Study on the chemical composition of teak wood extracts in different organic solvents
iForest - Biogeosciences and Forestry, Volume 14, Issue 4, Pages 329-336 (2021)
doi: https://doi.org/10.3832/ifor3717-014
Published: Jul 09, 2021 - Copyright © 2021 SISEF
Research Articles
Abstract
Teak wood (Tectona grandis Linn F.) is known for its high natural resistance to attack by microorganisms. For this reason, teak wood is used for restoration works. This paper provides an assessment of the extraction capacity of various organic solvents and the chemical and physical-structural characteristics of extracts of teak wood with an age of 40 years. On the basis of literature data, we selected the solvents with potential synergetic activity in preservation treatments. For this purpose we used the SEM-EDX and GC+MS methods, assisted by computerized processing software, and corroborated the data obtained from these two instrumental techniques.
Keywords
Teak Wood, Composition, Organic Solvents, Extracts, SEM-EDX, GC+MS
Introduction
Wood is an organic material that is degraded by bacteria, fungi, xylophagous insects and termites ([43]). The use of plant extracts has introduced new approaches for protection against fungi and insects ([37]). They include wood extracts, which are natural products rich in bioactive compounds such as tannins, polyphenols, alcohols, aldehydes, ketones, esters, and oxygenated hydrocarbons, which are toxic for the microorganisms that degrade wood ([46], [29]).
Regarding natural durability, expressed as the degree of physical deterioration and chemical degradation of the structural compounds under the action of biotic and/or abiotic agents, wood is affected differently along the three sections (R-radial, T-tangential, L-longitudinal - [33]). The specificity of the wood’s resistance to the attack of fungi and xylophagous insects (anobides, termites, beetles, and marine borers) is contingent on this anisotropy. Also, the presence of an extractable organic compound rises the resistance against fungi ([42], [14]).
It is known that wooden species with high natural durability have a wide range of uses and a much greater added value on the market. Among them, teak wood (Tectona grandis Linn F.) is renowned for its high resistance to the attack of many microorganisms ([18], [40], [6]).
Once it is processed into an object, wood generally deteriorates and/or degrades under the influence of climatic factors and of chemical (pollution), microbiological (fungi, xylophagous insects, rodents, etc.) and radiative agents (fire and very-high temperatures, UV and gamma radiation). The two effects are the result of processes that use different mechanisms (physical-structural destruction or chemical alteration), which are regulated by the presence of one or several components with insecto-fungal, hydrophobic or fire-retardant activity that originate from the native wood (natural product) or from the use of dispersed systems for mitigating or stopping the deterioration and/or degradation by means of preservation operations ([16], [33], [38]).
Concerning heritage goods, we cannot overlook human impact (the anthropic factor), which through unauthorized preservation or restoration interventions, through manipulation, transport or improper storage or through vandalism, explosions, fires, and floods (accidental or willful), can lead the artefact to the state of precollapse or collapse (end of use life - [33]).
The natural resistance of wood to exogenous factors and agents depends on the species, age of the tree, origin (area of harvest), time of felling, period and manner of processing and preservation, as well as the quantity and type of the active natural principle against insects and fungi or of the one introduced by the preservation treatments ([4], [23], [41], [33], [34], [10], [36]). As such, teak wood has a high commercial value, as it meets the requirements for its use as a wood with superior natural durability, which does not require preservation interventions ([10]).
It is known that the wooden species that have low natural durability require preservation treatments when turned into final products or later during their use life, in order to improve their performance as materials and extend their lifespan. The most common treatment procedures employ - depending on the species, the age of the tree, the age of the timber, the structural-ornamental complexity, and the size and state of the artifact - various ecological systems for preservation based on active principles anti insects and fungi, in the form of solutions of aqueous or alcoholic microdispersions or of other organic solvents. The procedures used for applying them are immersion, brushing or spraying, as well as vacuum or pressure technologies, which provide high protection against insecto-fungic attacks ([5], [21], [33]).
According to some authors ([22], [11], [17]), these active principles can include various chemical substances such as: arsenic salts, chrome salts, copper salts, zinc salts, and boron salts, which have the drawback of being expensive and toxic to the operator, owner and the environment. Moreover, these procedures required periodical re-treatments through time, which makes their use dangerous ([44]).
Currently, a wide range of synthetic ([23], [36]) or natural ([42]) substances are used for this purpose, as aqueous or organic solutions, emulsions or nanodispersions, which on account of being applied on the surface (as protection films) or inside the wood require periodic treatments that are hard to carry out once the artefact has been executed.
Given these aspects and the insecto-fungic protective role played by a number of natural extractable compounds from various plants (including flowers and seeds) or trees (oak, acacia, chestnut, mimosa, and teak), their investigation has become a very active area of research. Indeed, there is a large number of studies on the use of substances extracted from wooden species with high natural durability, as eco-friendly preservation agents ([26], [24], [39], [18], [40], [25]).
Many studies on teak wood are present in the literature, though most are focused on the base wood components (lignin, cellulose and hemicellulose - [15]). Few authors were concerned with the study of the nature and composition of the extracts from wooden species that are very resistant to insect or fungal attack ([8], [6]). In regards to teak wood, the antimicrobial effect of extracts is well known ([20], [27], [1], [19], [30], [6]). Another study very close to the present one ([31]) shows that extracts of teak wood in acetone contains 49 compounds with insecto-fungic activity, of which the most representative are: 4-tert-butil-2-fenil-fenol, 2-metilantraquinone, and 2.3-dimetil-1.4,4a, 9a-tetrahidro-9.10-antracenedione.
The specialized literature on treatments for preserving old processed wood using active principles extracted from teak is limited to hot water and ethyl alcohol extracts, without presenting the chemical composition of the components ([1]). In some studies the low natural durability of teak grown in plantation from Panama were investigated ([13]). In other studies, the heartwood extractive chemical components of teak from different provenances was studied by GC-MS analysis ([45]). Moreover, the effect of some heartwood extractives was studied in order to highlight the impact on symbiotic protozoan communities and termite species ([3])
This paper assesses the capacity to obtain the active principles of the teak wood in extraction processes using various solvents, determining the rate of extraction and the nature of the main components with various potential. Moreover, the purpose is the optimisation of the extraction conditions by using different organic solvents, alone or in mixture, in order to determine the efficacy of the extraction processes (quantity and number of extracted compounds). The experimental data highlighted the difference from the nature of the extracted compounds and their quantity for each studied extraction system.
Materials and methods
We used thin teak wood veneer (Tectona grandis L.f.) with no anatomical defects (knots, gnarls, torsades, etc.), with an age of 40 years. The samples were provided by a furniture factory in Iasi, Romania and imported from Thailand. Teak wood veener weighing 400 g were dried and mechanically divided by chopping, and subsequently ground in a ceramic ball mill, in order to obtain particles below 0.1 mm. For each sample 10 g were weighted, and the powder was dispersed into a series of preset solvents, from which the active components were extracted in the form of organic mixture. Organic solvents of the p.f.a. (pure for analysis) group were used for extraction, specifically: ethylic alcohol (96%), isopropyl alcohol (99.7%), acetone (99%), and acetone-isopropanol (60:40 ratio) co-solvent mixture.
The experiment consisted in 12 Erlenmeyer screw-cap flasks with each of the three solvents and the acetone-isopropanol mixture. In each flask 50.0 mL of solvent was added to 10 g of teak powder. The flask was shaken every hour for 60 sec, for a period of 10 h. The dispersion was subsequently decanted for 14 h and then filtered with a Büchner funnel. One mL of filtrate was taken and its solvent evaporated on a watch glass that was weighted beforehand (mg). The watch glass and the filtrate were weighted (mg+f), and after the evaporation of the solvent (normal conditions for 24-72 h, depending on solvent) the watch glass with the extract was weighted (mg+e). The concentration of the organic extract was determined according to the relation ([2] - eqn. 1):
For each solvent the evaluation was done as the arithmetic average of the three values obtained by evaporation.
The extract samples were analyzed by means of scanning electron microscopy, coupled with X-ray diffraction (SEM-EDX) and liquid chromatography, coupled with mass spectrometry (GC+MS).
The scanning electron microscopy produced microphotographs showing the morphology of the longitudinal and radial sections of the teak wood used for obtaining the powder and the extracts, and the EDX spectra provided the elemental composition in mass percentages of the main chemical components. For this purpose we used a scanning electron microscope (SEM), model VEGA II LSH (Tescan, Czech Republic), coupled with an EDX detector model Quantax QX2 (Bruker/Roentec, Germany). The analysis of the samples was performed at a magnification of 200× to 2500×, with an acceleration tension of 30 kV, and a work pressure lower than 1×10-2 Pa. The SEM image was acquisitioned using secondary (SE) and backscattered electrons (BSE).
The analysis of the chemical derivatives in the extracts was performed by GC (Gas Chromatography) technique (model 6890 N, (Agilent Technologies Inc., CA, USA) in conjunction with an Agilent 5975 mass spectrometer ([35]). The chromatographic conditions were differentiated according to the devices involved in the chromatography, as follows: the oven with a temperature 50 °C at the onset, then raised at a rate of 15 °C per minute firstly to 150 °C and then to 320 °C, which was retained for 15 min; the HP-5MS column, measuring 30 mm × 0.25 mm × 0.25 μm, with flow rate of 1.0 mL min-1; the sample injection was splitless (the entire sample) at a temperature of 270 °C. The mass spectrometry conditions were an EI (electronic ionization) source with an electron bombardment energy of 70 eV, an ion source temperature of 230 °C, and transfer line temperature of 280 °C. The compounds were identified by comparing the MS (mass spectrometry) spectra to the National Institute of Standards and Technology library ([28]) with retention time and indexes.
In this experiment, for the three components of the GC technique, the following parameters were used: (i) oven, which started from an initial temperature of 50 °C and reached a maximum temperature of 320 °C in 44.92 min; (ii) syringe for the splitless injection of sample (the entire sample is injected once, in order to obtain a higher concentration and a better selection of the component substances) at an initial temperature of 270 °C, a pressure of 7.12 psi, with the flow rate varying for 2 min from 20.0 to 24.1 mL min-1; (iii) column - product separation was achieved by using the carrier gas helium with a flow rate of 1 mL min-1.
Detection was achieved using an acquisitioning system, with the information processed according to an S975 Agilent database, in scan mode, with the mass unit from 30.0 to 550.0.
Results and discussion
To identify the main organic derivatives from teak wood that account for its great resistance to insect and fungal attack, we initially resorted to a wide range of solvents. After a series of preliminary tests on their extraction efficiency, only three solvent were selected (ethanol, isopropanol and acetone), along with the acetone-isopropanol mixture, which were used in the selective extraction of the organic compounds with insecto-fungicidal activity from teak wood.
Tab. 1shows that the acetone-isopropanol mix has the greatest extraction yield, followed by acetone, ethyl alcohol and isopropanol, respectively. This result was expected, as the available literature shows that acetone extracts better than ethyl alcohol ([9]), and among alcohols, isopropanol is miscible in water and nonpolar solvents, without evaporation traces left behind, making it the most recommended solvent for extraction protocols ([7], [32], [47], [12]).
Tab. 1 - Concentration of extracts from teak wood in the selected solvents.
| Sample no. | Solvent | Conc. (%) |
|---|---|---|
| S1 | Isopropanol | 0.4435 |
| S2 | Ethyl alcohol | 0.8888 |
| S3 | Acetone | 0.9393 |
| S4 | Volumetric mixture of acetone 60% and isopropanol 40% | 0.9634 |
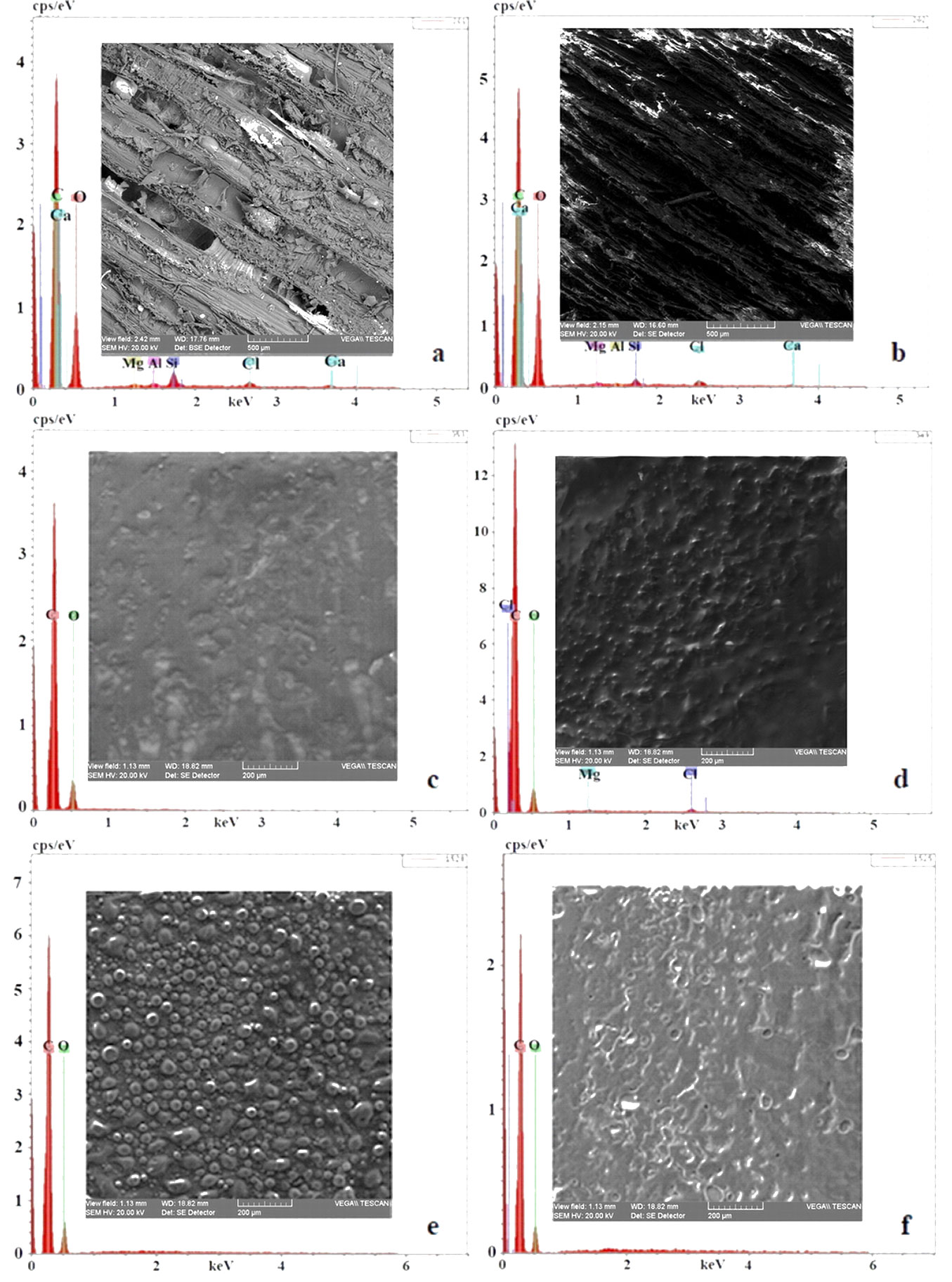
SEM-EDX analysis was first conducted on the two sections - radial and longitudinal - of the teak wood from which the powder was obtained, in structurally representative areas, for which the SEM microphotographs were made and incorporated into the EDX spectra (Fig. 1a, Fig. 1b). We continued with the analysis of the films of evaporated extract from the four organic solutions (Fig. 1c-f). The SEM-EDX analysis allowed assessing the texture, morphology and distribution of the anatomo-structural components of the wood fibers in the two sections, alongside the uniformity and morphology of the mixture of products from the evaporated extracts. On the basis of the EDX spectra, Tab. 2presents the elemental composition of the teak wood in the two sections and for the four organic extracts.
Fig. 1 - SEM images for the sample under analysis. (a): teak wood - radial section; (b): teak wood - longitudinal section; (c): extract in isopropyl alcohol; (d): extract in ethyl alcohol; (e): extract in acetone; (f): extract in a mixture of 60% acetone and 40% isopropyl alcohol.
Tab. 2 - The elemental composition in gravimetric percentages (%) for analysis samples.
| Sample | Elemental composition, gravimetric percentages (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Si | Al | Ca | Mg | Na | Cl | |
| Teak R | 49.6864 | 48.0086 | 1.2227 | 0.2451 | 0.3992 | 0.2657 | - | 0.1723 |
| Teak L | 45.3642 | 52.8382 | 0.6158 | 0.0636 | 0.6391 | 0.2686 | - | 0.2105 |
| S1 | 63.9852 | 36.0148 | - | - | - | - | - | - |
| S2 | 64.3797 | 34.8584 | - | - | - | - | - | 0.7620 |
| S3 | 65.0967 | 34.9033 | - | - | - | - | - | - |
| S4 | 66.0332 | 33.9668 | - | - | - | - | - | - |
We notice that in the radial section the microphotograph allowed a detailed rendering of the anatomo-structural components, with the parallel orientation of the fibers and of the resinophoric or gumiphoric channels, alongside the parenchymatous reserve cells with thickened and lignified walls, whereas in the longitudinal section only oriented arrangements of cellulose fibers and their defects were visible.
Conversely, the films of organic compounds obtained by evaporating the extracts appear as shinny resins, weakly colored in yellowish-brown, with a uniform arrangement of certain components that solidified differently when the solvent evaporated, in the form of glomerules and microalveoli, some of which were ruptured.
Tab. 2shows that the elemental composition in gravimetric percentages (%) differs in the two sections for all the elements identified, except for magnesium, which has approximately equal concentrations, and for chlorine, which is present in both sections of the wood sample. In the two sections (R) and (L) there is an inversion of carbon and oxygen, specifically about two-times diminishing of silicon and four times diminishing of aluminum, and again an inversion of calcium of about two times. This can be explained by the different distribution along the analyzed surface of the chemical compounds.
The four organic extracts were analyzed by GC-MS (Figs. 2-5), showing that among the large series of compounds present in the teak wood in the form of resins, sugars/polysaccharides, fatty acids, esters, phenols, terpenes, oils, and tannins had a greater capacity of selective extraction. Ethyl alcohol and acetone extracted a large number of derivatives, but acetone and the acetone-isopropyl alcohol mix had the highest efficiency.
The number of organic derivatives identified by GC-MS in the extracts obtained using the four solvent systems decrease as follows: acetone (8 derivatives), ethyl alcohol (8 derivatives), acetone-isopropanol mix (4 derivatives), and isopropanol (2 derivative); this was unexpected, given that the last is considered a solvent with a very high emollience and washing power for foulants, and which does not leave evaporation traces, but which increased the efficiency of the extraction in the mixture with acetone.
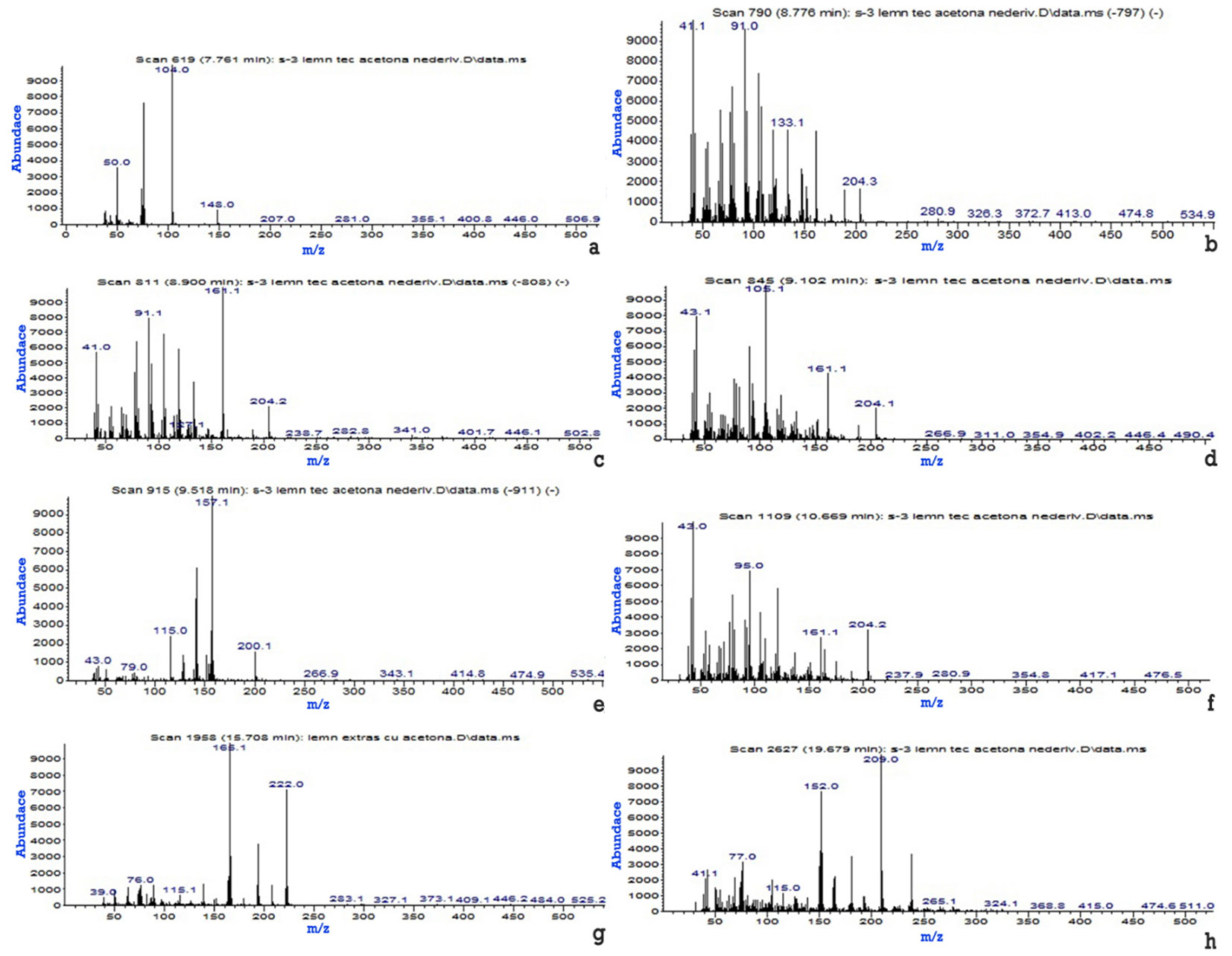
Of the eight derivatives extracted in acetone (Tab. 3, Fig. 2), four are also found in the ethyl alcohol extract (Tab. 4, Fig. 3), four in the acetone and isopropyl alcohol mix (Tab. 5. Fig. 4), which had the highest extraction efficiency and a single organic derivative, namely 9.10-Anthracenedione, 2-methyl- (Fig. 5) extracted in isopropanol, which can be found in all the extracts.
Tab. 3 - Organic derivatives identified by GC-MS, extracted in acetone.
| No. | Organic derivative | Minute | Spectrum |
|---|---|---|---|
| 1 | Phthalic anhydride- | 7.761 | Fig. 2a |
| 2 | 1H-Cycloprop[e]azulene, decahydro-1.1,7-trimethyl-4-methylene-, [1aR-(1a.alpha.,4a.beta.,7alpha.,7a)] | 8.776 | Fig. 2b |
| 3 | Naphthalene, 1.2,4a,5.6,8a-hexahydro-4.7-dimethyl-1-(methylethyl)- | 8.900 | Fig. 2c |
| 4 | Naphthalene, 1.2,4a,5.6,8a-hexahydro-4.7-dimethyl-1-(methylethyl)-, (1.alpha.,4a.alpha.,8a.alpha) | 9.102 | Fig. 2d |
| 5 | .alpha.-Calacorene | 9.518 | Fig. 2e |
| 6 | .alpha.-Cadinol | 10.669 | Fig. 2f |
| 7 | 9.10-Anthracenedione, 2-methyl- | 15.708 | Fig. 2g |
| 8 | 2-(Hydroxymethyl)anthraquinone- | 19.769 | Fig. 2h |
Fig. 2 - Mass spectra of the eight organic derivatives identified in the teak wood extract in acetone. (a): Phthalic anhydride; (b): 1H-Cycloprop[e]azulene, decahydro-1.1,7-trimethyl-4-methylene-, [1aR-(1a.alpha., 4a.beta.,7alpha.,7a)]; (c): Naphthalene, 1.2,4a,5.6, 8a-hexahydro-4.7-dimethyl-1-(methylethyl)-; (d): Naphthalene, 1.2,4a,5.6,8a-hexahydro-4.7-dimethyl-1-(methylethyl)-(1.alpha.,4a.alpha., 8a.alpha); (e): .alpha.-Calacorene; (f): .alpha.-Cadinol; (g): 9.10-Anthracenedione, 2-methyl-; (h): 2-(Hydroxymethyl)anthraquinone.
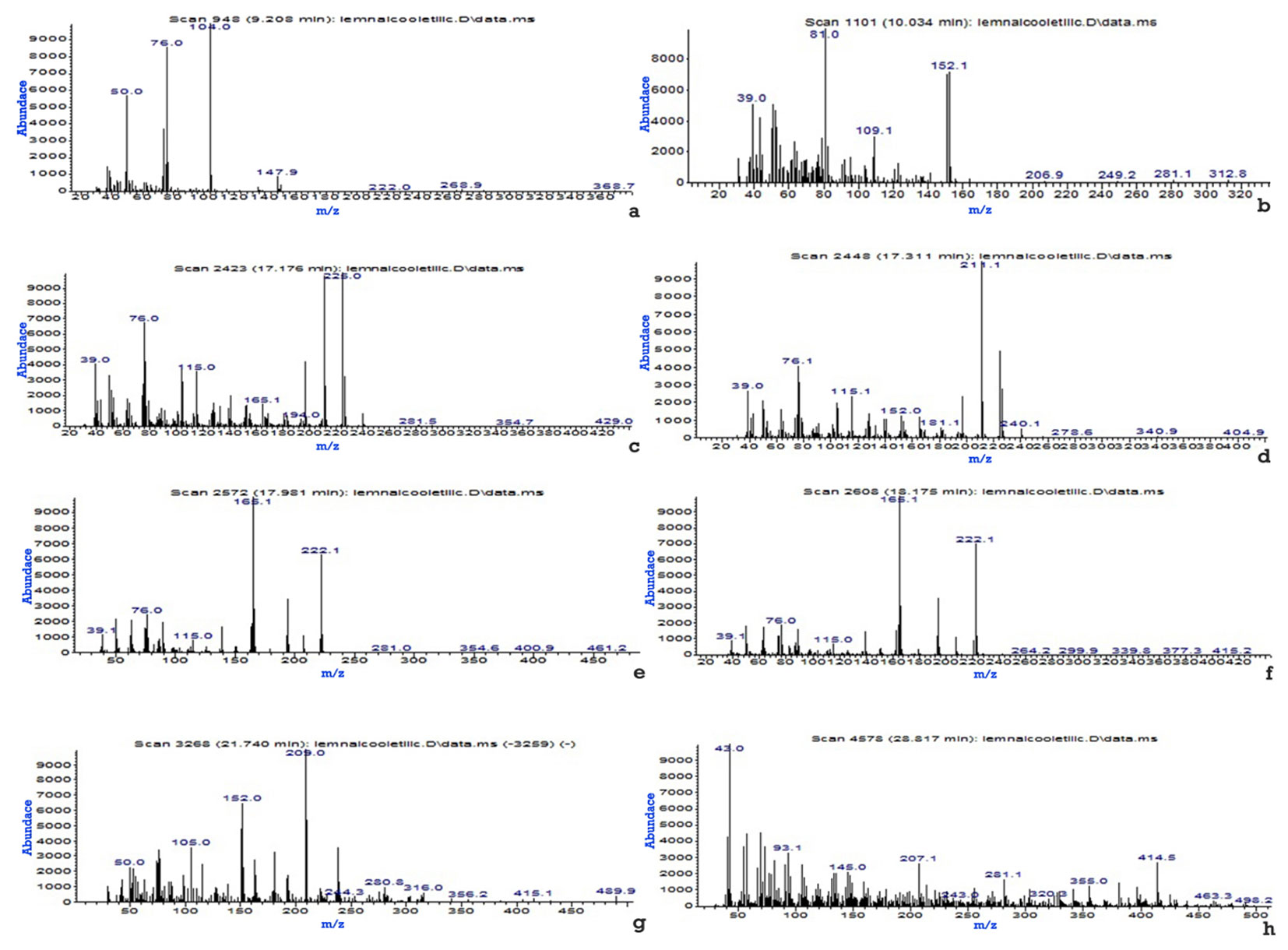
Tab. 4 - Organic derivatives from teak wood identified by GC-MS, extracted in ethyl alcohol.
| No. | Organic derivative | Minute | Spectrum |
|---|---|---|---|
| 1 | Phthalic anhydride | 9.208 | Fig. 3a |
| 2 | Vanillin | 10.034 | Fig. 3b |
| 3 | 2H-Naphtho [2.3-b]pyran-5.10-dione, 2.2-methyl- | 17.176 | Fig. 3c |
| 4 | [1.1’-Biphenyl]-2-ol, 5-(1.1-dimethylethyl)- | 17.331 | Fig. 3d |
| 5 | 9.10-Anthracenedione, 1-methyl - | 17.981 | Fig. 3e |
| 6 | 9.10-Anthracenedione, 2-methyl- | 18.175 | Fig. 3f |
| 7 | 2-(Hydroxymethyl)anthraquinone- | 21.740 | Fig. 3g |
| 8 | Butyl(2-chlorocyclohexyl) methyl phthalate | 28.817 | Fig. 3h |
Fig. 3 - Mass spectra of the eight organic derivatives identified in the teak wood extract in ethyl alcohol. (a): Phthalic anhydride; (b): Vanillin; (c): 2H-Naphtho [2.3-b]pyran-5.10-dione, 2.2-methyl-; (d): [1.1’-Biphenyl]-2-ol, 5-(1.1-dimethylethyl)-; (e): 9.10-Anthracenedione, 1-methyl-; (f): 9.10-Anthracenedione, 2-methyl-; (g): 2-(Hydroxymethyl)anthraquinone-; (h): Butyl(2-chlorocyclohexyl) methyl phthalate.
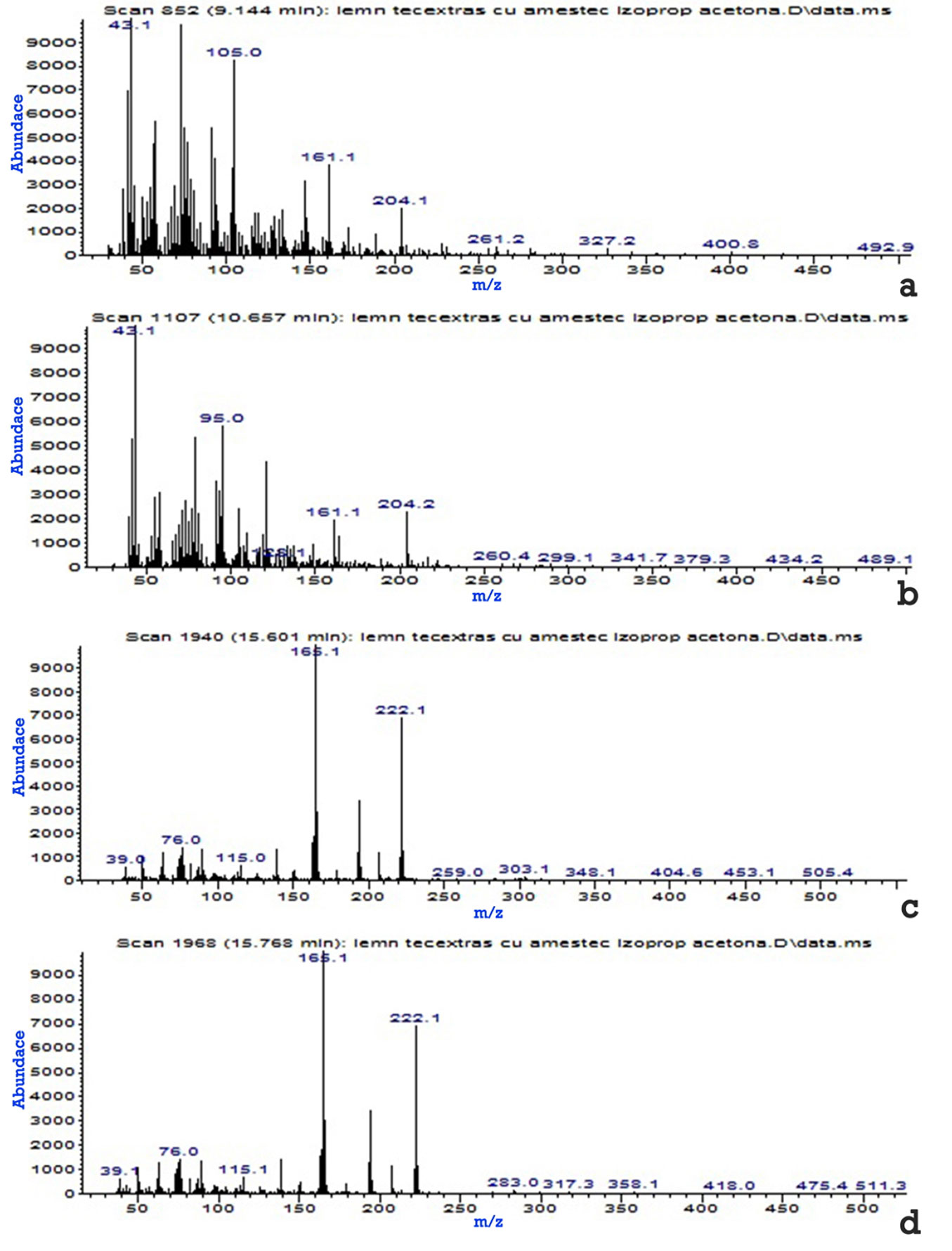
Tab. 5 - Organic derivatives identified by GC-MS, extracted in the acetone-isopropyl alcohol mixture.
Fig. 4 - The mass spectra of the four organic derivatives identified in the teak wood extract in the acetone-isopropyl alcohol mixture (6/4 vol.). (a): Naphthalene, 1.2, 4a,5.6,8a-hexahydro-4.7-dimethyl-1-(methylethyl)-(1.al pha.,4a.alpha., 8a.alpha); (b): .alpha.-Cadinol; (c): 9.10-Anthracenedione, 2-methyl-; (d): 9.10-Anthracenedione, 1-methyl-.
Fig. 5 - Mass spectra of the organic derivatives identified in the teak wood extract in isopropyl alcohol. (a): 2H-Naphtho [2.3-b]pyran-5.10-dione, 2.2-dimethyl-; (b): 9.10-Anthracenedione, 2-methyl-.
Tab. 3presents the organic derivatives extracted from the teak wood in acetone, identified by GC-MS, according to the mass spectra from Fig. 2.
Tab. 4presents the eight organic compounds from teak wood extracted in ethyl alcohol, identified according to the mass spectrum displayed in Fig. 3. Four of the compounds were also found in acetone (Phthalic anhydride, 2H-Naptho [2.3-b]pyran-5.10-dione, 2.2-dimethyl-, 9.10-Anthracenedione, 2-methyl- and 2-(Hydroxymethyl)anthraquinone-), but at somewhat lower minutes: 7.761, 14.812, 15.708, 19.769.
Tab. 5presents the organic derivatives extracted from the teak wood in the acetone-isopropyl alcohol mixture (6/4 vol.), identified by GC-MS, according to the mass spectra from Fig. 4.
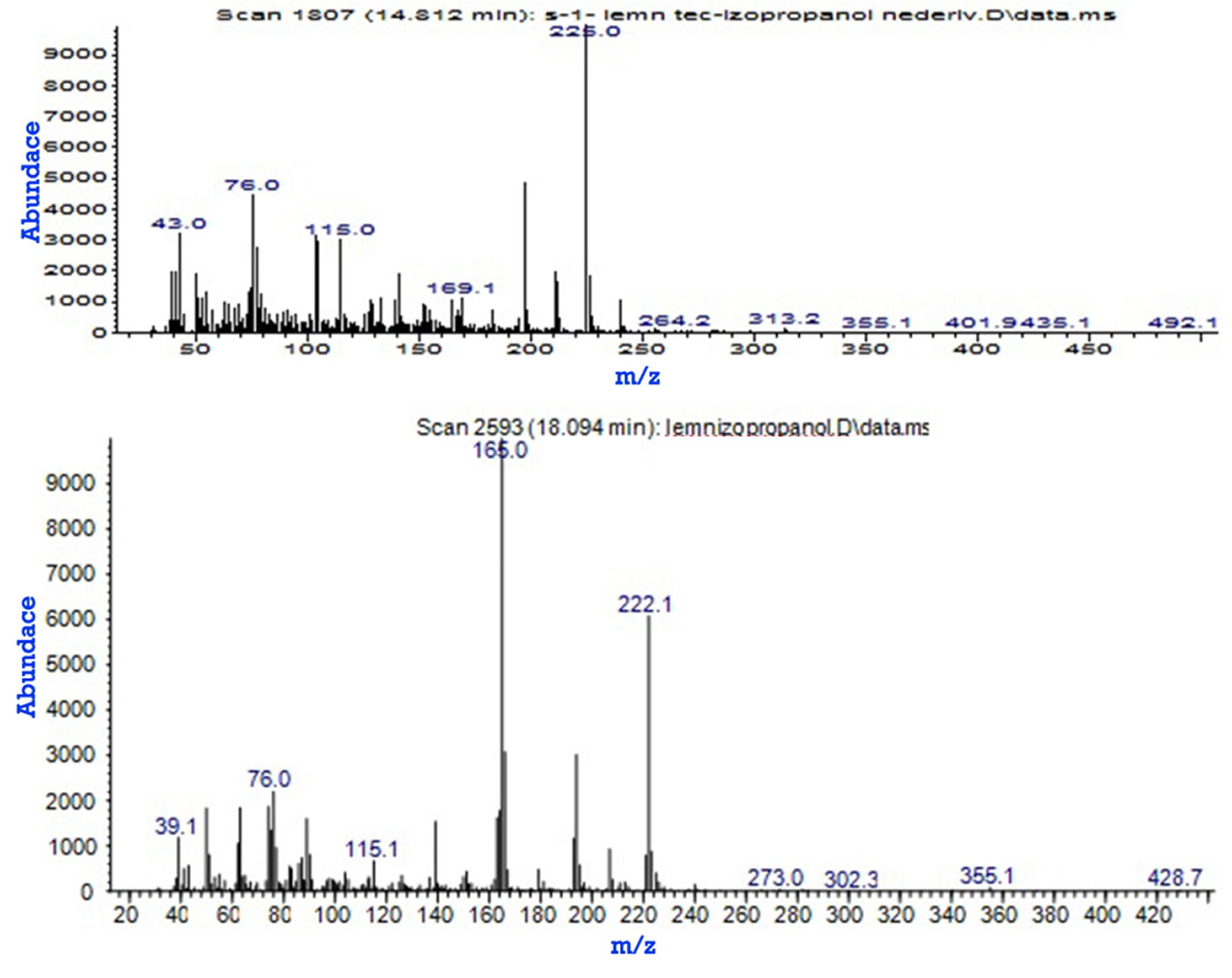
Fig. 5presents the two derivatives, 2H-Naphtho [2.3-b]pyran-5.10-dione, 2.2-dimethyl- and 9.10-Anthracenedione, 2-methyl-, extracted in isopropyl alcohol, identified from the mass spectrum at minutes 14.812 and 18.094, respectively, which were also found in the ethyl alcohol extract, but at different minutes (17.176 and 18.175).
Of the eight derivatives extracted in acetone, identified according to the mass spectra, four were found in the ethyl alcohol extract (Phthalic anhydride - 2H-Naphtho [2.3-b]pyran-5.10-dione, 2.2-methyl-, 9, 10-Anthracenedione, 2-methyl- and 2-(Hydroxymethyl)anthraquinone-), but at much higher minutes (9.208, 17.176, 18.175 and 21.740). Only four derivatives (Naphthalene, 1.2,4a,5.6,8a-hexahydro-4.7-dimethyl-1-(methylethyl)-(1.alpha.,4a.alpha.,8a.alpha)-, alpha.-Cadinol-, 9.10-Anthracenedione, 2-methyl- and 9.10-Anthracenedione, 1-methyl-) were extracted in the acetone-isopropyl alcohol mixture, three of which were also found in the acetone extract (Naphthalene,1.2,4a,5.6,8a-hexahydro-4.7-dimethyl-1-(methylethyl)-(1.alpha,4a.alpha, 8a.alpha); .alpha.-Cadinol- and 9.10-Anthracenedione, 2-methyl-), at approximately equal times, and only one (9.10-Anthracenedione, 1-methyl-) in the ethyl alcohol extract, but at minutes higher than 17.981.
The acetone-isopropyl alcohol mixture extracts fewer derivatives than acetone alone. By adding isopropanol a higher efficiency for extracting the four derivatives is provided.
We must underline that with the exception of isopropanol alone, acetone extracts selectively three derivatives (1H-Cycloprop[e]azulene, decahydro-1.1.7-trimethyl-4-methylene-, [1aR-(1a.alpha.,4a.beta.,7alpha., 7a)], Naphthalene, 1.2,4a,5.6,8a-hexahydro-4.7-dimethyl-1-(methylethyl)- and alpha-Calacorene), and ethyl alcohol two (Butyl(2-chlorocyclohexyl) methyl phthalate and [1.1’-Biphenyl]-2-ol, 5-(1.1-dimethylethyl)-), which are not found in other extracts, but which have a good insecto-fungicidal activity ([23]).
Among the derivatives extracted using the four selected organic solvent systems, most have good insecto-fungicidal activity, with the most active being the anthraquinones (9.10-Anthracenedione, 1-methyl-; 9.10-Anthracenedione, 1-methyl-; 2-(Hydroxymethyl)anthraquinone-; 2-Methyl-anthraquinone), naphthalenes (Naphthalene, 1.2, 4a,5.6,8a-hexahydro-4.7-dimethyl-1-(methyl ethyl)-(1.alpha.,4a.alpha.,8a.alpha); 2H-Naphtho [2.3-b]pyran-5.10-dione,2.2-dimethyl-), bisphenols ([1.1’-Biphenyl]-2-ol, 5-(1.1-dimethylethyl)-), and the chlorine derivative (Butyl(2-chlorocyclohexyl) methyl phthalate/or butyl phthalate (2-clorociclohexil) methylic). Noteworthy, the only halogenated derivative of the series extracted from the teak wood, Butyl(2-chlorocyclohexyl) methyl phthalate, is found only in ethyl alcohol and was highlighted in the EDX spectra ([23]).
Conclusions
The results obtained by two instrumental techniques (SEM-EDX and GC + MS) in order to identify the organic compounds with insect-fungicidal activity present in teak wood (Tectona grandis), separated by extraction with organic solvents, the following conclusions can be drawn:
- teak wood is part of the group of exotic species that are very resistant to biotic attacks, containing a large number of bioactive principles with insect-fungal activity, which so far are not sufficiently known;
- for their extraction from fine powder of old teak wood from 40-year-old trees, a working protocol was elaborated on the basis of preliminary experiments using a large number of organic solvents, which allowed to select the following four systems: isopropyl alcohol, ethyl alcohol, acetone and a mixture of acetone and isopropyl alcohol, with extraction capacity increasing in the above order;
- each extraction system presented a different selectivity for separating the bio-active principles, as follows: the extract in acetone separated eight organic compounds, the one in ethyl alcohol also eight, the acetone-isopropanol mixture four and only two in isopropanol;
- of the four extraction systems, the most efficient was acetone and the acetone-isopropyl mixture;
- of the eight organic compounds identified in the acetone extract, three are among the eight extracted in ethyl alcohol and four in the acetone-isopropyl alcohol mixture;
- of the two organic compounds identified in isopropyl alcohol, the first (2H-Naphtho [2.3-b] pyran-5.10-dione, 2.2-dimethyl-) is also found in the ethyl alcohol extract, and the second (9.10-anthracendion, 2-methyl-) is found in all four extracts;
- acetone selectively extracts three derivatives that are not found in other extracts (1H-Cycloprop[e]azulene, decahydro-1.1,7-trimethyl-4-methylene-, [1aR- (1a.alpha., 4a.beta.,7alpha.,7a)], naphthalene, 1.2,4a, 5.6,8a-hexahydro-4.7-dimethyl-1- (methylethyl)- and .alpha-Calacoren), and ethyl alcohol two (butyl (2-chlorocyclohexyl)) phthalate methyl and [1.1’-biphenyl] -2-ol, 5- (1.1-dimethylethyl)-);
- of the compounds extracted using the four selected organic solvent systems, the most active are: anthraquinones (9, 10-Anthracenedione, 1-methyl-; 9.10-Anthracenedione, 1-methyl-; 2- (Hydroxymethyl) anthraquinone; 2-Methyl-anthraquinone), naphthalenes (naphthalene, 1, 2.4a,5.6,8a-hexahydro-4.7-dimethyl-1-(methylethyl)) - (1.alpha, 4a.alfa, 8a.alfa); 2H-Naphtho [2.3-b] pyran-5.10-dione, 2.2-dimethyl-), bisphenol ([1.1’-biphenyl] -2-ol, 5- (1.1-dimethylethyl)-) and the chlorinated derivative (butyl (2-chlorocyclohexyl) methyl phthalate), the latter being found only in ethyl alcohol;
- of the four extracts the most indicated to be used in the preservation treatments of old wooden artifacts, with active biotic attack, is the concentrated extract in ethyl alcohol, which has the lowest toxicity and which extracts the chlorinated compound, the most effective in the biocide treatments.
References
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Faculty of Geography and Geology, Doctoral School of Geosciences, Alexandru Ioan Cuza University of Iasi, 20A Carol I St., Corp B, 700506 Iasi (Romania)
Viorica Vasilache 0000-0002-8866-3460
Institute of Interdisciplinary Research, ARHEOINVEST Center, Alexandru Ioan Cuza University of Iasi, 11 Carol I St., 700506 Iasi (Romania)
Faculty of Medicine and Pharmacy, “Dunarea de Jos” University of Galati, 35 Al.I. Cuza St., 800216 Galati (Romania)
Diana Iliescu Bulgaru 0000-0002-0847-8055
“Grigore T. Popa” University of Medicine and Pharmacy of Iasi, Institute of Forensic Medicine Iasi, 4 Buna Vestire St., 700455 Iasi (Romania)
Andrei Victor Sandu 0000-0002-9292-749X
Faculty of Material Science and Engineering, Gheorghe Asachi Technical University of Iasi, 41 D. Mangeron St., 700050 Iasi (Romania)
National Institute for Research and Development for Environmental Protection INCDPM, 294 Splaiul Independentei, 060031 Bucharest (Romania)
Corresponding author
Paper Info
Citation
Colbu DE, Sandu I, Vasilache V, Earar K, Paraschiv ED, Sandu IG, Iliescu Bulgaru D, Sandu AV (2021). Study on the chemical composition of teak wood extracts in different organic solvents. iForest 14: 329-336. - doi: 10.3832/ifor3717-014
Academic Editor
Luigi Todaro
Paper history
Received: Dec 09, 2020
Accepted: May 06, 2021
First online: Jul 09, 2021
Publication Date: Aug 31, 2021
Publication Time: 2.13 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2021
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 40580
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 33864
Abstract Page Views: 2960
PDF Downloads: 3054
Citation/Reference Downloads: 5
XML Downloads: 697
Web Metrics
Days since publication: 1694
Overall contacts: 40580
Avg. contacts per week: 167.69
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2021): 4
Average cites per year: 0.80
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Compositions of compounds extracted from thermo-treated wood using solvents of different polarities
vol. 10, pp. 824-828 (online: 25 September 2017)
Research Articles
Characterization of VOC emission profile of different wood species during moisture cycles
vol. 10, pp. 576-584 (online: 08 May 2017)
Research Articles
NIR-based models for estimating selected physical and chemical wood properties from fast-growing plantations
vol. 15, pp. 372-380 (online: 05 October 2022)
Research Articles
Validation of models using near-infrared spectroscopy to estimate basic density and chemical composition of Eucalyptus wood
vol. 17, pp. 338-345 (online: 03 November 2024)
Research Articles
Identification of wood from the Amazon by characteristics of Haralick and Neural Network: image segmentation and polishing of the surface
vol. 15, pp. 234-239 (online: 14 July 2022)
Research Articles
Physical, chemical and mechanical properties of Pinus sylvestris wood at five sites in Portugal
vol. 10, pp. 669-679 (online: 11 July 2017)
Research Articles
Modifying harvesting time as a tool to reduce nutrient export by timber extraction: a case study in planted teak (Tectona grandis L.f.) forests in Costa Rica
vol. 9, pp. 729-735 (online: 03 June 2016)
Research Articles
Examining the evolution and convergence of wood modification and environmental impact assessment in research
vol. 10, pp. 879-885 (online: 06 November 2017)
Research Articles
Mechanical and physical properties of Cunninghamia lanceolata wood decayed by brown rot
vol. 12, pp. 317-322 (online: 06 June 2019)
Research Articles
The potential of using xylarium wood samples for wood density calculations: a comparison of approaches for volume measurement
vol. 4, pp. 150-159 (online: 11 August 2011)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword