative humidity usually above 70%, and average annual precipitation around 1400 mm ([36]).
Data collection
In each sampling site, we recorded the height and diameter at ground height (DGH) for all individuals with a minimum height of 50 cm and a diameter at breast height (DBH) lower than 2.5 cm. Taxonomic identification of seedlings was carried out by comparing seedling samples with specimens stored in regional herbaria, such as the Herbário Capixaba (CAP) and virtual herbaria, with aid from the literature and specialists in taxonomic groups. Taxonomic classification followed the Angiosperm Phylogeny Group ([5]), and species nomenclature followed the Reflora Virtual Herbarium ([44]). Species were classified into ecological groups (shade-tolerant, intermediate shade-tolerant, and shade-intolerant species) according to Gandolfi et al. ([27]).
To characterize the canopy opening, we used digital hemispherical photographs captured by a smartphone with a hemispherical lens attached ([49]). The device was positioned at 1.30 m above the ground, with the upper part aligned with magnetic north, and the lens pointed towards the sky. Pictures were taken under cloudy or mist conditions and during the hours of the day when sunlight was not directly reaching the device’s lens, to increase the contrast between the sky and the foliage and guarantee the accuracy of the element classification in the image.
Data on the abundance of adult individuals and the soil chemical composition in the study area were taken from the study by Araújo ([6]) and data on the plant litter accumulation were taken from Castro ([17]). Araújo ([6]) inventoried all shrubby and arboreal individuals with DBH ≥ 2.5 cm to describe the adult floristic composition. Additionally, Araújo ([6]) collected subsamples of soil at a depth of 0-20 cm to characterize the chemical composition in each site of this study. To quantify plant litter accumulation, Castro ([17]) carried out monthly collections from November 2012 to October 2013. In each collection, 12 plant litter samples were obtained from the forest floor at each site.
Data analysis
Phytosociological parameters such as density, dominance, and frequency, Margalef richness index, Shannon diversity index, and Pielou’s evenness were estimated. Since the data on absolute density and species richness did not meet the assumptions of parametric statistics, the differences among the sites were tested using the non-parametric Kruskal-Wallis test (α = 0.05).
The Bray-Curtis index was used to assess floristic similarities among sites ([33]). This analysis was conducted considering only the morphotypes identified at least at the family level, excluding morphotypes that remained undetermined. Cluster analysis was performed using the UPGMA method based on Bray-Curtis index and species abundances. To assess the consistency of the groups, bootstrap resampling with 999 repetitions was performed ([22]). The fit between the graphical representation of similarity and the original abundance matrix was assessed by estimating their cophenetic correlation coefficient.
To identify the species characterizing the floristic clusters obtained by the UPGMA analysis, an Indicator Species Analysis (ISA) was carried out combining the abundance and relative frequency values of the species, calculated independently for each species ([20]). The significance of the results was verified by the Monte Carlo test ([16]).
A Redundancy Analysis (RDA) was performed to evaluate the interaction between the regenerating community and the environmental and spatial elements along the Santa Marta valley. To do this, two data matrices were created; the primary matrix contained the abundance values of the species, and the secondary matrix contained the environmental and spatial variables.
Species with less than 10 individuals in the total sample were disregarded from the abundance matrix, as rare species generate enormous statistical noise and add little to the ecological interpretation of the data. The Hellinger transformation method was then used on the abundance data ([29]), aiming to reduce the influence of highly abundant groups.
The environmental variables (Tab. S1 in Supplementary material) were standardized to mean of zero and standard deviation of 1 ([9]). Using correlation matrices (Tab. S2 in Supplementary material) and principal component analysis (PCA), the identified collinear environmental variables were removed from the set and the ecologically important predictors for this study were maintained ([23]), such as the potential soil acidity (H+Al), magnesium in the soil (Mg), plant litter accumulation (AS), and abundance of adult individuals in the plot (ABa). The collinearity of the variables selected from the Variance Inflation Factor (VIF < 10) was checked and then the global test was applied to assess the significance of the environmental covariates ([8]).
As proposed by Borcard & Legendre ([10]), geographical coordinates (latitude and longitude) were used to create spatial variables (MEMs - Moran’s Eigenvector Maps) and investigate the influence of space on the distribution of species in the community. Nine MEMs were created, of which the most significant (MEM 1) was chosen for the model using the forward selection method ([8]). These procedures were performed with the “vegan” and “spacemakeR” package in R environment ([43]).
The final matrix of species abundance (38 species) with transformed values (Hellinger transformation) and the matrix formed by the environmental variables (selected after correlation tests and PCA) and spatial variables (MEM 1 - selected by forward selection) were then submitted to RDA. The significance of the main axes of the RDA was evaluated by the Monte Carlo permutation test with 999 repetitions.
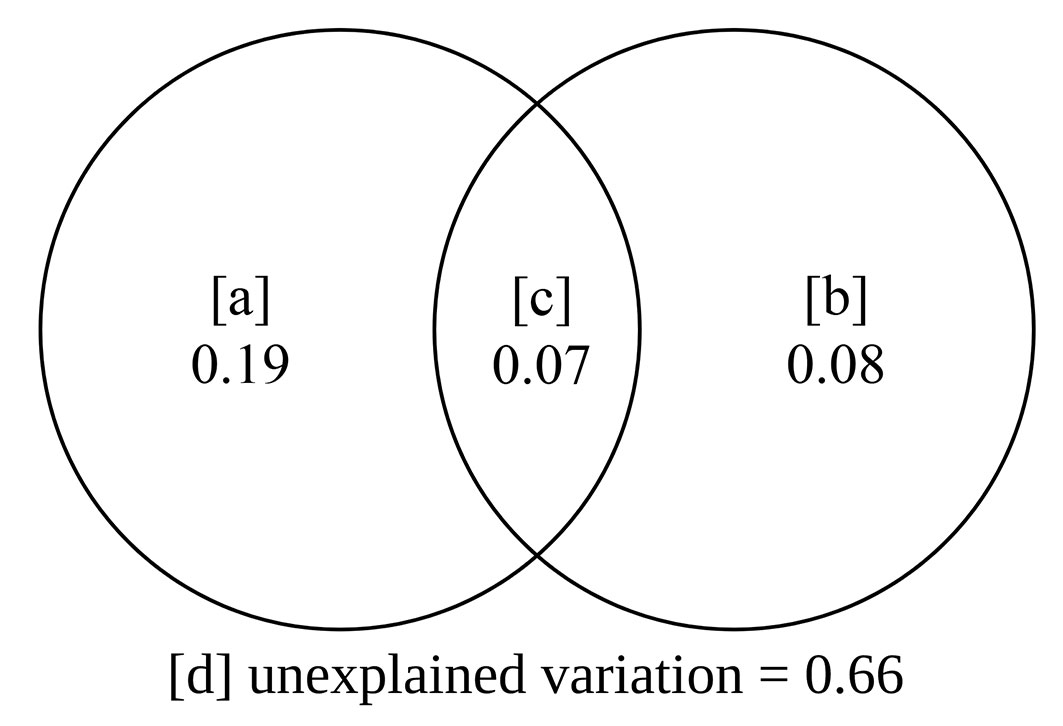
Based on the RDA results and the selected environmental and spatial variables, we verified how much of the variation in the composition of the regenerating community is explained by the environmental variables only (a), by the interaction between the environment and space (b), by spatial variables only (c), and by indeterminate variables (d). The significance of fractions (a), (b), and (c) was tested using the permutation test.
We adjusted the generalized linear models (GLM) to analyze how the number of individuals, species richness, and the regenerating diversity per plot are influenced by environmental variables. The environmental variables showing significant correlation were removed to ensure the model parsimony. All combinations between the independent variables were compared using the “glmulti” package in R environment and the best models were identified by the lowest values of Akaike Information Criterion (AICc). Due to the observed overdispersion, the negative binomial family was adopted to adjust the models for number of individuals and richness of species and Gaussian family for regenerators diversity. The normality of the residuals was verified by the Shapiro-Wilk and QQ-Plots tests.
Results
We found a total of 2055 individuals (18,348 ind ha-1), distributed in 136 species and 46 families, in addition to the morphotypes that remained unidentified and/or with taxonomic uncertainties (Tab. S3 in Supplementary material). Considering all the morphotypes identified at the species level, the Shannon diversity index (H’) was 3.98 nats ind-1 (one nat is equal to 1/ln 2 shannons ≈ 1.44 Sh), and the Pielou’s evenness index (J′) was 0.81. The highest values of H′ were observed in sites E4 (3.41 nats ind-1) and E5 (3.55 nats ind-1); and the lowest, in E7 (2.70 nats ind-1). The J′ index was higher in sites E3 and E5, with 0.90 and 0.92, respectively, and lower in E7 (0.81).
The group of shade-tolerant species were predominant (60%), followed by intermediate shade-tolerant trees (27%), and shade-intolerant (13%). The absolute density of individuals was higher in sites E1, E2, E4, and E6 (Tab. 2). The sites along the altitudinal gradient significantly differed in terms of species richness, forming small groups similar to each other. However, the Margalef Richness Index did not show any pattern reflecting site elevation.
Tab. 2 - Absolute density and Margalef’s richness index. The means followed by the same letter in the columns do not differ from each other, according to the Kruskal-Wallis non-parametric test (p < 0.05).
| Site | Elevation (m a.s.l.) |
Density (ind ha-1) |
Margalef Richness (DMg) |
|---|---|---|---|
| E1 | 1112 | 20,187.50 a | 6.19 a |
| E2 | 1219 | 25,187.50 a | 5.90 ab |
| E3 | 1302 | 9,250.00 b | 2.43 d |
| E4 | 1319 | 26,875.00 a | 6.03 a |
| E5 | 1391 | 12,937.50 b | 4.33 bc |
| E6 | 1420 | 20,625.00 a | 5.15 ab |
| E7 | 1550 | 13,375.00 b | 3.57 c |
The ten species with the highest Importance Value (IV) in the regenerating stratum were Palicourea sessilis (5.00%), Guapira opposita (3.68%), Leandra melastomoides (2.54%), Psychotria sp. 5 (2.14%), Bathysa australis (1.19%), Miconia latecrenata (1.70%), Cabralea canjerana (1.67%), Cupania racemosa (1.53%), Ocotea sulcata (1.32%), and Myrcia splendens (1.24%), summing up to 23% of the total IV of the community.
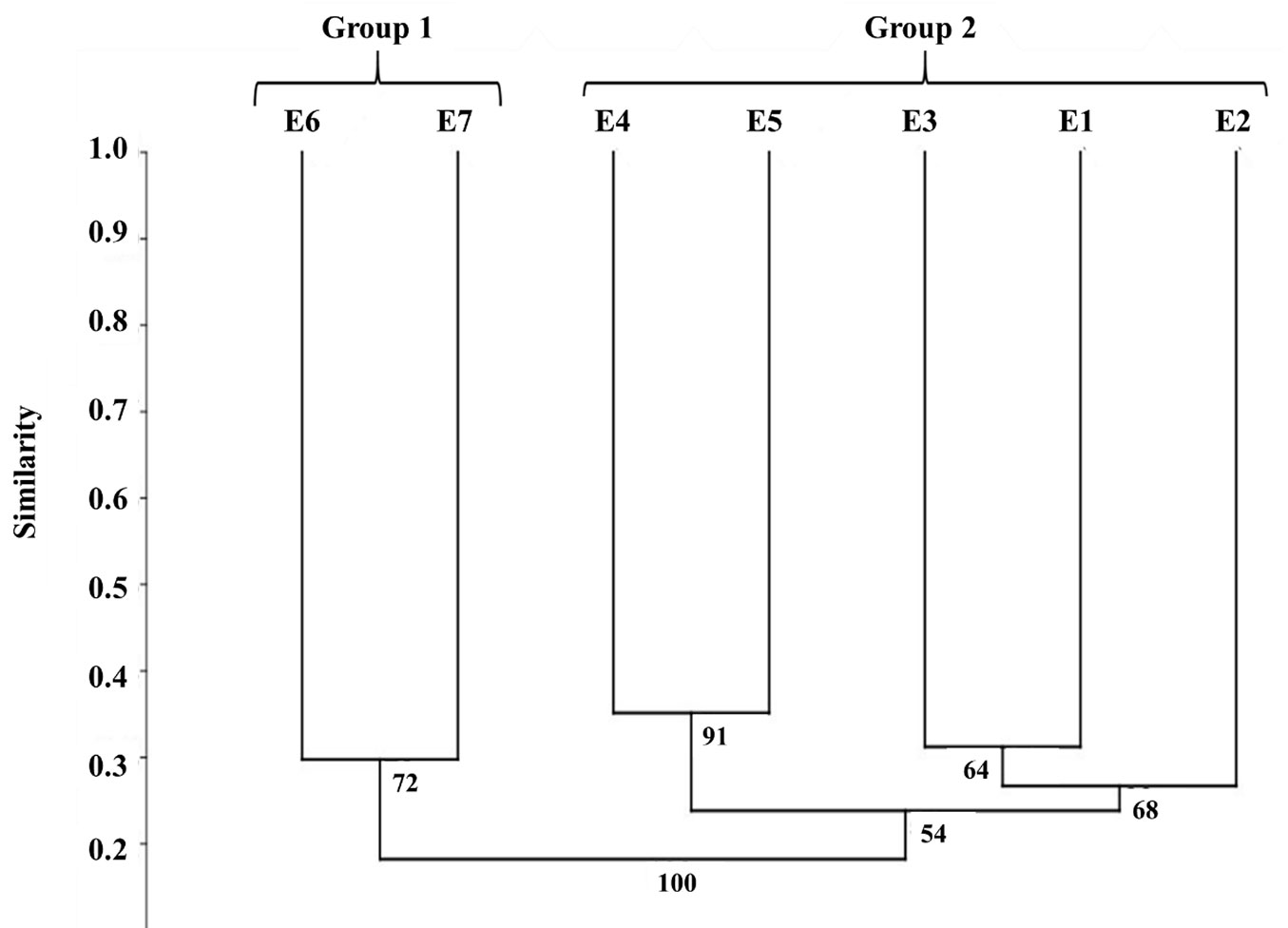
The cluster analysis revealed the formation of two groups with high species similarity (Fig. 2): group 1, formed by the highest sites (1420 to 1550 m); and group 2, composed of the lowest elevations (1112 to 1391 m). We also observed several subgroups within group 2, with sites E4 and E5 showing greater similarity between each other than with E1, E2, and E3, which formed a second subgroup. The subdivision within group 1 (between E6 and E7) showed a high replicability after bootstrap resampling (72%); whereas in group 2, the fork between E4 and E5 was obtained 91% of times, and the subgroup formed by E1, E2, and E3, was obtained in 68% of cases. The obtained dendrogram showed a cophenetic correlation coefficient of 0.7963, demonstrating that the grouping of individuals by the UPGMA method was not affcted by distortions.
According to the Indicator Species Analysis (ISA), 12 species showed significant (p<0.05) differences in abundance across the above groups: Cordiera cf. myrciifolia, Inga schinifolia, Maytenus sp. 2, Miconia cf. ibaguensis, Miconia sp. 7, Myrcia subcordata, Piper sp. 1, Primulaceae sp. 1, and Weinmannia pinnata are perfect indicators (Indicator Value = 100%) of group 1 in the cluster analysis. Additionally, Miconia cf. longicuspis and Begonia angularis also had a significantly differentiated distribution in this group. As for group 2, only Sorocea bonplandii was a perfect indicator. In this group, other species presented high indicator value, though they did not show statistical significance (Tab. 3).
Tab. 3 - Indicator Species (IV > 70) of the floristic groups of the Santa Marta valley from cluster analysis (UPGMA). (IV): importance value (%); (Prob): significance degree.
| Group (Elevation) | Indicator Species | IV | Prob. |
|---|---|---|---|
| Group 1 (1420 to 1550 m a.s.l.) |
Cordiera cf. myrciifolia | 100.00 | 0.048* |
| Inga schinifolia | 100.00 | 0.048* | |
| Maytenus sp. 2 | 100.00 | 0.048* | |
| Miconia cf. ibaguensis | 100.00 | 0.048* | |
| Miconia sp. 7 | 100.00 | 0.048* | |
| Myrcia subcordata | 100.00 | 0.048* | |
| Piper sp. 1 | 100.00 | 0.048* | |
| Primulaceae sp. 1 | 100.00 | 0.048* | |
| Weinmannia pinnata | 100.00 | 0.048* | |
| Miconia cf. longicuspis | 98.90 | 0.048* | |
| Begonia angularis | 97.60 | 0.048* | |
| Miconia cf. elegans | 89.30 | 0.094 | |
| Daphnopsis fasciculata | 81.80 | 0.095 | |
| Myrcia eugenioides | 76.90 | 0.144 | |
| Miconia latecrenata | 74.70 | 0.193 | |
| Miconia setosociliata | 71.40 | 0.245 | |
| Guapira opposita | 70.40 | 0.390 | |
| Group 2 (1112 to 1391 m a.s.l.) |
Sorocea bonplandii | 100.00 | 0.048* |
| Leandra melastomoides | 80.00 | 0.230 | |
| Meliosma itatiaiae | 80.00 | 0.294 | |
| Meriania tetrmera | 80.00 | 0.280 | |
| Mollinedia schottiana | 80.00 | 0.186 | |
| Ocotea aciphylla | 80.00 | 0.233 | |
| Psychotria leiocarpa | 80.00 | 0.142 | |
| Miconia tristes | 77.60 | 0.414 | |
| Cupania racemosa | 70.90 | 0.428 |
The eigenvalues of the first two axes of the RDA explained 51.02% of the total variation in species abundance (Tab. 4). The axes indicated strong positive correlations between species and covariates (values > 0.80); only the first axis, however, indicated a significant correlation according to the Monte Carlo test (p<0.05). Strong correlations between covariates and axes were also observed (Tab. 5).
Tab. 4 - Statistical summary of the canonical axes of Redundancy Analysis (RDA) for the relationship between the regenerating vegetation and the environment.
| Stats | Axis 1 | Axis 2 |
|---|---|---|
| Eigenvalue | 17.10 | 9.71 |
| Variation of species data explained by axis (%) | 32.54 | 18.48 |
| Cumulative percentage of variation | 32.54 | 51.02 |
| Pearson’s correlation (species - covariates) | 0.999 | 0.993 |
| F | 3.04 | 1.73 |
| Monte Carlo test (p-value) | 0.022 | 0.335 |
Tab. 5 - Correlations between the canonical axes of Redundancy Analysis (RDA) and predictor variables. (*): correlations with values > 0.8. (H+Al): potential acidity of the soil; (Mg): magnesium; (AS): plant litter accumulation; (Aba): abundance of adult individuals; (MEM 1): spatial filter (Moran’s Eigenvector Maps).
| Components | Correlation | |
|---|---|---|
| Axis 1 | Axis 2 | |
| H+Al | 0.961* | -0.168 |
| Mg | 0.319 | -0.186 |
| AS | -0.485 | 0.461 |
| ABa | 0.317 | 0.837* |
| MEM 1 | 0.917* | -0.082 |
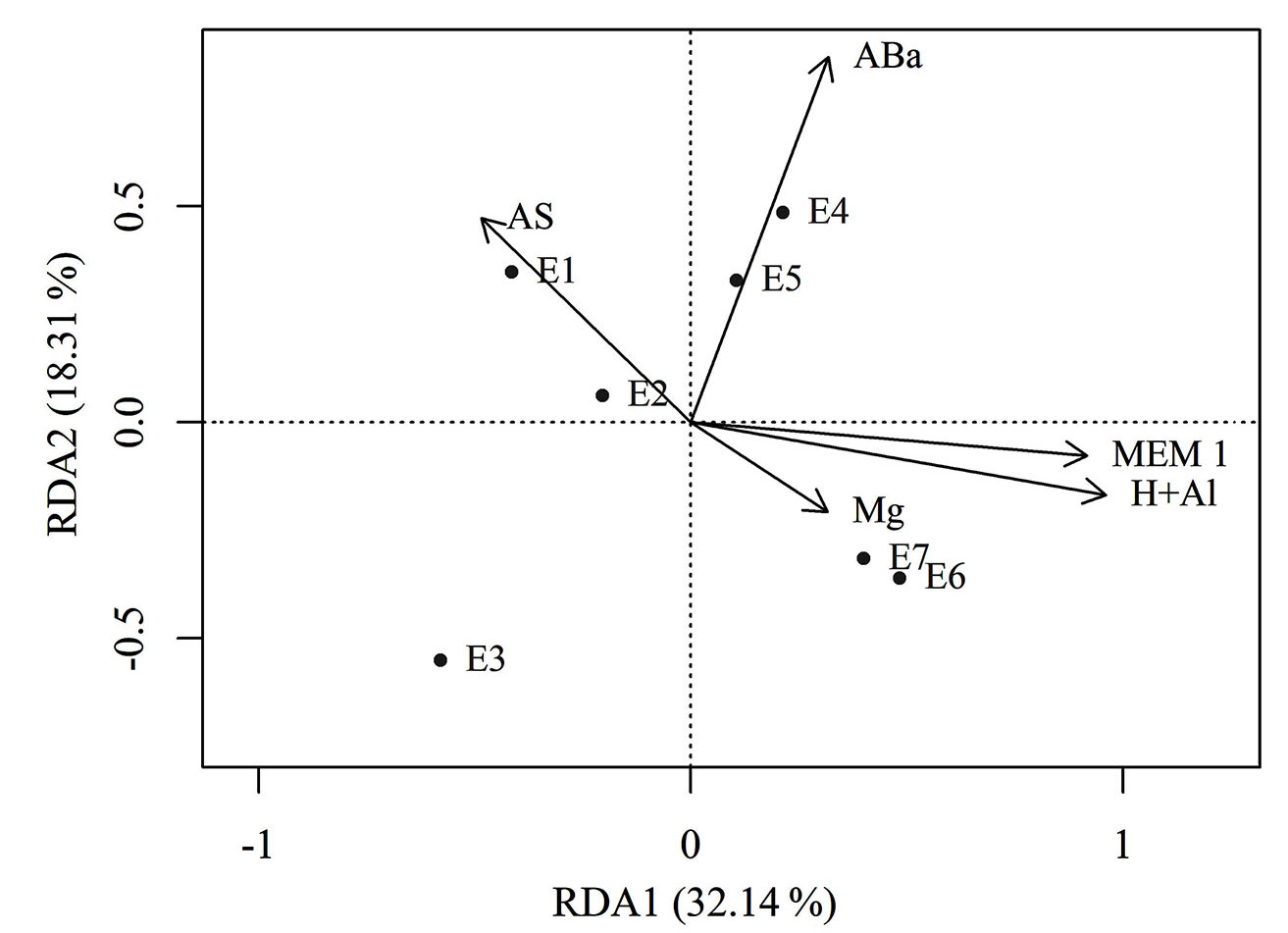
The distribution of sites along the first axis in the biplot of the environmental and spatial variables (Fig. 3) confirms the existence of the altitudinal gradient observed in the similarity dendrogram. Sites E6 and E7 are clearly differentiated from E1, E2, and E3, which are negatively related to the variables H+Al, Mg, ABa, and MEM 1, with E4 and E5 in an intermediate position. The position of sites along the RDA axis 1 revealed an environmental gradient directed by the increase in potential acidity (H+Al), magnesium, and MEM 1 spatial filter for the highest sites (E6 and E7), by the increase in the number of adult individuals in the intermediate sites (E4 and E5), and by the higher values of plant litter accumulation in the lowest sites (E1, E2, and E3).
Fig. 3 - Biplot of the environmental and spatial variables. (E1 to E7): sites distributed at different elevations; (AS): plant litter accumulation; (ABa): abundance of adult individuals; (MEM1): spatial filter (Moran’s Eigenvector Maps); (H+Al): potential acidity of the soil; (Mg): magnesium in the soil.
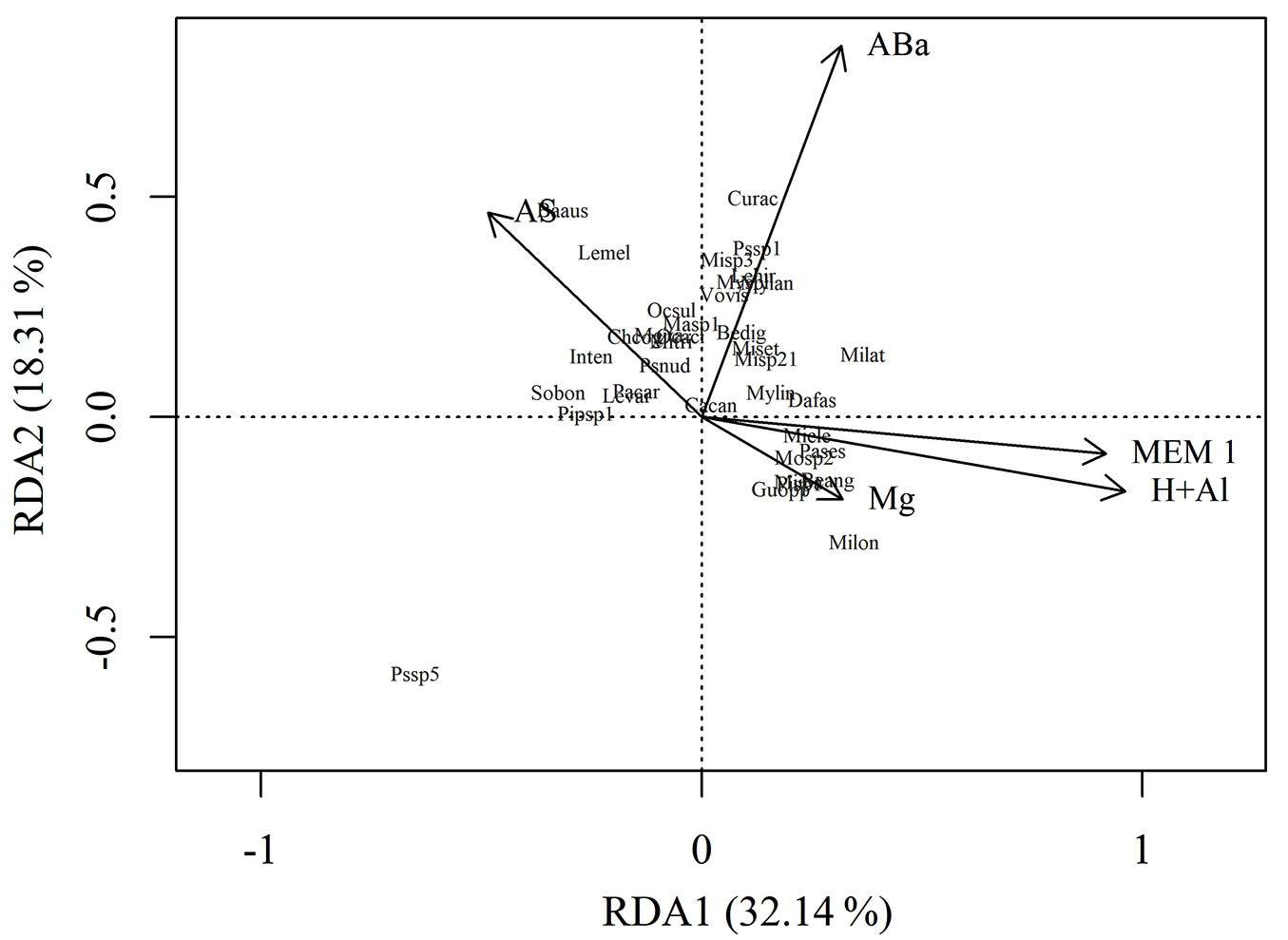
The distribution pattern of the species as a function of the environmental and spatial variables demonstrates that the first group of species is associated with the lowest and intermediate sites (1112 to 1391 m a.s.l.), this environment being characterized by higher values of plant litter accumulation and more basic soils (Fig. 4). We highlight the occurrence of the S. bonplandii species in this group (represented by Sobon in the diagram), which is considered an indicator of this environment. On the other hand, some species were more strongly associated with higher altitudes (1420 to 1550 m), where there are soils with higher potential acidity, magnesium content, and higher spatial filter (MEM 1). In this group, we observed the occurrence of many species from the family Melastomataceae, mainly from the genus Miconia, with M. cf. ibaguensis (Miiba) and M. cf. longicuspis (Milon) having significantly differentiated distribution in this environment, according to the Indicator Species Analysis.
Fig. 4 - Biplot of the environmental and spatial variables with the ordering of natural regeneration species. (AS): plant litter accumulation; (ABa): abundance of adult individuals; (MEM1): spatial filter (Moran’s Eigenvector Maps); (H+Al): potential acidity of the soil; (Mg): magnesium in the soil; (Baaus): Bathysa australis (A.St.-Hil.) K. Schum.; (Beang): Begonia angularis Raddi; (Bedig): Begonia digitata Raddi; (Cacan): Cabralea canjerana (Vell.) Mart.; (Chcog): Cheiloclinium cognatum (Miers) A.C.Sm.; (Curac): Cupania racemosa (Vell.) Radlk.; (Dafas): Daphnopsis fasciculata (Meisn.) Nevling; (Guopp): Guapira opposita (Vell.) Reitz; (Inten): Inga tenuis (Vell.) Mart.; (Lehir): Leandra cf. hirta Raddi; (Levar): Leandra cf. variabilis Raddi; (Lemel): Leandra melastomoides Raddi; (Masp1): Matayba sp. 1; (Meita): Meliosma itatiaiae Urb.; (Miele): Miconia cf. elegans Cogn.; (Miiba): Miconia cf. ibaguensis (Bonpl.) Triana; (Milon): Miconia cf. longicuspis Cogn.; (Milat): Miconia latecrenata (DC.) Naudin; (Miset): Miconia setosociliata Cogn.; (Misp21): Miconia sp. 21; (Misp3): Miconia sp. 3; (Mitri): Miconia tristis Spring; (Mosp2): Monimiaceae sp. 2; (Myeug): Myrcia eugenioides Cambess.; (Myspl): Myrcia splendens (Sw.) DC.; (Mylan): Myrsine lancifolia Mart.; (Ocaci): Ocotea aciphylla (Nees & Mart.) Mez; (Ocsul): Ocotea sulcata Vattimo-Gil; (Pacar): Paullinia carpopoda Cambess.; (Pisp1): Piper sp. 1; (Pipsp1): Piptocarpha sp. 1; (Psnud): Psychotria nuda (Cham. & Schltdl.) Wawra; (Pssp1): Psychotria sp. 1; (Pssp5): Psychotria sp. 5; (Pases): Palicourea sessilis (Vell.) C.M.Taylor; (Sobon): Sorocea bonplandii (Baill.) W.C.Burger et al.; (Vovis): Vochysia cf. vismiifolia Spruce ex Warm.
The partitioning of variance revealed that the environmental (fraction “a+c”, p = 0.028) and spatial (fraction “b+c”, p = 0.012) variations explained 19% and 8% of the variation in the floristic composition of the valley, respectively (Fig. 5). The interaction between the environmental and spatial variables (fraction “c”) indicated that the spatially structured environment accounts for 7% of the floristic variation in the valley; however, the isolated variables (fractions “a” and “b”) do not significantly explain this variation. The high percentage of the fraction “d” (66%) denotes that a large part of the variation could be explained by other important predictors, which were not considered in this study, and/or stochastic events.
Fig. 5 - Partitioning of variance between fractions that explain the distribution patterns of species of natural regeneration. (a): Percentage of variation explained by environmental factors; (b): percentage of variation explained by the spatially structured environment; (c): percentage of variation explained by the “pure” space.
The better adjustment of the GLM to understand the variations in the number of regenerating individuals in the valley was due to calcium, sodium, and the abundance of adult individuals (AICc = 142.46, R2 = 0.81, p < 0.05). The prediction of species richness occurred as a function of the abundance of adult individuals and altitude (AICc = 86.86, R2 = 0.63, p < 0.05). The model for regenerating diversity per plot was adjusted according to the abundance of adult individuals and potassium (AICc = 24.95, R2 = 0.52); however, the independent variables were not significant (p > 0.05).
Discussion
The Pielou’s evenness index (0.81) and diversity (3.98 nats ind-1) indicate low species dominance and high diversity in the regenerating stratum when compared to other studies in ombrophilous forests, where the values varied from 1.5 to 3.5 nats ind-1 ([31], [39]). The indexes did not show a clear pattern of change along the gradient. The plots with greater diversity, however, were associated with the adult component and the plant litter accumulation in the RDA, indicating that the shade tolerance, given the predominance of a late-successional ecological group, and the gentler slope, represented by the greater accumulation of plant litter, contribute to the high species richness at intermediate altitudes. Less abundant species were determinant in the high diversity found ([33]).
The species Palicourea sessilis showed the highest Importance Value (IV) in the regenerating stratum of the vegetation. This species was represented by a large number of individuals (126) well-distributed among the sample units along the environmental gradient. Palicourea sessilis has a wide distribution throughout the Atlantic Forest. This species tends to form a bank of seedlings and young plants rather than extensive seed banks in the area ([1]). Furthermore, in the adult component, where the species had the second highest IV in the community, most individuals were found under the forest canopy, with an average height of 5.5 m, typical behavior of shade tolerant species ([50], [6]).
The aspect of the terrain and the orientation of slopes in the valley affects the local microclimate, the patterns of deposition and germination of propagules, thus influencing the establishment and growth of certain species ([42]). In the valley, the luminous incidence on the North and Northeast slopes, especially in winter, is more perpendicular and constant, implying a greater amount of energy in these areas ([25]). Indeed, the lowest absolute density values were recorded on these slopes (with the exception of site E1), indicating that the highest luminosity may affect the establishment of species belonging to the mature stages of succession, predominant in the valley ([13]).
The number of regenerating individuals was also influenced by the abundance of adult individuals and sodium and calcium contents in the soils, which varied in conjunction with the regenerating individuals in different sites and slopes of the valley. We observed higher species richness and larger abundance of adult individuals at intermediate altitudes, both declining at higher elevation ([35]). Indeed, places higher in the mountain tend to have lower species richness, although there is a tendency to have a higher proportion of endemic individuals, likely due to ecological limitations such as frosts ([35]).
Altitude clearly influenced the formation of the floristic groups in the cluster analysis. We found a floristic distinction between the sites at highest altitude, E6 (1420 m) and E7 (1550 m), and the sites at lower elevation, from 1112 (E1) to 1391 m (E5), which form a second group. Moreover, we noticed that the floristic similarity among sites was low, which may indicate that the valley has high beta diversity (β - [33]). Considering that the similarity indexes represent the inverse of beta diversity, the low similarity between sites suggests the occurrence of a substitution of species along the elevation gradient, determining its high floristic heterogeneity ([15]).
The RDA results showed that the species composition in the regenerating community has a significant relationship with environmental and spatial characteristics, and is influenced by the amount of plant litter accumulated on the forest floor, potential soil acidity (H+Al), magnesium content, abundance of adult individuals, and the spatial variable MEM 1. Additionally, the reduction of air temperature with increasing altitude plays an important role in the structure and composition of the plant community along the gradient ([41]).
The higher plant litter accumulation observed in sites E1, E3, and E5 is probably due to a high productivity due to the higher luminous incidence in the plots, while the lower accumulation in plots E6 and E7 may be related to the lower abundance and size of adult individuals as well as to the steep slope ([6]), which causes the litter to be dragged by the surface runoff of rainwater ([17]). Plant litter seems to affect the germination and seedling development of some species, influencing the structure and dynamics of the community ([47]).
Variations in elevation lead to changes in the chemical and physical characteristics of the soil, which can influence the structure of the vegetation. In fact, the analysis of soil chemical attributes revealed an increase in organic matter (OM) and magnesium which increases with altitude. Environments richer in OM tend to present higher levels of H+Al, as observed in this study ([14]). This behavior, associated with other edaphic and environmental factors, influenced the occurrence of species exclusive to the highest sites (1420 to 1550 m).
The space influences the patterns of ecological distribution of species along environmental gradients ([24], [40]). In this study, we found that the environmental characteristics of the studied sites did not fully explain the floristic variation observed. However, spatially structured environmental variables (joint action of environment and space) acounted for a significant fraction of the total variance, indicating that biotic factors, such as dispersion and competition, may also affect species composition ([30]).
The association of abiotic factors with biotic interactions is commonly considered as a predictor of species distribution ([12]). The seed dispersal process, for example, can determine differentiated spatial patterns of floristic composition, especially in the regenerating stratum, since plant species have varied dispersal mechanisms. Additionally, the density of individuals of a species may also influence its spatial distribution ([32]).
Fluctuations in the number of regenerating species in the different slope orientations were associated with the abundance of adult component, especially in the intermediate sites that presented the smallest (E3) and the largest (E4) numbers of individuals in both strata. Soil conditions and geomorphological characteristics, such as elevation, slope and aspect, may have affected both the regenerating and the superior stratum ([19]). Along the gradient, the richness of regenerating stratum was also associated with the adult component, which acts directly as a source of propagules and indirectly by affecting the microclimate, increasing plant litter deposition, and altering the edaphic conditions ([2]).
The high percentage of unexplained variation (66%) in this investigation has been frequently reported in studies using environmental and spatial variables as predictors of the floristic composition of an area ([6], [32]), due to the environmental heterogeneity and the stochasticity of various processes ([37]). Baldeck et al. ([7]) suggested, for example, that environmental variables related to luminosity and soil moisture may be important to explain patterns of species distribution in tropical forests. The results of this study highlight that factors not investigated in this research probably contribute substantially to the variation in regenerating species composition.
Conclusions
Natural regeneration showed environmental preferences along the Santa Marta Valley, being influenced by luminosity, mainly due to the orientation of the slopes, abundance in adult components, calcium and sodium contents, plant litter accumulation, altitude, and spatial structure of the environment. The altitude, however, did not seem to influence a pattern of increase or decrease in the abundance or richness of regenerating species. The largest fraction of the floristic variation along the valley was not explained by factors investigated in this study. We suggest that future studies test the influence of other predictor variables, such as dispersal, competition, soil moisture, among others.
Acknowledgments
The authors would like to thank the Federal University of Espírito Santo (UFES) for the logistic support and the Caparaó National Park (ICMBIO) for their permission to carry out the research. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the support through the Universal Public Call 14/2011 (475471/2011-3) and the Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (FAPES) Universal Public Call no. 03/2017 (T.O.169 - SIAFEM: 80709605/18) and PDPG/FAPES-CAPES (T.O.131 - Process 2021-FDGS5).
Author contributions
VBRD and VSA collected and analyzed the field data with support of HMD. VBRD and VSA wrote the manuscript. HMD, SHK, and EVDB discussed the results and revised manuscript versions. All authors designed the study, read, and approved the final manuscript. The authors declare no conflict of interest.
References
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Henrique Machado Dias 0000-0003-2217-7846
Sustanis Horn Kunz 0000-0001-6937-7787
Department of Forest and Wood Sciences, Federal University of Espírito Santo, 29550-000, Jeronimo Monteiro, Espírito Santo (Brazil)
Department of Forest Sciences, Federal University of Lavras, 37200-900, Lavras, Minas Gerais (Brazil)
Department of Ecology and Conservation, Federal University of Lavras, 37200-900, Lavras, Minas Gerais (Brazil)
Corresponding author
Paper Info
Citation
Braga Rodrigues Duarte V, Abreu de Souza V, Machado Dias H, Horn Kunz S, Van Den Berg E (2024). How environmental factors condition natural regeneration in the altitudinal gradient of a montane rainforest. iForest 17: 132-139. - doi: 10.3832/ifor4319-017
Academic Editor
Susanna Nocentini
Paper history
Received: Jan 31, 2023
Accepted: Feb 08, 2024
First online: May 04, 2024
Publication Date: Jun 30, 2024
Publication Time: 2.87 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2024
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 6677
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 2161
Abstract Page Views: 1893
PDF Downloads: 2305
Citation/Reference Downloads: 3
XML Downloads: 315
Web Metrics
Days since publication: 637
Overall contacts: 6677
Avg. contacts per week: 73.37
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
A silvicultural stand density model to control understory in maritime pine stands
vol. 10, pp. 829-836 (online: 25 September 2017)
Research Articles
Effects of planted European beech on the understory in Scots pine forests of Lithuania
vol. 7, pp. 12-18 (online: 07 October 2013)
Research Articles
Understory vegetation dynamics and tree regeneration as affected by deer herbivory in temperate hardwood forests
vol. 10, pp. 837-844 (online: 26 October 2017)
Research Articles
Exposure elevation and forest structure predict the abundance of saproxylic beetles’ communities in mountain managed beech forests
vol. 16, pp. 155-164 (online: 08 June 2023)
Research Articles
Potential natural vegetation pattern based on major tree distribution modeling in the western Rif of Morocco
vol. 17, pp. 405-416 (online: 22 December 2024)
Research Articles
Environmental niche and distribution of six deciduous tree species in the Spanish Atlantic region
vol. 8, pp. 214-221 (online: 28 August 2014)
Research Articles
Distribution of juveniles of tree species along a canopy closure gradient in a tropical cloud forest of the Venezuelan Andes
vol. 9, pp. 363-369 (online: 08 December 2015)
Research Articles
Relationships between overstory and understory structure and diversity in semi-natural mixed floodplain forests at Bosco Fontana (Italy)
vol. 9, pp. 919-926 (online: 21 August 2016)
Research Articles
Remote sensing of Japanese beech forest decline using an improved Temperature Vegetation Dryness Index (iTVDI)
vol. 4, pp. 195-199 (online: 03 November 2011)
Research Articles
Stomatal morphometry of Andean species and their relationship with spatial variation
vol. 18, pp. 327-334 (online: 03 November 2025)
iForest Database Search
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword