Exposure elevation and forest structure predict the abundance of saproxylic beetles’ communities in mountain managed beech forests
iForest - Biogeosciences and Forestry, Volume 16, Issue 3, Pages 155-164 (2023)
doi: https://doi.org/10.3832/ifor4264-016
Published: Jun 08, 2023 - Copyright © 2023 SISEF
Research Articles
Abstract
In the managed beech forests of Central Italy (Molise), the diversity of saproxylic species is potentially under threat by intensive management. To evaluate the impact of forestry on the biodiversity of these ecosystems, we analyzed the relationship between abundance of saproxylic beetles and (i) forest stand exposure and elevation, (ii) deadwood availability (coarse woody debris - CWD - and stumps), (iii) abundance of microhabitats. Four sampling sectors with different altitudes and exposure were identified in a 400-ha study area in the Appenine mountains. Fifteen circular plots (13 m radius) were established in each sector where deadwood and microhabitats were surveyed and saproxylic beetles sampled. We fitted joint species distribution models to quantify the relationship between forest attributes and saproxylic species’ abundance, including the interactions with their family and trophic category. Overall, 2334 specimens belonging to 64 species of saproxylic beetles were collected. Both abundance and species richness were higher in the sectors with high elevation (respectively, 55% and 44%) and South exposure (respectively, 28% and 44%). Average deadwood volumes were low (stumps: 7.6 m3 ha-1; CWD: 0.3 m3 ha-1; snags: 0.4 m3 ha-1), and insect galleries were the most abundant microhabitat (380 records over a total of 434). The most important variables affecting abundance were stump characteristics (model deviance = 81.2), elevation (deviance = 64.7), and CWD characteristics (deviance = 58.0). Our results show that topographical variables and forest structure jointly affect the abundance patterns of saproxylic beetle communities in managed beech forests. These ecological interactions imply that management has different impacts on the saproxylic communities in different topographic conditions. To acknowledge this complexity we advocate for a landscape-level forest management supporting the local beetle diversity maintaining a mosaic of semi-natural forest characteristics in different topographic contexts. The ecological value of the forest landscape will be further enhanced by the application of closer-to-nature management interventions based on deadwood retention, microhabitat creation and tree retention, in line with the guidelines of the new EU Forest Strategy for 2030.
Keywords
Deadwood, Forest Heterogeneity, Fourth-corner Problem, Italy, Joint Species Distribution Models, Microhabitats, Trophic Categories
Introduction
Forests provide multiple ecosystem services and are important for biodiversity conservation ([30], [47]). To preserve forest goods and services, their management should combine sustainable forest production with retention of high value habitats for biodiversity conservation. The fast pace at which pristine forests are vanishing, along with their associated species, has moved the attention of the forest science community to revert this loss by improving the status of biodiversity even in managed forests ([2]).
In forest ecosystems, saproxylic species (i.e., related to deadwood and microhabitats) are an important component of biodiversity, which is negatively affected by changes induced by intensive management. This is because such ecological group is an indicator of forest naturalness, in terms of deadwood amount and diversity ([15]). In particular saproxylic beetles, both as larvae and adults, play a fundamental role in the forest, being involved in decomposition processes and nutrient cycling ([33]). However, as saproxylic beetle species are associated with deadwood, which is scarce in managed forests, 25% of them is threatened in Italy, i.e., a total of 2049 beetle species belonging to 65 families assessed by the Red List of Italian Saproxylic Coleoptera ([10]). The positive relationships of saproxylic species with deadwood characteristics and habitat trees in old-growth forests are well known ([33], [45]). However, the relationships between saproxylic species and attributes of managed forests have been analyzed only in recent times ([7]). Interest in the relationships between saproxylic beetles and forest management type has increased significantly when related to deadwood and microhabitats’ abundance ([37], [52], [1]). According to Paletto et al. ([32]), forest managers have often perceived deadwood as a negative component and considered it as a potential source of pests and diseases ([27]), a cause of risk for fires ([43]), and an obstacle to silvicultural practices ([48]). For this reason, coarse woody debris and standing or dead downed trees have usually been removed in many forests, decreasing the availability of suitable habitats for saproxylic communities, including birds, small mammals, and reptiles ([29]).
Retaining deadwood in managed forests is a conservation measure that should be considered to find a trade-off between economic benefits from timber harvesting and biodiversity conservation ([52],[28]). Indeed, the availability of a low quantity of habitat trees or dead trees, harboring many tree-related microhabitats, represents a serious threat to saproxylic communities ([29]). However, biodiversity loss in forests is not only related to deadwood removal ([41]). In many forest areas, ecosystem simplification and large-scale homogenization of tree cover have often reduced the availability of particular microclimatic conditions for saproxylic species, only available for local combinations of topographic conditions. For example, when intensive forestry has interested South-exposed localities, this has induced the disappearance of sun-exposed dead trees, which host an important portion of the saproxylic community ([44]). On the other hand, old trees exposed to North are more likely to support special microhabitats like dendrotelms - small temporary rain-fed water bodies on trees - and saproxylic fungi which host highly specialized beetle species. Both these microhabitats occur in deadwood and old trees, which are scarce in managed forests, and are available under specific topographic conditions ([45], [46]).
To achieve suitable environmental conditions for maintaining saproxylic insect diversity in managed forests, knowledge about the microclimatic characteristics of their substrate is crucial. Insects are ectotherms, and their activity, survival, and development are highly dependent on environmental temperature. It has been suggested that temperature variations in different parts of the trunk of standing or dead downed trees can significantly affect the occurrence of saproxylic beetles ([23]). Indeed, many threatened and endangered saproxylic species have shown a positive association with sun-exposed microhabitats and logs ([41], [23]). Accordingly, many studies have suggested that sun-exposed substrates can support higher species richness compared with shaded substrates ([6], [42]).
Despite the importance of exposure in sustaining saproxylic beetle diversity is clear, the microenvironmental preferences of these organisms are still poorly understood across climatic regions, as most of the available studies have been conducted in boreal and temperate forests in Europe ([51]). For instance, in northern Sweden, Johansson et al. ([19]) found that topographical variables (altitude, solar radiation, and soil humidity) affected beetle assemblages, with differences between clear cuts and mature forest stands which locally affect canopy closure. Indeed, the effects of topographical variables on species abundance and richness are mediated by forestry practices that affect forest structural characteristics. In Germany, Edelmann et al. ([12]) found that decreasing deadwood volume and increasing canopy cover negatively affect species abundance and richness of saproxylic beetles. In contrast, Leidinger et al. ([21]) found a higher saproxylic beetle richness in managed forests characterized by a lower canopy cover than in unmanaged forests.
However, the effects of topographical variables on species abundance may also differ among saproxylic trophic groups. The likely reason is that the trophic characteristics of each saproxylic beetle species result from complex combinations of traits related with resource exploitation, which link the species mechanistically to its potential habitat ([11]). For example, although subcortical beetles do not show a clear preference for sun-exposed conditions ([50]) they could be associated to this microhabitat because they exploit resources available under bark. Therefore, the functional composition of communities of saproxylic organisms may vary significantly along a gradient of sunlight exposure ([6]).
We may also expect that the different responses to topographic variables from species belonging to similar trophic categories align with the responses of closely-related families. This expectation derives from the concept of phylogenetic niche conservatism, which postulates that species tend to retain their ancestral traits ([17]). Consequently, the species response to the environment will be similar not only if they share similar trophic categories, but also if they belong to related families which presumably have similar trophic habits. To test this hypothesis, we evaluated the relationship between environmental factors and beetles’ families.
In order to achieve a better comprehension of the interplay between topographic variables, forest management and insects’ community composition, it is necessary to investigate saproxylic beetles in forest stands with different topographical conditions (in terms of combinations of exposure and elevation), in forests encompassing a gradient of management intensities. To acknowledge the role of the interaction between topography and management in shaping the composition of saproxylic communities, our aims were two-fold: (i) to investigate the relative effects of variables related with topography (exposure and elevation) and forest structure (microhabitats’ abundance, coarse woody debris - CWD - and stump characteristics) on the abundance of saproxylic beetles; (ii) to analyse the link between variables related with topography / forest structure and species characteristics (family and trophic level).
Material and methods
Study area
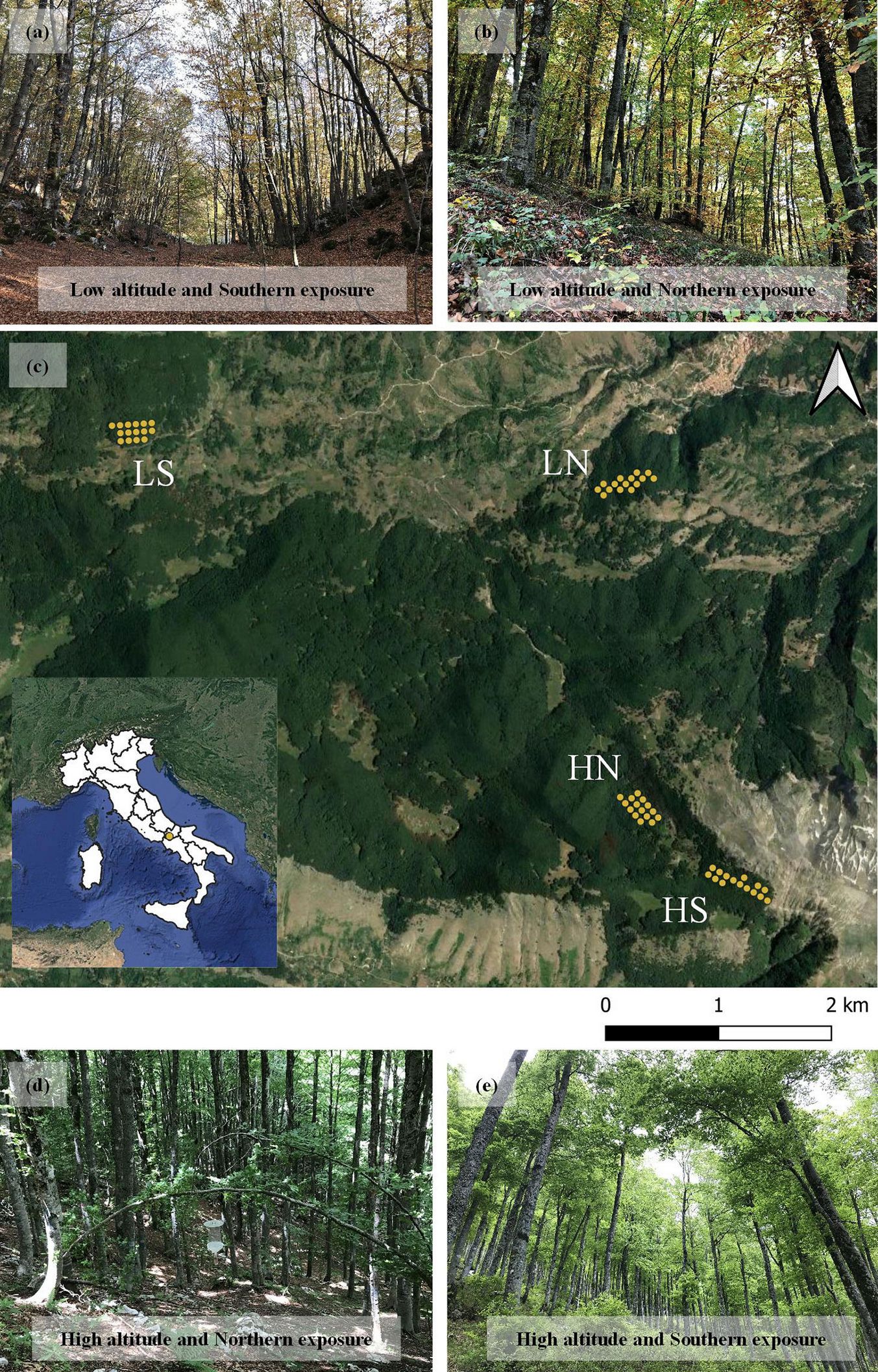
The study was conducted in the Roccamadolfi (Molise, Italy) beech forest in the Central Apennines (Fig. 1), within the Site of Community Importance (SCI, ⇒ http://natura2000.eea.europa.eu/) “La Gallinola - Monte Miletto - Monti del Matese” (Cod. IT 7222287). This forest is mainly characterized by mountainous and sub-mountainous beech forest types ([38]). The forest is regularly managed for timber production, and is mostly characterized by even-aged stands of gamic and agamic origin. About 70% of the total area is covered by forests, which belong to nine different forest types ([13]), the most common being beech forest (8000 ha), composed of coppices resulting from past forest management ([38]). The experimental area covers about 400 ha, its altitude ranges from 1180 to 1737 m a.s.l., and its climate is warm-summer Mediterranean, according to the Koppen-Geiger classification ([38]).
Fig. 1 - Study sites and distribution of sampling plots in the four sectors. Distribution of the four sectors and sampling plots in Roccamadolfi (Molise, Italy) beech forest within the Site of Community Importance “La Gallinola - MonteMiletto - Monti del Matese” (Cod. IT 7222287). (c). (LS): low altitude and southern exposure (a); (LN): low altitude and northern exposure (b); (HN): high altitude and northern exposure (d); (HS): high altitude and southern exposure (e).
Sampling scheme
The ground survey sampling scheme followed Parisi et al. ([38]) and was settled within the context of the project LIFE “AForClimate” (LIFE15418 CCA/IT/000089). Four sectors were selected, according to the north and south facing slopes, and to the altitude, high (1400 - 1700 m a.s.l.) and low (1200 - 1300 m a.s.l.): LS, low altitude and southern exposure; LN, low altitude and northern exposure; HN, high altitude and northern exposure; and HS, high altitude and southern exposure (Fig. 1). Fifteen circular sample plots (radius 13 m) located at a regular distance of 70 m to each other, were established in each sector following a systematic aligned grid ([38]). UTM-WGS84 coordinates (Zone 32T) and elevation were recorded through a Juno SB Global Positioning System (Trimble, Sunnyvale, CA, USA).
Deadwood and microhabitats survey
The survey protocol followed Lombardi et al. ([25]). Dead standing trees, dead downed trees, CWD, and stumps were sampled, measuring their length and height, minimum (> 5 cm) and maximum diameter, and recording the tree species, when possible. The volume of snags, CWD, and stumps, dead standing trees and dead downed trees was calculated through the cone trunk formula ([24], [39]).
Tree-related microhabitats on deadwood were identified following the protocol developed by Kraus et al. ([20]). Microhabitat types were separated into two main categories, namely saproxylic and epixylic microhabitats, and were censused carefully observing each deadwood element occurring in the sampling plot. The microhabitats censused for the presence of saproxylic beetles in our samples were grouped into the following groups: woodpecker breeding cavities (abbreviation: Woodcavi), rot holes (Rothole), concavities (Concavts), insect galleries and bore holes (Insehole), exposed sapwood only (Expoonly), exposed sapwood and heartwood (Expohear), crown deadwood (Crowdead), twig tangles (Twigtang), burrs and cankers (Burrcank), perennial fungal fruiting bodies (life span > 1 year, Pere1y), ephemeral fungal fruiting bodies and slime moulds (Ephemoul), epiphytic or parasitic crypto- and phanerogams (Epipphan), nests (Nests), fresh exudates (Fresexud) and microsoils (Microsls).
Saproxylic beetles
Adult saproxylic beetles were sampled in the 60 plots using window flight traps ([4], [8]), positioned at the center of each plot. Traps were checked every 30 days for five surveys in 2018 (from 6th May to 4th October). All the monitoring systems were then removed after the end of the sampling period.
Systematics and nomenclature followed Bouchard et al. ([3]). Species were grouped according to their family and trophic categories and IUCN risk categories, defined by Carpaneto et al. ([10]). In particular, species were grouped into five trophic categories of organisms: (i) xylophagous (XY), feeding exclusively or mainly on wood; (ii) saproxylophagous (SX), feeding exclusively or largely on fungus-infected wood; (iii) mycophagous (MY), feeding exclusively or mainly on fungi; (iv) mycetobiontic (MB), feeding on carpophores of large Polyporales and other fungi living on old trees and stumps; and (v) predator (PR), organisms that primarily obtain food by killing and consuming other organisms. Finally, species were grouped into four IUCN risk categories: (i) Data Deficient (DD), species with inadequate information to make a direct or indirect assessment of their risk of extinction; (ii) Least Concern (LC), species not facing an imminent threat; (iii) Near Threatened (NT), species close or likely to qualify for one of the Threatened categories in the near future; and (iv) Vulnerable (VU), species that are expected to go extinct within a medium time interval.
Statistical analyses
We fitted joint species distribution models (JSDMs - [31]) to quantify the relationship between forest attributes and the abundance of saproxylic species, explicitly evaluating the interactions with their family and trophic categories, solving the fourth-corner problem (solved fitting GLMs with a LASSO penalty parameter). Specifically, we fitted JSDMs via GLMs based on maximum-likelihood, testing separately for the effect of the two topographic variables (elevation and exposure), and the variables related with forest structure: the microhabitats with the greatest abundance (Insect galleries and bore holes - Insehole; and Perennial fungal fruiting bodies, life span > 1 year - Pere1y) and CWD and STUMP variables (see Tab. S2 in Supplementary material). As we dealt with over-dispersed count data, we fitted the GLMs with the negative binomial family with a log link function. Moreover, we accounted for potential non-independence of sampling sites by including the sector (i.e., the combination of elevation and exposure) as a random effect (i.e., random “site” effect - [18]). JSDMs were fitted using the function “manyglm” and the fourth corner problem was solved using the function “traitglm”, both within the “mvabund” R package ([49]).
We fitted the JSDMs for the most abundant 17 species, i.e., 27% of the initial 64 species. We excluded species present in five or fewer sampling sites because they were too rare to reliably estimate their niches ([31]). For each of the 17 most abundant species, it was also possible to calculate the relationship between their abundance and the variables related with topography and forest structure (see Figs. S1-S6 in Supplementary material). We also excluded from the analysis those variables sampled only in a few number of sampling plots, like snags and standing and downed dead trees.
The five CWD characteristics (minimum diameter, maximum diameter, length, decay state, volume) and the five stump characteristics (base diameter, top diameter, height, decay state, volume) were aggregated with a Principal Component Analysis, and the first Principal Component (PC1) was used in the models to predict species abundance.
Results
Deadwood and microhabitats
Deadwood amounts varied both among the four sectors and deadwood types. Across the four sectors, the median volumes (5th and 95th percentiles in parentheses) for stump was 4.9 · 10-5 (3.7 · 10-6, 3.2 · 10+1) m3 ha-1, for CWD was 9.3 · 10-2 (9.3 · 10-6, 6.4 · 10-1) m3 ha-1, for snags was 8.2 · 10-4 (1.1·10-5, 1.6) m3 ha-1, for standing dead trees was 1.35 (0.16, 40.88) m3 ha-1 and for downed dead trees was 1.17 (0.02, 16.75) m3 ha-1 (Tab. 1). The CWD and STUMP PC1 were positively correlated with all the five CWD and stump characteristics (Pearson correlation ranging between 0.25 and 0.52 - Tab. S2 in Supplementary material).
Tab. 1 - Mean volume values ± standard deviation (m3 ha-1) of the deadwood type sampled in each sector. (HS): high altitude, southern exposure; (HN): high altitude, northern exposure; (LS): low altitude, southern exposure; (LN): low altitude, northern exposure.
| Deadwood types | HS | HN | LS | LN |
|---|---|---|---|---|
| Stumps | 1.0-3 ± 8.4-3 | 9.7-5 ± 1.4-4 | 6.81 ± 1.32 | 7.5-3 ± 7.9-2 |
| CWD | 4.1-5 ± 4.6-5 | 2.9-5 ± 5.4-5 | 2.1-1 ± 3.0-1 | 7.2-1 ± 3.80 |
| Snags | 1.5-4 ± 5.6-5 | - | 9.5-1 ± 1.90 | 1.6-4 ± 2.8-4 |
| Standing dead trees | - | 2.2 ± 1.8 | - | 12.8 ± 26.8 |
| Dead downed trees | 4.3 ± 7.5 | - | - | 5.2 ± 8.5 |
Moreover, 434 microhabitats were censused only on deadwood and not on living trees. The most abundant microhabitat types were saproxylic (e.g., insect galleries and bore holes, woodpecker breeding cavities, and rot holes), censused 396 times. In contrast, epixylic microhabitats, i.e., perennial fungal fruiting bodies, were less abundant (censused 38 times - Tab. 2).
Tab. 2 - Types of microhabitats sampled in deadwood per sector. (HS): high altitude, southern exposure; (HN): high altitude, northern exposure; (LS): low altitude, southern exposure; (LN): low altitude, northern exposure.
| Microhabitat | HS | HN | LS | LN | Total |
|---|---|---|---|---|---|
| Woodpecker breeding cavities | 3 | 2 | - | - | 5 |
| Rot holes | - | 2 | 2 | 1 | 5 |
| Concavities | 3 | - | - | - | 3 |
| Insect galleries and bore holes | 95 | 83 | 77 | 125 | 380 |
| Exposed sapwood only | 2 | - | - | - | 2 |
| Exposed sapwood and heartwood | - | 1 | - | - | 1 |
| Perennial fungal fruiting bodies (life span > 1 yr) | 14 | 8 | 8 | 8 | 38 |
Saproxylic beetle survey
Overall, 2334 specimens were collected belonging to 64 species referring to 27 families of saproxylic Coleoptera (see Tab. S1 in Supplementary material). The most abundant species were Ernoporicus fagi (Curculionidae) with 1232 specimens and Hemicoelus costatus (Ptinidae) with 214 specimens, representing 62% of the total sampled beetles. Indeed, the most abundant families were Cerambycidae (12.5%, 8 species) and Curculionidae (12.5%, 8 species), followed by Ptinidae (7.8%, 5 species) and Zopheridae (6.3%, 4 species - Tab. 3).
Tab. 3 - Number of species and their abundance for each beetle family.
| Family | No. Species | Abundance |
|---|---|---|
| Biphyllidae | 1 | 1 |
| Cerambycidae | 8 | 138 |
| Cerylonidae | 1 | 1 |
| Ciidae | 1 | 1 |
| Clerideae | 2 | 5 |
| Cryptophagidae | 1 | 7 |
| Curculionidae | 8 | 1470 |
| Elateridae | 2 | 106 |
| Endomychidae | 1 | 3 |
| Erotylidae | 3 | 14 |
| Eucnemideae | 1 | 17 |
| Latriididae | 2 | 6 |
| Lucanidae | 2 | 17 |
| Lycidae | 1 | 1 |
| Melandryidae | 2 | 2 |
| Melyridae | 3 | 181 |
| Monotomidae | 1 | 12 |
| Mycetophagidae | 3 | 13 |
| Nitidulidae | 1 | 1 |
| Oedemeridae | 1 | 1 |
| Ptinidae | 5 | 229 |
| Salpingidae | 3 | 85 |
| Scarabaeidae | 2 | 6 |
| Scraptiidae | 1 | 1 |
| Staphylinidae | 1 | 1 |
| Tenebrionidae | 2 | 2 |
| Trogossitidae | 1 | 6 |
| Zopheridae | 4 | 7 |
| Total | 64 | 2334 |
Concerning the different exposure and elevation of the sampling plots, the highest abundance and species richness of the specimens were found in the HS sector, with 1084 individuals (46% of the total abundance) and 24 species (38% of the total species), with the most abundant families being Curculionidae and Melyridae. The second sector in terms of abundance and richness was LN, with 810 individuals (35% of the total) and 21 species (33% of the total species), with the most abundant families being Curculionidae and Ptinidae. A lower abundance and richness were found in the other two sectors, respectively: in the HN we found 194 individuals (8% of the total abundance) and 8 species (22% of the total richness) and in the LS sectors, 246 individuals (11% of the total) and 12 species (19% of the total richness).
Regarding the trophic categories, Xylophagous represented 28% of the total sampled beetles, followed by Saproxylophagous (25%), Mycophagous (21.8%), Predators (11%), and Mycetobiontic (6.2%).
As for the IUCN risk categories, the sampled saproxylic beetles were classified as follows: Vulnerable (VU, 2 species), Near Threatened (NT, 8 species), Data Deficient (DD, 1 species), and Least Concern (LC, 53 species).
Relative importance of environmental drivers for species abundance
Variables related with topography and forest structure had different importance in explaining the patterns of abundance of saproxylic beetles. The most important factors affecting the abundance of single species of saproxylic beetles were: (i) stump-related variables, with deviance for the model related with the first Principal Component (PC1) of the STUMP variables = 81.2 (Tab. S2 in Supplementary material) and 5 species significantly associated with the STUMP PC1 (see below for details and Fig. S6); (ii) the topographic variable elevation, with model deviance = 64.7 (Tab. S2) and 5 species significantly associated with elevation (Fig. S1); and (iii) the CWD-related variables, with deviance for the model related with the first Principal Component of the CWD variables = 58.0 (Tab. S2) and 6 species significantly associated with the CWD PC1 (Fig. S5 in Supplementary material). Lower importance had the exposure (deviance = 43.6 - Tab. S2) and only two species significantly associated with elevation (Fig. S2), followed by the abundance of the two most important microhabitats: (i) perennial fungal fruiting bodies, with deviance = 34.1 (Tab. S2) and two species significantly associated (Fig. S4); and (ii) insect galleries and bore holes, with deviance = 23.2 (Tab. S2) and two species significantly associated (Fig. S3 in Supplementary material).
Among the most abundant species in the saproxylic assemblage, only a few of them showed a clear significant association with low (Melanotus villosus, Elateridae; Hemicoelus costatus, Ptinidae) and high elevation (Salpingus planirostris, Salpingidae; Trypodendron domesticum, Curculionidae; and Dasytes plumbeus, Melyridae). Rhagium mordax was the only species positively associated with southern exposure and M. villosus was the sole associated with northern exposure (Figs. S1-S2 in Supplementary material). Most of the other species showed either a less clear preference for low or high elevation and S/N exposure or no preference at all. Regarding microhabitats, only Melasis buprestoides (Eucnemidae) and H. costatus (Ptinidae) were positively associated with a high abundance of insect galleries and bore holes. At the same time, most of the other species tended to be positively but less strongly associated (Fig. S3 in Supplementary material). R. mordax (Cerambycidae) and D. plumbeus (Melyridae) were positively associated with a high abundance of perennial fungal fruiting bodies, while most of the other species did not show a clear preference for this microhabitat (Fig. S4 in Supplementary material). Four species (M. villosus, Elateridae; Scolytus intricatus, Curculionidae; Stenurella sennii, Cerambycidae; H. costatus, Ptinidae) were positively associated with CWD-related variables, while other two species (T. Domesticum, Curculionidae and D. plumbeus, Melyridae) showed a negative association, but other species showed either a less strong positive or negative relationship with CWD, or were indifferent to CWD characteristics (Fig. S5 in Supplementary material). Finally, five species were negatively associated with stump-related variables: E. fagi, Anisandrus dispar, T. domesticum (Curculionidae), D. plumbeus (Melyridae), and S. planirostris (Salpingidae), while most of the other species showed a negative but less clear association (Fig. S6 in Supplementary material).
Interactions of topographic and structural variables with trophic categories and families
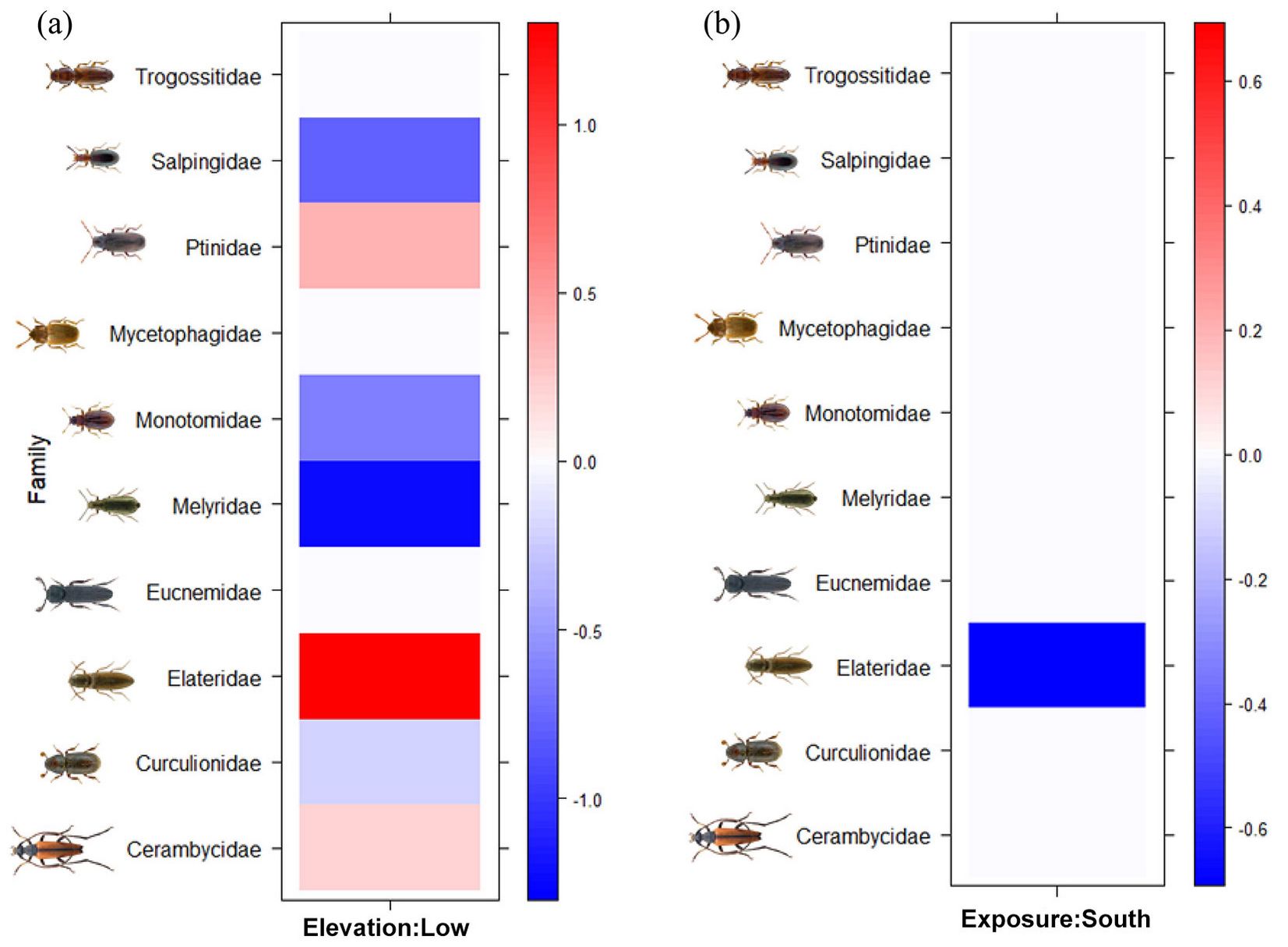
The interactions between topographical characteristics of the sample sites (elevation and exposure) with saproxylic beetles’ families and trophic categories were investigated. In particular, Cerambycidae, Elateridae and Ptinidae families were positively associated with low elevation, while Curculionidae, Melyridae, Monotomidae and Salpingidae with high elevation (Fig. 2a). Regarding plot exposure, Elateridae was the sole family positively associated with Northern exposure (Fig. 2b). On the other hand, the trophic categories were not associated either with elevation or exposure.
Fig. 2 - Interactions of (a) elevation and (b) exposure of the sampling plots with saproxylic beetles’ families according to the Fourth-corner modeling results. Standardized coefficients for all environment-trait interaction terms are presented from a generalized linear model (GLM). Brighter squares show stronger associations than paler ones, positive associations are showed in red and negative associations are in blue.
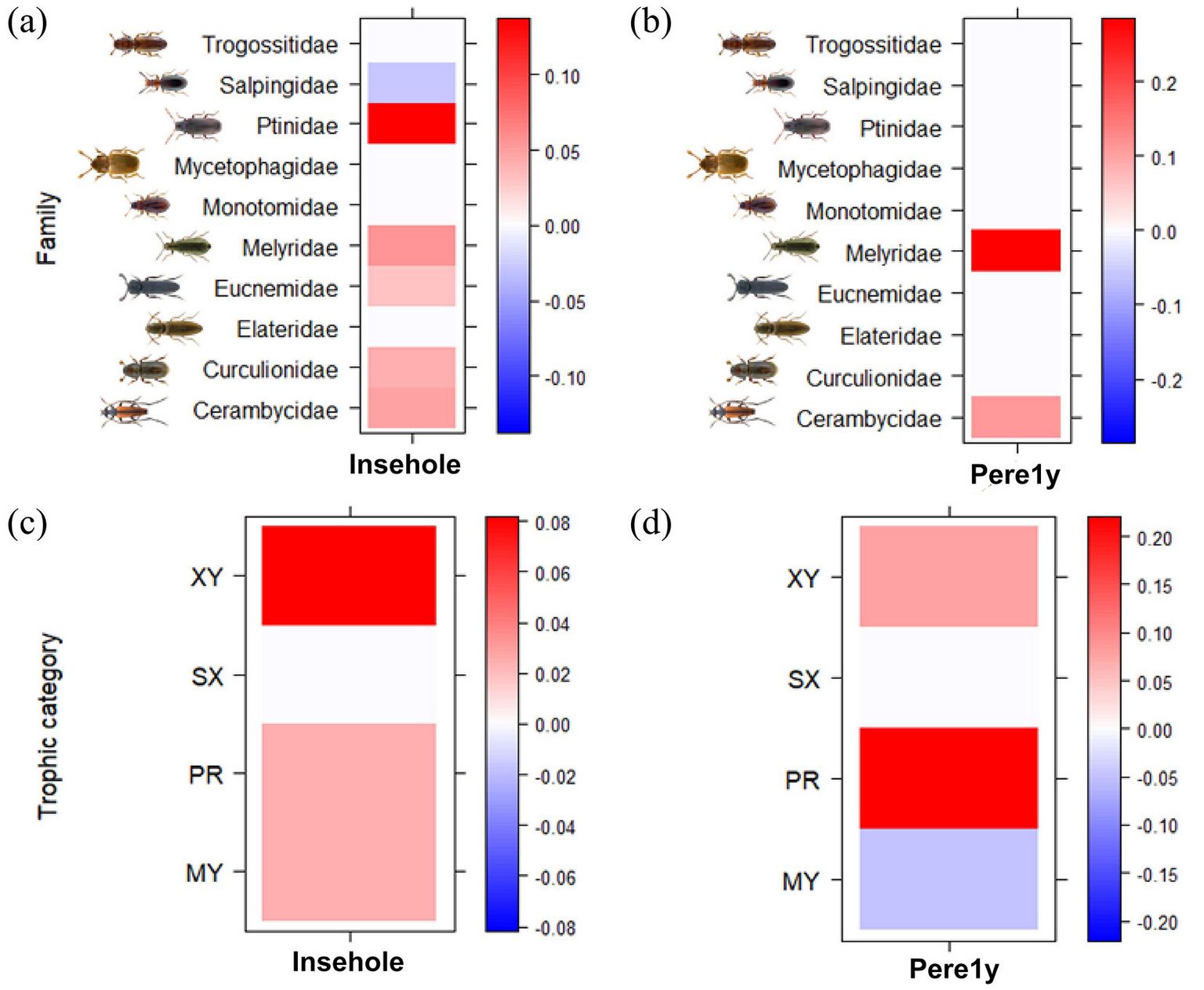
For what concerns the interactions between microhabitats and saproxylic beetles’ families, a high frequency of insect galleries and bore holes were positively associated with the families Cerambycidae, Curculionidae, Eucnemidae, Melyridae, and especially Ptinidae, and negatively associated with Salpingidae (Fig. 3a). A high frequency of perennial fungal fruiting bodies was positively associated with the families Cerambycidae and Melyridae (Fig. 3b). As for the trophic categories, a high frequency of insect galleries and bore holes was positively associated with mycophagous, predator and xylophagous species (Fig. 3c). Instead, a high frequency of perennial fungal fruiting bodies was positively associated with predator and xylophagous species and negatively associated with mycophagous species (Fig. 3d).
Fig. 3 - Interactions of the two most abundant microhabitats with saproxylic beetles’ families (a-b) and trophic categories (c-d) according to the Fourth-corner modeling results. GLMs and legend are the same as in Fig. 1. Abbreviations: Insehole, insect galleries and bore holes; Pere1y, perennial fungal fruiting bodies (life span > 1 year).
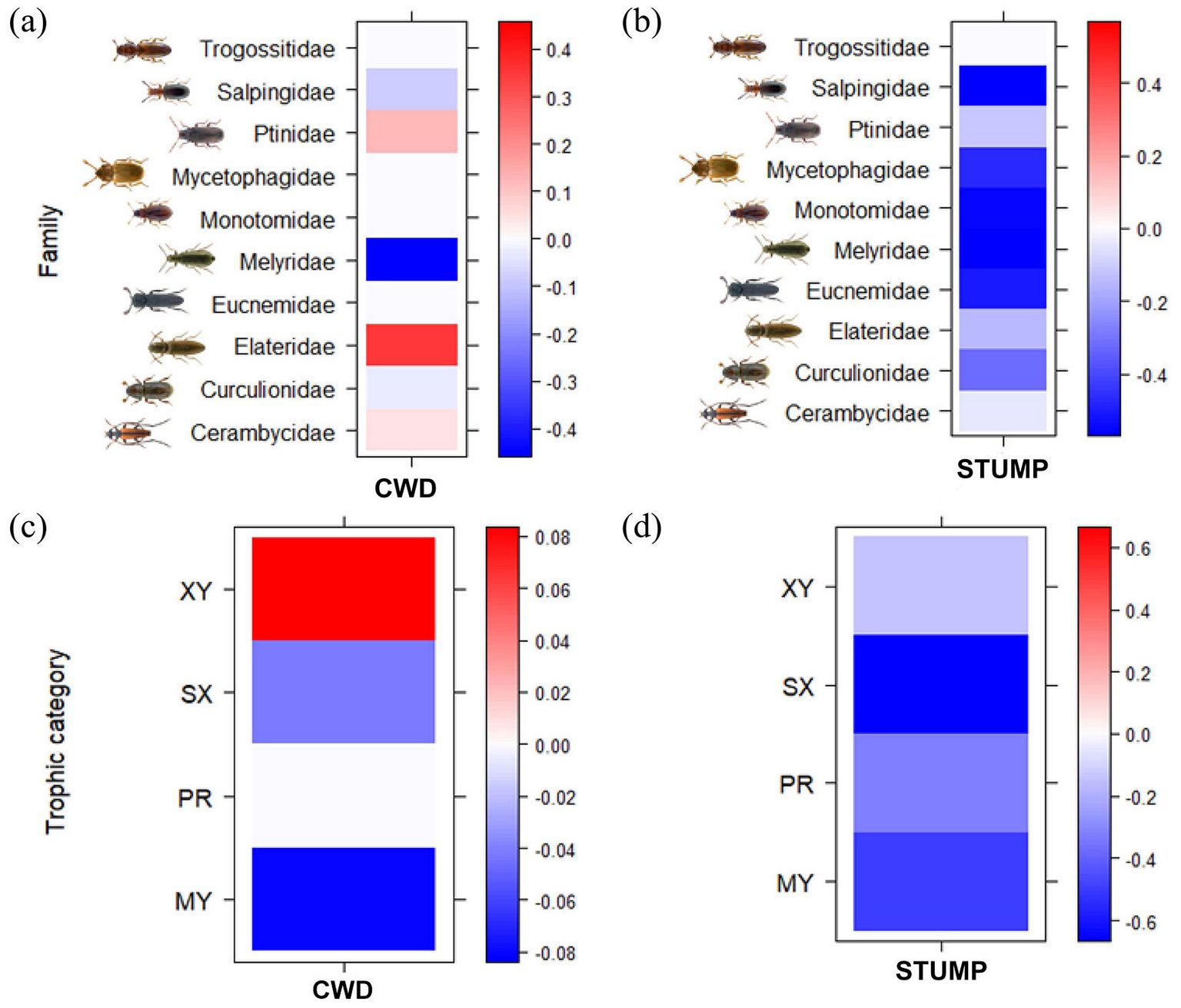
A high score of the CWD PC1 was positively associated with Cerambycidae, Elateridae and Ptinidae families, and negatively associated with Curculionidae, Melyridae and Salpingidae (Fig. 4a). As for the trophic categories, a high CWD PC1 score was positively associated with xylophagous species and negatively associated with micetobiontic, saproxylophagous and xylophagous species (Fig. 4c).
Fig. 4 - Interactions of deadwood characteristics (CWD) with saproxylic beetles’ families (a) and trophic categories (c) and interactions of stump characteristics (STUMP) with saproxylic beetles’ families (b) and trophic categories (d) according to the Fourth-corner modelling results. GLMs and legend are the same as in Fig. 1.
Finally, a high score of the STUMP PC1 was negatively associated especially with Eucnemidae, Melyridae, Monotomidae and Salpingidae (Fig. 4b). All the trophic categories were negatively associated with a high STUMP PC1 score (Fig. 4d).
Discussion
We studied the relationship between the most abundant species in a saproxylic beetle community and their niche dimensions, including topographic variables, microhabitats, abundance, and deadwood amount in a Mediterranean-managed beech forest. We also evaluated the role of the family and trophic level of the species in explaining these abundance-environment associations. Specifically, we found that the predictors affecting the abundance of single saproxylic species were, in order of importance, stump-related, elevation, and CWD-related variables (Fig. 5). Topographic preferences and resource availability had a different impact on beetle species belonging to different families and trophic categories: xylophagous species (mostly Cerambycidae and Ptinidae) were positively associated with low elevation and insect galleries, whereas Melyridae and Eucnemidae were negatively associated only with stump characteristics.
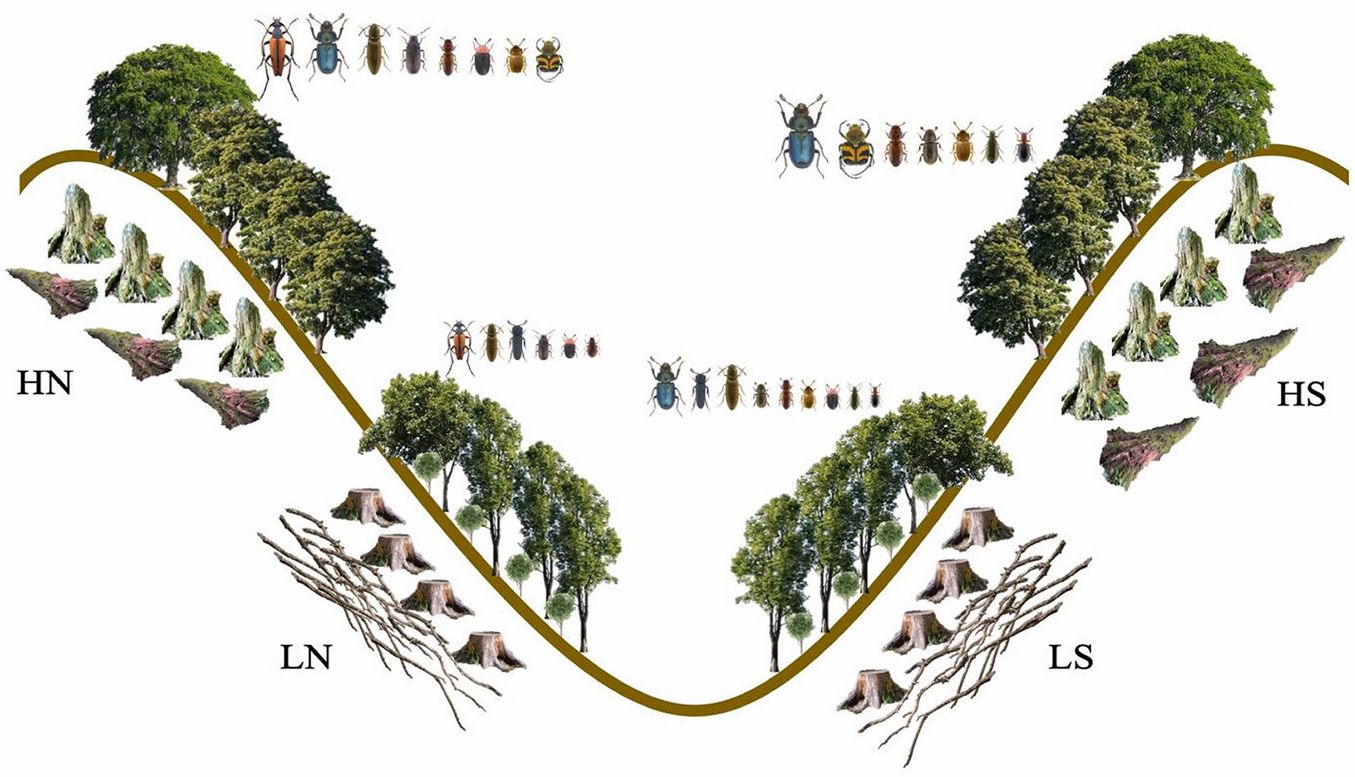
Fig. 5 - Graphic representation of the ecological dynamics of saproxylic beetles in managed beech forests (elaboration: F. Parisi). (LS): Low altitude and southern exposure (in order of decreasing size: Lucanidae, Eucnemidae, Elateridae, Curculionidae, Trogossitidae, Mycetophagidae, Erotylidae, Melyridae, and Salpingidae); (LN): Low altitude and northern exposure (in order: Cerambycidae, Elateridae, Eucnemidae, Ptinidae, Erotylidae, and Monotomidae); (HN): High altitude and northern exposure (in order: Cerambycidae, Lucanidae, Elateridae, Ptinidae, Trogossitidae, Erotylidae, Mycetophagidae, and Scarabaeidae); (HS): High altitude and southern exposure (in order: Lucanidae, Scarabaeidae, Trogossitidae, Curculionidae, Mycetophagidae, Melyridae, and Salpingidae).
Relative importance of environmental drivers of species abundance and richness
The most important drivers of beetle abundance and richness in the four sectors were the characteristics of the stumps produced by intensive forest management, which occurred more often in our sample plots, while the characteristics of CWD, which was very scarce, played a limited role. In general, high stumps and snags might host higher saproxylic diversity than lying deadwood, due to the different microclimatic conditions found along the standing deadwood, i.e., being wetter at the basis and drier and warmer at the top ([5]). Indeed, we found that deadwood volume can be a poor predictor for saproxylic species abundance and richness, in agreement with what Götmark et al. ([16]) and Basile et al. ([1]) found in the boreal and Mediterranean managed forests, respectively.
Although forest characteristics had a large importance in determining beetle abundance and richness patterns, elevation also played an important role. In fact, saproxylic beetle abundance and species richness were greater in the two sectors at higher elevations (overall, 55% of the abundance and 44% of the species) than in the two sectors at lower elevations (overall, 45% of the abundance and 38% of the species). The higher productivity in high-elevation sectors - in terms of species abundance and diversity near the upper limit of the forest - is surprising, given the scarce availability of resources for saproxylic species in terms of CWD, stumps, and snags (Tab. 1). However, at higher altitudes different types of microhabitats were found, which were absent at lower altitudes, like woodpecker breeding cavities, concavities, exposed sapwood and heartwood, that may have a positive effect in enhancing species abundance and diversity. Furthermore, the high-altitude sectors were located at the forest edges, which are important ecotones recognized as suitable habitats for saproxylic beetles. Indeed, the presence of a consistent herbaceous layer in the forest understorey at high altitude could be the reason for the presence of saproxylic beetle species depending on flowering plants ([50]) together with saproxylic species completing their entire life cycle on deadwood, and this might have increased locally species abundance and diversity ([8]).
Exposure also had a minor but still important role in driving species abundance and richness patterns. We observed that saproxylic beetle abundance and species richness were higher in the two south facing sectors (overall, 28% of the abundance and 44% of the species) than in the two north facing sectors (overall, 24% of the abundance and 38% of the species). This higher productivity in terms of biomass and diversity is certainly driven by the higher availability of resources on the southern than on the northern slopes, both in terms of stumps and snags, even though on the southern slopes CWD was lower than on the northern slopes. The microhabitats were substantially of the same type on both southern and northern slopes, but more abundant on the latter. Overall, on the southern slopes, saproxylic species may have benefited from the joint availability of more substrates and the positive impact of warm microclimate on their reproduction and development ([51]). This is particularly true for species like R. mordax (Cerambycidae), which was positively associated with southern exposed slopes. This is also in agreement with what Lindhe et al. ([22]) found in boreal forests. Specifically, Lindhe et al. ([22]) found that sun-exposed areas support a higher species abundance and richness, since beetle activity and larval development are correlated with solar radiation. For instance, it is known that a good solar radiation is essential for early successional saproxylic species, belonging to Cerambycidae (e.g., R. mordax) and Curculionidae (e.g., E. fagi), and specimens for these species are frequently found on snags and sun-exposed deadwood ([33], [38]). Indeed, Seibold et al. ([42]) found a higher number of specimens and species in sunny forest plots than in shady areas in central European temperate forests.
The lower importance of microhabitats in driving species richness and abundance patterns likely derives from their scarce abundance and diversity in all four sectors. This can be a consequence of intensive forest management and limited availability of data on microhabitats. In fact, forest management for timber production has certainly led to the lack of habitat trees and large deadwood ([29]). However, also the fact that the inventory of microhabitats was available only for deadwood limited its importance in describing patterns in species abundance. Including the data for microhabitats on living trees would have certainly increased their importance in characterizing the niche of some of the saproxylic beetle species.
Interactions of environmental variables with trophic categories and families
Xylophagous species, like H. costatus (Ptinidae), were positively associated with low elevation, probably because of the higher number of stumps, snags, and CWD available in those sectors (Tab. 1). In fact, at higher altitudes forest stands have been historically less impacted by management ([40]). In accordance with Johansson et al. ([19]) for boreal forest, we observed that fungivore and mycetophagous families (e.g., Monotomidae) were negatively linked to high elevation, where the vegetative period is generally shorter, which reduces the time window available for these species to complete their life cycle in the sporocarps. Consequently, predator species like M. villosus (Elateridae), feeding on xylophagous and fungivorous organisms, may have found better feeding opportunities in the low-altitude sectors. On the contrary, Melyridae species (e.g., D. plumbeus) preferred ecotone meadows at high elevations, especially in the HS sector, probably due to a rich herbaceous layer. Indeed, Melyridae species are rhyzophagous as larvae and frequently occur on flowers as adults, since they are pollinators ([26]).
Mycetophagous species such as Rhizophagus bipustulatus (Monotomidae) were positively associated with southern exposure. In boreal forests, Johansson et al. ([19]) confirmed this pattern, observing that fungivore species’ density was positively linked with south-exposed slopes, probably due to a higher decomposition rate and a higher fungal production.
We found that xylophagous and saproxylophagous species, such as H. costatus (Ptinidae) and M. buprestoides (Eucnemidae), were positively associated with specific microhabitats (i.e., insect galleries), as their larvae, feeding on wood, might expand cavities and build galleries ([35]). On the other hand, predator species (e.g., D. plumbeus, Melyridae) feeding on xylophagous and fungivore organisms were associated with fungal fruiting bodies. Thus, trophic categories and families were generally assorted in specific microhabitats, which represented dimensions of the spatial and feeding niche of the species.
Positive associations were detected between strict xylophagous families (e.g., S. sennii, Cerambycidae and H. costatus, Ptinidae) and CWD, and negative/less positive associations between non-strictly xylophagous (i.e., micetobiontic/saproxylophagous) families such as Curculionidae, Melyridae and Salpingidae, and CWD ([34]). Moreover, a strong positive association was also found between predator species, like M. villosus (Elateridae), and CWD-related variables, as large amounts of lying deadwood might host a great variety of larvae upon which this trophic group feeds ([33]).
Eventually, negative associations linked stump-related variables to several families, in particular to species belonging to Eucnemidae (e.g., M. buprestoides), Melyridae (e.g., D. plumbeus), Monotomidae, Mycetophagidae, and Salpingidae (e.g., S. planirostris). This negative effect is explained by the fact that these families are frequently linked to standing deadwood, typical of unmanaged or old-growth forests, which was poorly available in our study sectors.
Conclusions
Our results show that the patterns of abundance and richness of our saproxylic beetle communities were jointly affected by the interaction of topographic variables (elevation and exposure) with variables related to forest structure in this Mediterranean managed forest ecosystem in Central Italy. The existence of these significant interactions means that management affecting forest structure has different impacts on the availability of resources for saproxylic communities in different topographic conditions. For this reason, both these drivers must be taken into account when planning for forest management. To acknowledge this complexity, we advocate for landscape-level forest management which supports the local diversity of beetle assemblages maintaining a mosaic of seminatural forest characteristics in different topographic contexts. We recommend that closer-to-nature forest management targeting deadwood retention and accumulation and increasing the occurrence of microhabitats should be practiced in forests of different altitudes (i.e., forest edges at high altitudes and forest cores at low altitudes) and exposures (i.e., sunny and shaded areas - [36]). Furthermore, “passive” management should be finalized to the retention of large, old trees ([9]) and favored in different topographic contexts. These two measures will favor the saproxylic communities in two ways: increasing locally the amount and diversity of available microhabitats in living and dead wood, and reducing local tree density, which increases habitat heterogeneity ([35]).
Even in our managed forests, 15% of the saproxylic species sampled were listed in the Italian IUCN Red List ([10]) as “high conservation priority”, belonging to Near Threatened (8 species) and Vulnerable (2 species) categories (Tab. S1 in Supplementary material). To guarantee the survival of these threatened species, the EU Forest Strategy for 2030 ([14]) has recommended the development of sustainable forest management strategies, like those mentioned above, and the improvement of monitoring activities, as conducted in our study areas. In fact, to be cost-effective, these two activities should go hand-in-hand, as the capacity of closer-to-nature management interventions of restoring the habitat of threatened species can be evaluated only implementing rigorous biodiversity assessment and ecological monitoring programs over the whole forest landscape.
Acknowledgements
This work was supported by the LIFE program in the framework of the project “AForClimate-417 Adaptation of FORest management to CLIMATE variability: an ecological approach” (LIFE15418 CCA/IT/000089), and PNRR project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for tender no. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union - NextGenerationEU; Project code CN_00000033, Concession Decree no. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP H73C22000300001, Project title “National Biodiversity Future Center - NBFC”.
AM received funding from the Academy of Finland Flagship Forest-Human-Machine Interplay “Building Resilience, Redefining Value Networks and Enabling Meaningful Experiences” (UNITE) 337653.
The authors thank the specialists of the various taxonomic groups for the identification of the specimens: Paolo Audisio (Nitidulidae), Alessandro Bruno Biscaccianti (Biphylidae, Cerylonidae, Erotylidae, Lucanidae, Mycetophagidae, Salpingidae, Tenebrionidae, Trogossitidae), Enzo Colonnelli (Curculionidae), Massimo Faccoli (Curculionidae, Scolytinae), Fabrizio Fanti (Lycidae), Gianfranco Liberti (Melyridae), Emanuele Piattella (Scarabaeidae), Giuseppe Platia (Elateridae), Pierpaolo Rapuzzi (Cerambycidae, pars), Adriano Zanetti (Staphylinidae, pars). We are grateful to Bruno Lasserre (Università degli Studi del Molise, Italy) for technical support.
Authors contribution
FP and AM have contributed in equal measure to the writing of this article.
FP, AM: conceptualization, methodology, analysis, project administration; FP, AM, EDS: data curation, investigation, visualization, writing and original draft preparation, writing, review and editing; EV, RT, DT, MM, GD, SF, CB, writing, review and editing; RT, DT, MM, GC: supervision.
References
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Marco Marchetti 0000-0002-5275-5769
Dipartimento di Bioscienze e Territorio, Università degli Studi del Molise, c.da Fonte Lappone, 86090 Pesche (IS), Italy
NBFC, National Biodiversity Future Center, 90133 Palermo (Italy)
Davide Travaglini 0000-0003-0706-2653
Giovanni D’Amico 0000-0002-2341-3268
Saverio Francini 0000-0001-6991-0289
Costanza Borghi 0000-0002-5334-7548
Gherardo Chirici 0000-0002-0669-5726
geoLAB - Laboratorio di Geomatica Forestale, Dipartimento di Scienze e Tecnologie Agrarie, Alimentari, Ambientali e Forestali, Università degli Studi di Firenze, v. San Bonaventura 13, 50145 Firenze (Italy)
National Research Council of Italy, Institute for Agriculture and Forestry Systems in the Mediterranean - ISAFOM, 06128, Perugia (Italy)
Dipartimento Agricoltura, Ambiente e Alimenti, Università degli Studi del Molise, v. De Sanctis, 86100 Campobasso (Italy)
CREA Research Centre for Forestry and Wood, 52100 Arezzo (Italy)
Gherardo Chirici 0000-0002-0669-5726
Fondazione per il Futuro delle Città, Firenze (Italy).
Corresponding author
Paper Info
Citation
Parisi F, Mazziotta A, Vangi E, Tognetti R, Travaglini D, Marchetti M, D’Amico G, Francini S, Borghi C, Chirici G (2023). Exposure elevation and forest structure predict the abundance of saproxylic beetles’ communities in mountain managed beech forests. iForest 16: 155-164. - doi: 10.3832/ifor4264-016
Academic Editor
Marco Borghetti
Paper history
Received: Nov 14, 2022
Accepted: Mar 14, 2023
First online: Jun 08, 2023
Publication Date: Jun 30, 2023
Publication Time: 2.87 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2023
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 23138
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 17671
Abstract Page Views: 3195
PDF Downloads: 1822
Citation/Reference Downloads: 1
XML Downloads: 449
Web Metrics
Days since publication: 968
Overall contacts: 23138
Avg. contacts per week: 167.32
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2023): 2
Average cites per year: 0.67
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Review Papers
Linking deadwood traits with saproxylic invertebrates and fungi in European forests - a review
vol. 11, pp. 423-436 (online: 18 June 2018)
Research Articles
Stand structure and deadwood amount influences saproxylic fungal biodiversity in Mediterranean mountain unmanaged forests
vol. 9, pp. 115-124 (online: 08 September 2015)
Research Articles
Early responses of biodiversity indicators to various thinning treatments in mountain beech forests
vol. 11, pp. 609-618 (online: 25 September 2018)
Research Articles
The Habitat-Trees experiment: using exotic tree species as new microhabitats for the native fauna
vol. 8, pp. 464-470 (online: 22 October 2014)
Research Articles
Factors affecting the quantity and type of tree-related microhabitats in Mediterranean mountain forests of high nature value
vol. 14, pp. 250-259 (online: 21 May 2021)
Research Articles
Biodiversity conservation and wood production in a Natura 2000 Mediterranean forest. A trade-off evaluation focused on the occurrence of microhabitats
vol. 12, pp. 76-84 (online: 24 January 2019)
Research Articles
Diversity of saproxylic beetle communities in chestnut agroforestry systems
vol. 13, pp. 456-465 (online: 07 October 2020)
Research Articles
Rewilding beech-dominated temperate forest ecosystems: effects on carbon stocks and biodiversity indicators
vol. 18, pp. 1-9 (online: 02 February 2025)
Research Articles
Fluctuation of the ecological niche of Moringa peregrina (Forssk.) Fiori with topoclimatic heterogeneity in southern Iran
vol. 16, pp. 53-61 (online: 16 February 2023)
Research Articles
Concordance between vascular plant and macrofungal community composition in broadleaf deciduous forests in central Italy
vol. 8, pp. 279-286 (online: 22 August 2014)
iForest Database Search
Google Scholar Search
Citing Articles
Search By Author
- F Parisi
- A Mazziotta
- E Vangi
- R Tognetti
- D Travaglini
- M Marchetti
- G D’Amico
- S Francini
- C Borghi
- G Chirici
Search By Keywords