Compositions of compounds extracted from thermo-treated wood using solvents of different polarities
iForest - Biogeosciences and Forestry, Volume 10, Issue 5, Pages 824-828 (2017)
doi: https://doi.org/10.3832/ifor2360-010
Published: Sep 25, 2017 - Copyright © 2017 SISEF
Research Articles
Collection/Special Issue: COST action FP1407
Understanding wood modification through an integrated scientific and environmental impact approach
Guest Editors: Giacomo Goli, Andreja Kutnar, Dennis Jones, Dick Sandberg
Abstract
How well modified wood products perform may be influenced by their chemical compositions. Wood extractives are nonstructural constituents, many with specific biological properties, which affect the color, fragrance, hygroscopicity, durability, and acoustic properties and the drying and adhesion processes of wood. However, incomplete information is available on the extraction techniques and potential use of extractives as value-added chemical products. The main goal of this research was to explore the effects of thermo-vacuum treatment of Deodar cedar (Cedrus deodara Roxb.) and Italian alder (Alnus cordata Desf.) woods on the content and composition of extractives. Solvents with different polarities were used, including water, hexane, dichloromethane, methanol, and a benzene/ethanol mixture. Component groups in extracts were determined by gas chromatography in combination with mass spectrometry. Regardless of the treatment and solvent, the most representative extracts to be obtained from alder were acids/esters, whereas hydrocarbons were most frequently obtained from cedar. Our results revealed an interesting differential species-specific effect of solvents on the composition of extracts. Aside from benzene/ethanol, greater amounts of extracts were obtained from treated than from untreated alder, whereas the opposite was true for cedar, aside from methanol.
Keywords
Introduction
In the last decade several research groups have developed heat-treatment processes to create wood that would be suitable for industrial application. Heat treatment is an effective method to improve wood properties (e.g., performance, dimensional stability, durability, color) by changing low-value species into higher value materials without the use of chemical additives ([8]). However, heat treatment can also have downsides, such as a reduction in wood strength ([17]).
Many changes of wood properties are related to modifying of the wood’s physical and chemical compositions. Heating alters the microstructure and cell-wall components of wood, among other changes. Chemical modifications begin with the deacetylation of hemicelluloses followed by depolymerization, which leads to low-mass extractable compounds ([29], [22], [23]). Carbohydrate dehydration reduces the content of hydroxyl groups to form aldehydes, such as furfural and hydroxymethylfurfural from pentoses and hexoses, respectively ([36]). Thermal treatment also affects wood characteristics by causing the progressive degradation of cellulose and lignin ([30], [38], [3], [15]).
Extractives represent the nonstructural chemical components of plants. They are mainly produced in the heartwood in response to environmental stress ([35]). Extractives may influence the performance of wood-based products by affecting the decay, color, odor ([26]), as well as gluing and finishing of wood ([37]). During heat treatment, the most volatile extractives may leave the wood or be degraded ([7]), with new chemical compounds being obtained, e.g., due to polymer degradation ([6]). Researchers have identified and analyzed numerous extracts, representing many classes of organic compounds, from different trees and plant materials ([14], [12]). Extractive composition is highly variable both within and among species ([28]), and it depends on the age, seasonality, and location of the tree ([2]). In addition, the variability of extractives obtained from different chemical substrates presents an enormous challenge in terms of standardization.
Extractives can be drawn from wood by different approaches, and specific methods have been applied to influence the yield and type of extracted compound. Currently, the most commonly used extraction techniques require either the Soxhlet apparatus with solvents or autoclaving with hot water as a simple green method. Extractives are generally classified as water-soluble, toluene-ethanol-soluble, or ether-soluble, according to the extractive solvent. The amount of extractive compound dissolved in each solvent will differ, and the choice of the appropriate solvent will depend on the application. Organic solvents are able to extract resin compounds, whereas cold and hot water are useful to extract phenolic compounds, carbohydrates, glycosides, and soluble salts.
Promising evidence from experimental studies has highlighted that different nonpolar and polar solvents can be used to isolate different types of extractives from woods ([25]). Nevertheless, the effect of solvent polarity on extractive phases is not well understood, nor are the differences in extractive yields between heat-treated hardwood and softwood. Therefore, in the present study we performed qualitative and quantitative analyses of extracts obtained from untreated and thermo-vacuum-treated Deodar cedar (Cedrus deodara Roxb.) and Italian alder (Alnus cordata Desf.) woods. We examined species-specific changes in the amounts and compositions of extracts as a function of (i) the solvent used in the laboratory, and (ii) the treatment applied to the wood material.
Materials and methods
Wood material
Boards (50 × 6 × 180 mm) of alder and cedar woods were dried under vacuum to a moisture content of 0%. Wood samples were firstly dried for 4 h in vacuum conditions (185-200 mbar) at a temperature of 90 °C. Then, thermal treatment was done at 200 °C for 4 h in a thermo-vacuum cylinder (WDE-Maspell s.r.l., Italy). Further information on the technology can be found in Ferrari et al. ([13]).
Determination of extractive compositions
Soxhlet method
Treated and untreated wood samples were powdered and extracted in a Soxhlet apparatus by using the TAPPI test method T204 ([34]). This method can be used to determine the amount of solvent-soluble nonvolatile material in wood. One gram of milled wood sample was extracted by using two different solvent systems: a 2:1 mixture of 99% benzene: 96% ethanol, and different organic solvents with increasing polarity.
For extraction, the Soxhlet apparatus was used with 300 ml of a 2:1 mixture of benzene: ethanol and 1 g of milled wood for 7 h. One gram of milled wood was sequentially extracted by solvents with increasing polarity (hexane < dichloromethane < methanol) for 7 h per solvent. The extraction apparatus consisted of a 500-ml flask, Soxhlet tube, and 300-mm Allihn condenser. Samples were put in cellulose thimbles (33 × 80 mm) of medium porosity. After extraction, the solution was dried in a previously weighed 25-ml flask, by using a rotary evaporator connected to a vacuum pump (Vacuumbrand PC3001). Extraction percentage was obtained by weighing the flask containing the residue and comparing the weight to that of the initial wood. The weight of each dried extract was measured by using a Gibertini E42 analytical balance (precision ± 0.1 mg).
Autoclave method
Treated and untreated wood samples were split into small pieces and extracted with water as solvent in a Vapor Matic 770 sterilization autoclave. This completely automatic and thermoregulated autoclave system is equipped with a microprocessor, which permits time and temperature programming. The following autoclave cycle was used: 121 °C, 1 atm, 20 min. At the end of the process, the solution was dried by a lyophilization. The extraction percentage was calculated as described above.
Gas chromatography-mass spectrometry (GC-MS)
GC-MS analyses were performed on an HP 6890 GC system equipped with an HP 5963 MS selective detector, with a high-temperature capillary column (HP-5MS, 30 m × 0.25 mm I.D., 0.25-μm film thickness; J&W Scientific, CA, USA) and helium as carrier gas. Samples were injected directly into the column at a temperature of 80 °C. After injection, the temperature was held at 80 °C for 3 min, and then heated to 250 °C at a rate of 20 °C min-1 and held for 20 min. Compounds were identified by computer comparison of the mass spectra with NIST libraries and by mass fragmentation patterns.
Results and discussion
Extractive yield
Amounts of extracts obtained from alder wood by any solvent, excluding benzene/ethanol, were smaller for untreated than for thermo-treated wood (Tab. 1). Conversely, amounts of extracts obtained from cedar wood by any solvent, excluding methanol, were not higher for thermo-treated than for untreated wood. These data are in agreement with the reported greater cross-linking of thermo-treated wood compared to untreated wood ([3]). During thermo-treatment, polycondensation reactions with other cell-wall components occur, resulting in further cross-linking. These reticulation reactions make it difficult to extract chemical compounds from wood.
Tab. 1 - Extraction yield in percentage (%) for untreated (C) and thermo-treated (TH) wood species.
| Extraction Method | Italian alder | Deodar cedar | ||
|---|---|---|---|---|
| C | TH | C | TH | |
| Water | 0.8 | 1.6 | 2.9 | 1.5 |
| Hexane | 0.5 | 0.8 | 2.6 | 1.0 |
| Dichloromethane | 0.5 | 1.3 | 1.3 | 1.0 |
| Methanol | 4.5 | 7.1 | 3.4 | 4.8 |
| Benzene/Ethanol | 8.2 | 2.2 | 10.8 | 9.0 |
For both untreated wood species, greater amounts of extracts were obtained by the benzene/ethanol blend than by solvents with different polarities.
Larger amounts of polar extracts were obtained with the methanol solvent for thermo-treated wood (Tab. 1), consistent with findings in the literature ([25]). During thermal treatment, hemicellulose undergoes reactions that produce polar compounds that are easily extracted by polar solvents such as methanol.
Chemical compounds in alder wood
Tab. S1 (Supplementary material) summarizes characteristics of the GC-MS peaks of untreated and thermo-treated alder wood samples subjected to different techniques and solvents. Differences in the chemical compositions of extracts obtained from untreated vs. treated samples can be attributed, in large part, to the heating process, which modifies the chemical composition of wood by degrading wood biopolymers through pyrolysis and thermolysis ([5]).
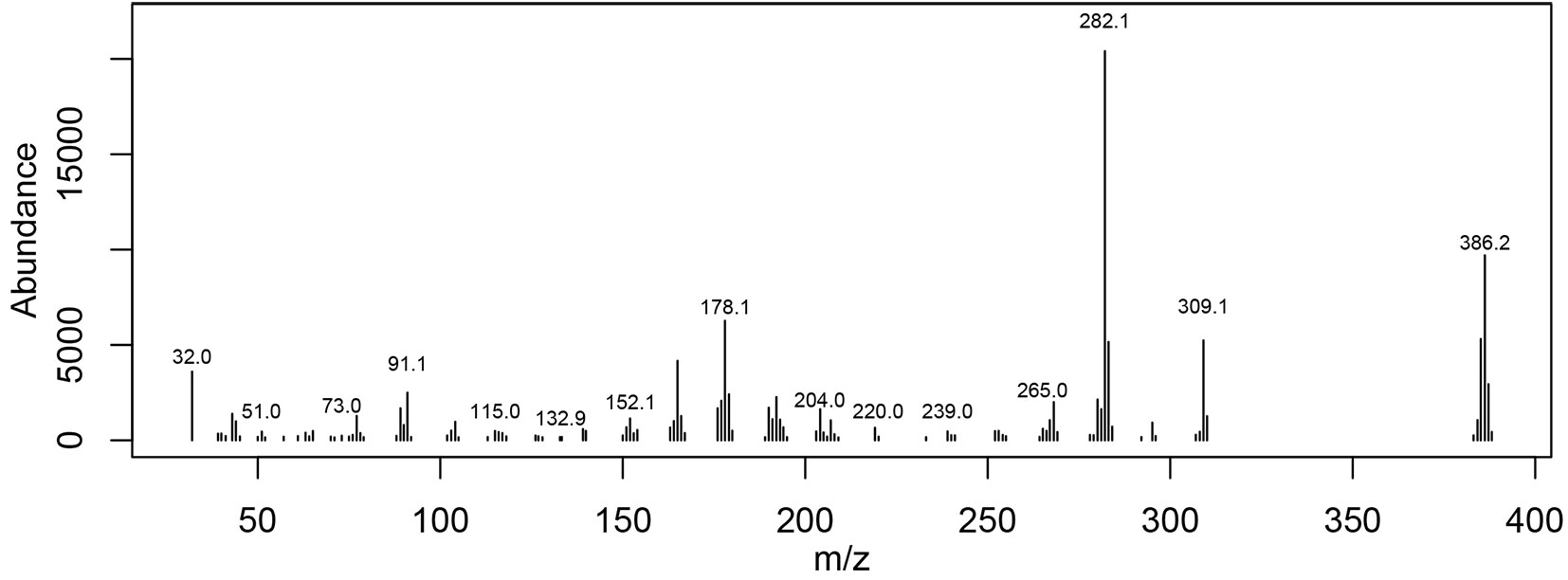
Different results were obtained from consecutive extractions of untreated vs. thermo-treated samples. For thermo-treated alder wood extracted by using hexane, only one relevant peak was obtained, which was attributed to isobutyl octyl phthalate. Although large quantities of compounds were extracted from thermo-treated samples by methanol, identifying these compounds was difficult (Fig. 1). Using the autoclave method and water, we were able to identify a large number of compounds from thermo-treated alder. When using the Soxhlet technique, the quantities of extracts obtained from both untreated and thermo-treated samples appeared to be related strictly to the type of solvent and its polarity.
We observed differences among the results in the classes of extracts obtained (row in bold, Tab. S1 in Supplementary material). Alkene compounds were obtained from untreated samples by using the Soxhlet apparatus. When using the water technique, alkenes were obtained only from thermo-treated samples. Extraction of untreated samples by methanol and benzene/ethanol resulted in extracts with longer aliphatic chains. Both dichloromethane and benzene/ethanol solvent yielded higher amounts of carbonyl and phenolic compounds from thermo-treated samples. These compounds could have resulted from the decomposition of volatile organic compounds (VOCs), terpenes, polyosides, and lignins ([10], [11], [21]).
Fatty acids were generally extracted from untreated wood by using hexane and methanol. However, with water and the benzene/ethanol mixture, fatty-acid derivatives (octadecanoic acid and 1-methylethyl dodecanoate, glycerol 1.2-diacetate, respectively) were extracted from thermo-treated samples. Octadecanoic acid is a saturated fatty acid, which may have resulted from the hydrolysis or degradation of fatty acids during heat treatment.
Building blocks of some wood structural components were obtained only after thermo-treatment, excluding diphenyldisulfide, extracted from untreated wood with methanol. Most of building block were obtained when using dichloromethane as solvent. No building block were obtained when using hexane as solvent. These compounds could derive from the thermal degradation of other structural components of wood.
Among the extracts from heat-treated alder, vanillin is an important extract that was obtained by the benzene/ethanol mixture. Vanillin is commonly employed in the biological and biotechnological industries as a flavoring agent in foods and pharmaceuticals products and as a fragrance in perfumes. According to Fache et al. ([9]), vanillin has the potential to be a key intermediate for the synthesis of biopolymers. Syringaldehyde (4-hydroxy-3.5-dimethoxybenzaldehyde) is similar in structure to vanillin and has comparable uses ([4]). This compound was extracted from thermo-treated samples by using water or benzene/ethanol as solvent. Syringaldehyde has antioxidant, antioncogenic, antimicrobial, and antifungal activities, which make the compound important commercially ([16]). Another relevant compound, methoxycinnamaldehyde, was extracted by dichloromethane from thermo-treated alder samples. This compound is related to ferulic acid, an important precursor in the manufacture of aromatic compounds ([18]). The aforementioned compounds were not found in untreated alder samples, reflecting the importance of thermo-treatment in generating mainly polar compounds ([25]).
Chemical compounds in cedar
Tab. S2 (Supplementary material) summarizes the characteristics of the GC-MS peaks of untreated and thermo-treated cedar wood subjected to different techniques and solvents. In contrast to alder, cedar produced many similar extractive compounds from both untreated and thermo-treated samples. This finding suggests that cedar is more heat resistant and less degradable when subjected to heating. We were able to identify predominant compounds from thermo-treated vs. untreated cedar samples by using the autoclave method with water. As for alder when the Soxhlet apparatus was used for extraction, the amounts of extracts from both thermo-treated and untreated samples depended on the type of solvent used and its polarity.
Acids and esters were extracted from cedar mainly by using water or the benzene/ethanol mixture with thermo-treated samples (row in bold, Tab. S2). While benzenepropanoic acid, 3.5-bis(1.1-dimethylethyl)-4-hydroxy-methyl ester (methanol), bis(2-ethylhexyl) phthalate, and isobutyl-octyl phthalate (hexane) were extracted from untreated samples. Alkene or alkene-like compounds were found in all extracts from cedar, regardless of heat treatment or solvent. Alkenes were most abundantly extracted when using benzene/ethanol with untreated and thermo-treated samples, or using water with thermo-treated samples.
Surprisingly, we found that long-chain compounds were extracted from thermo-treated samples. These extracts could be a building block component of the wood and not derived from thermal degradation or other alkenes.
Carbonyl compounds were obtained when thermo-treated samples were extracted with water, while phenylbenzeneacetic acid, methyl ester were obtained from untreated sample. Fatty acids were obtained only with water. Two different saturated fatty acids were obtained from untreated vs. thermo-treated samples, with the fatty acid obtained from the thermo-treated samples having fewer carbon atoms.
Compounds belonging to the organic building block class were obtained when thermo-treated samples were extracted with water, hexane, dichloromethane, or methanol. For untreated samples, 3.5-dimethoxy-4-hydroxycinnamaldehyde was extracted by using water, and diphenyldisulfide was extracted by using methanol.
Some potentially valuable bioactive compounds were extracted from thermo-treated samples. For example, the terpene artumerone was extracted from thermo-treated samples by using hexane and dichloromethane solvents. Artumerone is a compound found in curcumin, a perennial plant cultivated throughout tropical Asia, India, and China. This compound has been investigated in clinical trials for its potential therapeutic properties, including anticancer activity ([32], [24], [31]). Artumerone acts through various mechanisms, including the induction of apoptosis, inhibition of angiogenesis, and modulation of tumor suppressor genes ([1], [20]). The organic building block butyl citrate was extracted with water from thermo-treated samples. This compound has possible use as a phthalate substitute plasticizer ([33]). For the alkene class, different benzocycloheptene compounds were extracted from untreated and thermo-treated samples with only organic solvents. Benzocycloheptene has antioxidant properties that make the compound important for humans and animals ([19], [27]).
Conclusions
This paper describes the analysis of compounds extracted from untreated and thermo-treated alder and cedar woods, by using polar and nonpolar solvents. The processes applied, wood material, and equipment used all affect the species and amounts of extracts. We also found that for both wood species the extractive composition was strongly related to the polarity of the extractive solvent.
The preliminary results showed that different types of polyaromatic compounds were produced from thermo-treated woods, with some difference between species, which was probably due to their different wood structures and thermal reaction rates.
Higher quantities of extracts were obtained from thermo-treated than from untreated alder wood samples, whereas the amounts of extracts obtained from untreated cedar wood were greater than those obtained from treated wood aside from methanol. For cedar, cross-linking probably takes place after polycondensation reactions between the chemical components of wood, which makes it difficult to obtain extractives.
For both alder and cedar, lower total percentages of compounds could be identified from untreated vs. treated wood. Polar components were the most commonly obtained extracts from cedar and alder thermo-treated woods. This result probably arises from the fact that thermo-transformation generates mainly polar products.
Different compounds were extracted from treated vs. untreated samples mainly in case of alder.
Extracts obtained from alder wood predominantly belonged to the acid/ester class, whereas extracts from cedar wood mostly belonged to the terpene and hydrocarbon classes.
We found many extracts that might have industrial applications as antimicrobial and antifungal agents or as important precursors in the manufacture of other aromatic compounds. Appropriate solvent selection is crucial for the extraction process and will depend on the intended application. To understand the appropriate procedure, a detailed analysis should be done that considers the chemical properties and final uses of the extracts, as well as the structural differences between wood species.
Acknowledgements
The authors would like to acknowledge the contribution of the COST Action FP1407. The Ph.D. program in “Agricultural, Forest and Food Science” at the University of Basilicata, supported T. Lovaglio.
References
Gscholar
Online | Gscholar
Online | Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Angelo Rita
Luigi Todaro
Scuola di Scienze Agrarie, Forestali, Alimentari e Ambientali, Università della Basilicata, v.le dell’Ateneo Lucano 10, 85100 Potenza (Italy)
Dipartimento di Scienze, Università della Basilicata, v.le dell’Ateneo Lucano 10, 85100 Potenza (Italy)
Corresponding author
Paper Info
Citation
Lovaglio T, D’Auria M, Rita A, Todaro L (2017). Compositions of compounds extracted from thermo-treated wood using solvents of different polarities. iForest 10: 824-828. - doi: 10.3832/ifor2360-010
Academic Editor
Giacomo Goli
Paper history
Received: Jan 17, 2017
Accepted: Jul 04, 2017
First online: Sep 25, 2017
Publication Date: Oct 31, 2017
Publication Time: 2.77 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2017
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 50571
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41806
Abstract Page Views: 3426
PDF Downloads: 4126
Citation/Reference Downloads: 17
XML Downloads: 1196
Web Metrics
Days since publication: 3063
Overall contacts: 50571
Avg. contacts per week: 115.57
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2017): 16
Average cites per year: 1.78
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Short Communications
Influence of thermo-vacuum treatment on bending properties of poplar rotary-cut veneer
vol. 10, pp. 161-163 (online: 13 June 2016)
Research Articles
Hardness and contact angle of thermo-treated poplar plywood for bio-building
vol. 14, pp. 274-277 (online: 29 May 2021)
Research Articles
Improving dimensional stability of Populus cathayana wood by suberin monomers with heat treatment
vol. 14, pp. 313-319 (online: 01 July 2021)
Technical Notes
Improving impregnation properties of fir wood to acid copper chromate (ACC) with microwave pre-treatment
vol. 8, pp. 89-94 (online: 01 April 2014)
Review Papers
Wood modification technologies - a review
vol. 10, pp. 895-908 (online: 01 December 2017)
Short Communications
Changes in Populus sp. wood subjected to heat treatment: anatomy and silica content
vol. 18, pp. 223-226 (online: 09 August 2025)
Research Articles
Life cycle assessment of tannin extraction from spruce bark
vol. 10, pp. 807-814 (online: 25 September 2017)
Research Articles
Assessment of timber extraction distance and skid road network in steep karst terrain
vol. 10, pp. 886-894 (online: 06 November 2017)
Research Articles
Pre-treatment with sodium silicate, sodium hydroxide, ionic liquids or methacrylate resin to reduce the set-recovery and increase the hardness of surface-densified Scots pine
vol. 10, pp. 857-864 (online: 26 October 2017)
Research Articles
Thermo-modified native black poplar (Populus nigra L.) wood as an insulation material
vol. 14, pp. 268-273 (online: 29 May 2021)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword