Is there an effect of storage depth on the persistence of silver birch (Betula pendula Roth) and rowan (Sorbus aucuparia L.) seeds? A seed burial experiment
iForest - Biogeosciences and Forestry, Volume 14, Issue 3, Pages 224-230 (2021)
doi: https://doi.org/10.3832/ifor3685-014
Published: May 06, 2021 - Copyright © 2021 SISEF
Research Articles
Abstract
Sorbus aucuparia L. (rowan) and Betula spp. (birch) are the most common of the early successional pioneer tree species in central Europe with the ability to form a soil seed bank. Little is known about the reasons for the high variations observed in the persistence in the soil of rowan and birch seeds. The objective of this study was to assess the ability of buried birch and rowan seeds to form short-term persistent soil seed banks and to analyse the influence of burial depth on seed persistence. An artificial seed burial experiment was initiated to study the persistence of birch seeds and rowan seeds, with and without pulp, stored at depths of 2, 5 and 10 cm in mineral soil over 2.5 years. The predicted maximum storability periods for buried birch seeds was 13 years, compared to 4.5 years for rowan seeds with pulp and 3.5 years without pulp. The lower storage capacity of rowan seeds was demonstrated by germinations in the darkness within soil of 3-22% of seeds without pulp and 4-48% of seeds with pulp. Germination percentages of birch and rowan with and without pulp did not differ between depths. Only burial duration had an effect for either tree species. Birch and rowan seeds are able to form short-term persistent soil seed banks. Birch accumulates a seed reserve in the soil over time, until a change in conditions conducive to germination occurs, while rowan seeds germinate promptly after overcoming seed dormancy. The pulp provides no benefits in relation to the persistence of rowan seeds; rather, it appears to act as a physical inhibitor of germination. Therefore, annual input of fresh seeds is required for the success of rowan. Seed input every few years seems sufficient to guarantee a minimum number of viable birch seeds.
Keywords
Soil, Propagule Bank, Seed Longevity, Germination Within Soil, Pioneer Trees, Dormancy
Introduction
Soil seed banks are reservoirs of viable seeds stored in the soil ([10]). The adaptive value of a soil seed bank is to prevent germination under unfavourable site conditions ([11], [40]) and thus enable a seed crop to germinate over an extended period of time, i.e., “dispersal in time” ([18]). Once optimal site conditions arise, for example, following a storm, the buried seed reserves make feasible a fast colonisation of the disturbed site. Soil seed banks are an important component of the regeneration of disturbed areas, but in general they also are an important driver of succession dynamics in ecosystems ([26], [10]).
Soil seed banks mainly contain light-demanding species and disturbance-adapted species of early or middle successional stages with small and compact seeds ([45], [22]). The largest group of light-demanding species in forest soils is herbaceous plants ([29], [55]), but there are also light-demanding, early successional pioneer tree species ([15], [26]). Some tropical pioneer tree species are known for the long-term persistence of seeds in soil ([15]) and also some early successional pioneer species in temperate woodlands of America, for example, Prunus pensylvanica (up to 30 years) and Robinia pseudoacacia (from just a few years to 40 years - [52], [2], [26], [30]). In the temperate forests of Europe, the most common woody colonisers of disturbed sites are the pioneer species Betula spp., Salix spp., Populus spp. and Sorbus aucuparia L. These species have received little consideration in earlier soil seed bank studies. Rowan (Sorbus aucuparia) and birch (Betula spp.) were chosen for this study because these are the most common pioneer tree species in central Europe with the ability to form a soil seed bank ([50]). Hill ([25]) and Erlbeck ([17]) espoused the opinion that rowan seeds remain viable in the soil for up to five years due to embryo and seed coat dormancy ([39]). However, Tiebel et al. ([50]) rarely found rowan seeds in soil seed banks in forests and assumed that the persistence of rowan seeds in the soil might be less than one year ([16]). The assumptions about the duration of birch seed storage in the soil vary from less than one year to more than 13 years ([46], [13]). The high degree of variation of rowan and birch seed persistence begs the question whether contrasting soil moisture or burial depth may lead to these differences. It is well known that in moist soil and flooded areas the seeds of dry-habitat species lose their viability more rapidly due to pathogen attack and predation in wet than in dry soil ([46], [7], [26], [53]). Generally, little is known about the storability of buried tree seeds in the soil as a function of mineral soil depth ([52], [53]). Zhang et al. ([54]) and Mennan ([31]) observed for some weed species a trend of reducing seed viability loss with increasing soil depth. This evidence is lacking for tree species of temperate woodlands, thus it deserves specific investigations. In the case of rowan another question arises: does the pulp have an influence on seed longevity in soil by triggering fruit-induced secondary dormancy (Devillez 1979, cited in [39], [8])? Granström ([23]) observed rowan seed germination within soil before excavation of the buried seeds.
To answer these questions, we performed an artificial seed burial experiment aimed to study whether birch and rowan establish a short-term persistent soil seed bank. The effect on seed viability of different storage depths within the mineral soil was also assessed for birch seeds and rowan seeds with and without pulp. The following hypotheses were formulated at the outset of the study: (1) birch seeds remain viable in the soil longer than rowan seeds with and without pulp, because rowan seeds will germinate within the soil during the storage period; (2) the pulp surrounding rowan seeds offers benefits in terms of seed longevity in the soil, because the pulp prevents early germination of seeds within soil; (3) rowan and birch seed germination percentages shows no differences between deep and shallow soil.
Materials and methods
Characterisation of study species
Silver birch (Betula pendula Roth) is a light-demanding pioneer tree species, which produces small and light wind-dispersed seeds with wings ([35]). The seeds are 1.5-2.0 mm in size ([12]), weigh 0.12-0.25 mg ([3]) and are mainly dispersed from June to November ([35]) over mean distances of 40-400 m ([27], [51]). Birch seed trees usually have a mast year every 2-3 years, at which time seed viability is increased ([41]). The light-demanding seeds commonly germinate in spring, as snow is melting and the temperature increasing. Birch seeds exhibit no dormancy but the germination percentage benefits from moist pre-chilling of seeds. More favourable germination conditions are temperatures between 20-30 °C and a long-day photoperiod ([35], [3], [32]). Birches are tolerant to different soil conditions, therefore, the seeds can germinate and establish on sites with soil pH between 3.5 and 7.8 ([3]). Litter and soil cover hinder the germination of birch seeds ([35], [3]).
Rowan (Sorbus aucuparia L.) is also a pioneer tree species. It is shade-tolerant when young and becomes increasingly light-demanding with age ([37]). Rowan trees produce red fruits with small seeds (1.2-4.0 mm in size - [12]), whose masses range between 1.15-4.14 mg ([39]). Mast years occur every 2-5 years ([42]). The fruits ripen from August to October and are endozoochorously dispersed by small mammals and birds ([39]). Birds, the main vectors of long distance seed dispersal, mostly defecate rowan seeds at distances of 40-100 m from the seed tree ([48], [57]). Embryo and seed coat dormancy are well documented for rowan. When the fruits are not eaten by animals, dormancy must be broken under natural conditions ([39]). A second period of dormancy can be caused by the temperature rising above 10 °C within a short period of time after the winter ([47]). Rowan seeds can germinate even at temperatures as low as 2 °C ([6]) to 5 °C ([24]). Germination can also occur in light as well as in darkness and at soil pH < 7.0 (Devillez 1979, cited in [39]).
Study area
The burial experiment was located at the colline and submontane belts of the Tharandter Forest (50° 57′ N, 13° 30′ E) in the German Federal State Saxony ([20]). The study area is situated between 200-460 m a.s.l., on the northern edge of the eastern Ore Mountains (Erzgebirge), at the transition from hill country to low mountain range ([20], [34]). The annual average temperature in the region varies between 7.3 and 7.7 °C and the mean annual precipitation ranges from 819 to 850 mm ([21]). The area is moderately subcontinental ([34]). The geology of the region has given rise to medium to deep brown soils that predominate on the forest sites, as well as dry sands and podzols with low nutrient contents and silty brown earths ([34], [44]). For the seed burial experiment, an old, pure coniferous forest in the Tharandter Forest with a closed canopy and no ground vegetation (only litter) was chosen. These coniferous forests are particularly exposed to storms and soil seed banks are important for natural regeneration. The soil type of the forest stand is pseudogley-brown earth, whose pH value generally varies between 3.5 and 5.5.
Experimental design and data collection
Birch (Betula pendula) seeds and rowan (Sorbus aucuparia) fruits were collected from mature seed trees on 2-12 September 2015, a few days before they were buried in the soil. At each of the three sampling locations in Germany (Tab. 1), 10 inflorescences each were harvested from three birch and three rowan seed trees. As the seeds and fruits were mixed, the seeds could not later be traced back to individual trees or locations.
Tab. 1 - Overview of birch and rowan seed collection.
| Species | Date of collection |
Landscape | Approximate location |
Seed trees |
Inflorescences per tree |
|---|---|---|---|---|---|
| Betula pendula | 02.09.2015 | Göttingen - road site | 51°33′ N - 09°56′ E | 3 | 10 |
| 07.09.2015 | Thuringian forest | 50°40′ N - 10°45′ E | 3 | 10 | |
| 10.09.2015 | Tharandter forest | 50°57′ N - 13°30′ E | 3 | 10 | |
| Sorbus aucuparia | 03.09.2015 | Göttingen - road site | 51°33′ N - 09°56′ E | 3 | 10 |
| 12.09.2015 | Thuringian forest | 50°40′ N - 10°45′ E | 3 | 10 | |
| 10.09.2015 | Tharandter forest | 50°57′ N- 13°30′ E | 3 | 10 |
A total of 400 fresh birch and rowan seeds were used to test the initial germination capacity, according to the methods of the International Seed Testing Association ([28]). Four germination samples (4 replicate with 100 seeds) were prepared by placing birch seeds in germinators on moist filter paper. The germinators were placed in a climatic chamber for 21 days under constant conditions (25 °C, 80% humidity, 16 h light and 8 h dark) and checked weekly. The rowan seeds were cold-wet stratified for 4 months at 5 °C in trays with moist sand and darkness before starting the germination test. After stratification the four trays (4 replicate with 100 seeds) were placed in the cold greenhouse for 28 days, where daily temperatures varied between 10-15 °C. The germination process was checked weekly.
On 25 September 2015, the birch and rowan seeds and fruits were inserted separately into 10 × 20 cm net bags filled with soil and sewn up (hereinafter referred to as seed sets). The bags had a mesh size of 1.5 × 1.5 mm. After six to twelve months the seams of the bags had opened and access to the seeds for mesofauna was no longer impeded. While removing rowan seeds from the pulp for the purposes of the experiment, it was possible to determine an average seed number per fruit and calculate the number of fruits required to give 50 seeds per bag. Each bag contained either 50 birch seeds, 50 rowan seeds or 18 rowan fruits (approx. 50 rowan seeds). On 25 October 2015, in total 90 bags were buried in mineral soil at depths of 2 cm, 5 cm and 10 cm at two separate plots in the same coniferous stand (8 m apart) to spread the risk of damage to the seeds or plots by mice or wild boars. After burial of the bags, the humus and litter layer were carefully restored to their prior state.
At intervals of six months between April 2016 and April 2018, seed sets (one bag per seed set, layer and plot) were removed from the soil to collect 100 seeds from each seed set and layer to test the viability of the stored seeds by germination tests. The “seedling emergence method” was used for both species ([19]), but it was adapted to the particular germination ecology of each species. The seeds in the bags with soil were filled separately into trays after each excavation. The trays with the birch seed sets were always placed in a climate chamber at a constant 25 °C, 80% relative humidity and 16 h lighting per day for three months to assess germination percentage. The trays with the rowan seed and rowan fruit sets were always placed in a greenhouse under varying temperatures, relative humidity and day-night light regime for one year. The mean day-night temperature ranged from -5 to 26 °C in winter and from 9 to 34 °C in summer, and the mean day-night humidity varied between 34% and 99% in winter and 51% and 100% in summer. The soil of all samples was kept continuously moist through regular watering in climate chamber and greenhouse. The frequency of watering depended on temperature, air humidity and sunlight in the greenhouse. In the first month, every week the number of successfully germinated seeds was recorded. Thereafter, germination was checked every 14 days.
Statistical analysis
The recorded germination percentages were not corrected for previous germination percentages and seed mortality or time intervals to represent the observed germination loss over the storage time of seeds in soil.
The aim of the study was to assess the effect of storage time and burial depth on the quantity of germinated seeds after excavation. Due to the nested experimental design a mixed modelling approach was used. The response variable (germination percentage) was not normally distributed, thus we used generalized linear mixed models (GLMM). The nested random effects in the model was the plot number and net bag number. The net bags were included as a random effect because each of the 50 seeds in a net bag were considered as one germination observation in the data frame. We tested separately for each tree species seed set whether the number of germinated seeds within a seed set depends on the fixed effects: storage time (continuous variable) and depth in the soil (3-level categorical variable). The interaction between the fixed effects was also tested. The “glmmADMB” package version 0.8.3.3 was applied to model the GLMM in R software version 3.3.2 ([38]). The package “glmmADMB” used the automatic differentiation model builder (ADMB) to fit the parameters ([9]). The advantages of ADMB are: (i) the range of distribution families; (ii) the range of link functions; and (iii) the use of the MCMC method (Markov chain Monte Carlo) to summarise uncertainties ([56], [9]). As seed germination is an absence-presence event, that is, the data distribution is binomial, the model link function was set to logit ([56]). Significant differences in all models were accepted at p<0.05. The necessary homoscedasticity of variance and normality were checked and confirmed with plots of residuals and quantiles from fitted GLMM. Overdispersion has no relevance in a binomial model with binary data.
The terms “initial germination capacity (%)” and “percentage of germination (%)” were used to delimit the initial germination percentage of the collected fresh birch and rowan seeds and the regularly recorded remaining viability percentage of buried seeds.
Results
Birch seeds
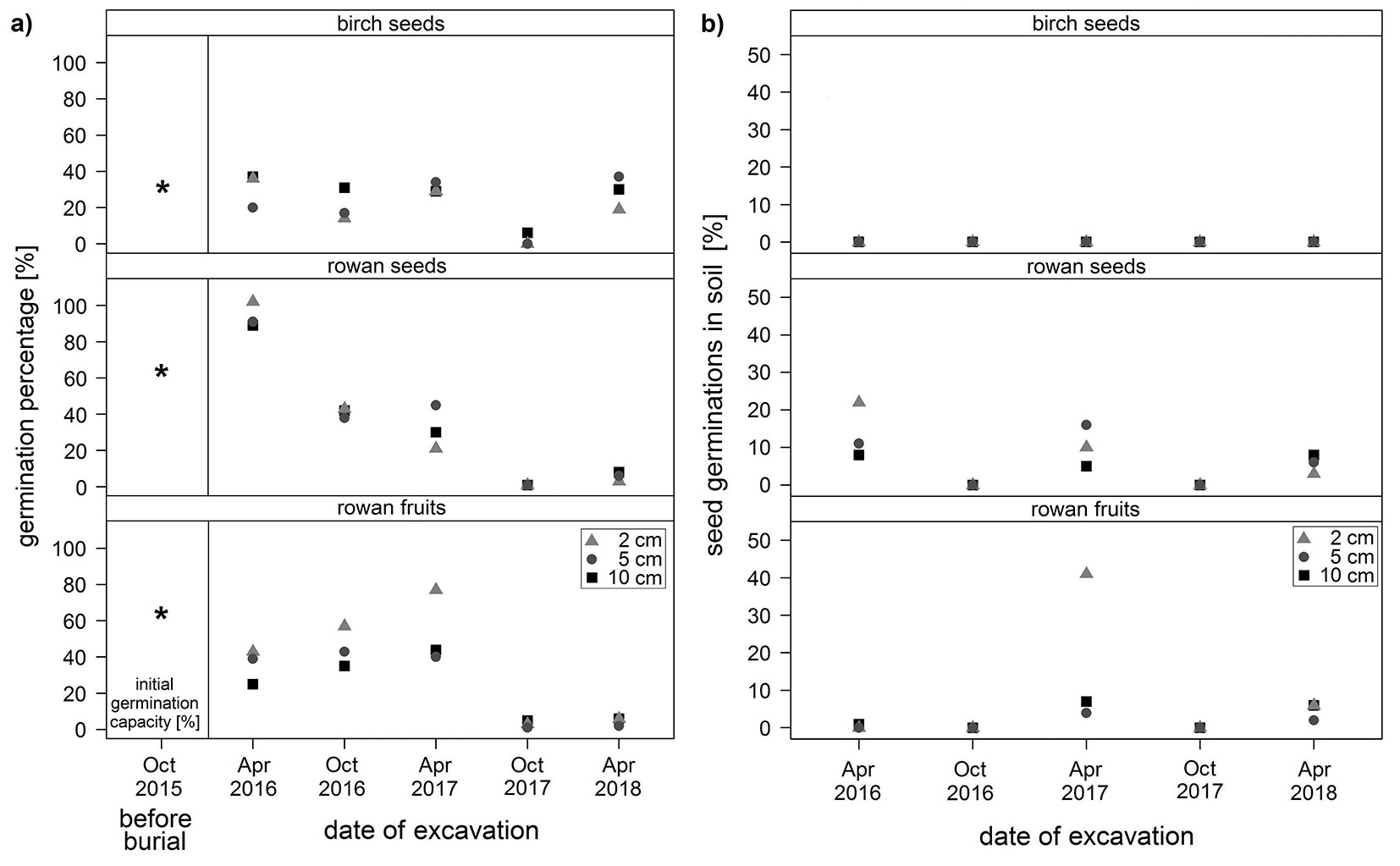
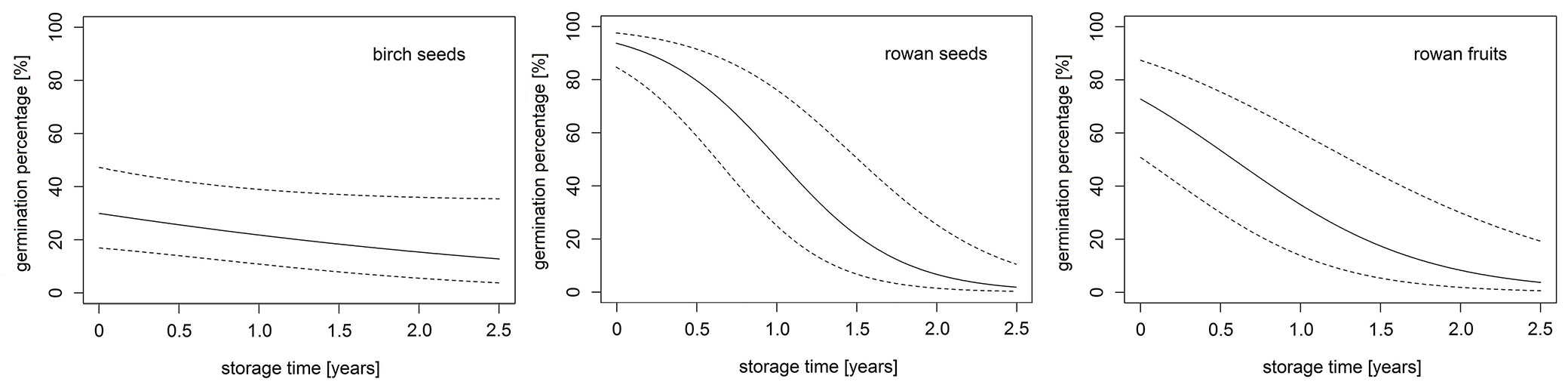
The initial germination capacity of freshly harvested birch seeds before burial was 32% (Fig. 1a). Over the period of storage in the soil, the percentage of germination varied between 0-37% without a detectable effect of storage depth (Fig. 1a, Tab. 2). The quantity of germinated seeds was always higher in spring (19-37%) than in autumn (0-31%). In April 2018, after 2.5 years in the soil, the percentage of germination was as high as at the beginning of the experiment. The GLMM results reveal a weak significant influence of storage time (p-value = 0.074), but there was no influence of burial depth or interaction between these two effects on seed germination (Tab. 2, Fig. 2). The model predicts that the birch seeds would have lost all viability after 13 years. No germination in soil was observed for birch (Fig. 1b).
Fig. 1 - (a) Percentage of germination (%) of birch seeds (top), rowan seeds (middle) and rowan fruits (bottom) stored in mineral soil at depths of 2, 5 and 10 cm in a coniferous forest. (b) Early seed germination within soil (%) relative to the total seeds buried in mineral soil at depths of 2, 5 and 10 cm for birch seeds (top), rowan seeds (middle) and rowan fruits (bottom) before excavation of the seed sets. The percentage of germination and the early seed germination within the soil were checked at intervals of 6 months from April 2016 to April 2018. Mature seeds and fleshy fruits were buried in October 2015 after the initial germination capacity (%) had been tested according to ISTA ([28]). Note the different scales on the y-axes.
Tab. 2 - GLMM results for the percentage of germination of the buried seed sets, stored over 2.5 years in mineral soil at depths of 2, 5 and 10 cm in two study plots in a coniferous forest. (f): fixed effects; (r): random effects; (ns): not significant; (SD): standard deviation; (plot): plot number; (net bag): number of the net bag for each plot and storage depth. The reference (intercept) is the seed set buried at depths of 2 cm in the mineral soil.
| Seed sets |
Effects | Estimate | Std. error | z-value | p-value | Variance | SD |
|---|---|---|---|---|---|---|---|
| birch seeds | f intercept | -0.917 | 0.401 | -2.29 | 0.022 * | - | - |
| f storage time | -0.213 | 0.119 | -1.79 | 0.074 | - | - | |
| f storage depth 5 cm | 0.036 | 0.258 | 0.14 | 0.888 ns | - | - | |
| f storage depth 10 cm | 0.161 | 0.257 | 0.63 | 0.530 ns | - | - | |
| r plot | - | - | - | - | 0.000 | 0.003 | |
| r net bag | - | - | - | - | 0.935 | 0.967 | |
| rowan seeds | f intercept | 2.698 | 0.530 | 5.09 | <0.001 *** | - | - |
| f storage time | -1.331 | 0.166 | -8.03 | <0.001 *** | - | - | |
| f storage depth 5 cm | 0.012 | 0.269 | 0.04 | 0.960 ns | - | - | |
| f storage depth 10 cm | 0.003 | 0.269 | 0.01 | 0.990 ns | - | - | |
| r plot | - | - | - | - | 0.000 | 0.006 | |
| r net bag | - | - | - | - | 1.620 | 1.273 | |
| rowan fruits | f intercept | 1.089 | 0.501 | 2.17 | 0.030 * | - | - |
| f storage time | -0.843 | 0.160 | -5.27 | <0.001 *** | - | - | |
| f storage depth 5 cm | -0.173 | 0.269 | -0.64 | 0.520 ns | - | - | |
| f storage depth 10 cm | -0.152 | 0.268 | -0.57 | 0.570 ns | - | - | |
| r plot | - | - | - | - | 0.000 | 0.005 | |
| r net bag | - | - | - | - | 1.546 | 1.244 |
Fig. 2 - GLMM predictions of germination percentage (%) for birch seeds (left), rowan seeds (center) and rowan fruits (right) in soil over the 2.5-year study period.
Rowan seeds
The initial germination capacity of freshly harvested rowan fruits was 64%. After the first winter period in soil, the percentage of germination of buried rowan seeds reached 89-99%, which clearly exceeded the initial germination capacity and was not observed in any other seed sets over the whole observation period (Fig. 1a). From April 2016 to April 2018, the number of germinated rowan seeds decreased significantly with increasing storage time (3-8%), which was confirmed by the model results (Tab. 2, Fig. 2). The predicted maximum storability period was 3.5 years. The model revealed no significant influence of storage depth and interaction between fixed effects.
Seeds germinated within the soil were observed during the excavation. The highest germination within the soil occurred in the first spring after burial and were exhibited by the seeds buried at 2 cm (22% - Fig. 1b). After 2.5 years of storage the germination at 2 cm decreased to 3%. At 10 cm soil depth the number of seeds germinated within the soil remained similar over time. The model results showed that overall there was no effect of storage time, storage depth, or any effect of their interaction, on seed germination within the soil (result not shown).
Rowan fruits
Rowan seeds with pulp exhibited an initial germination capacity of 64% in October 2015. After the first winter period, only 25-43% of the buried seeds with pulp germinated (Fig. 1a). The germination subsequently increased in all soil layers until April 2017 (40-77%) before most seeds in pulp lost viability after two years of storage in the soil (1-6%). The negative influence of storage time is also shown by the model results (Tab. 2, Fig. 2), which predict a complete loss of seed viability after 4.5 years in soil. The germination percentage at 2 cm soil depth was always higher than in the deeper soil layers, but the model results confirmed no significant differences in germination numbers on the basis of storage depth. No interactions between the fixed effects were detected.
The presence of germinated seeds in the soil was also observed for the rowan seeds with pulp prior to the excavation of the net bags. The first time this observation was made in the second spring after burial, when the germination within the soil reached 4-48%. The highest germination percentages within soil always occurred at 2 cm soil depth, after the pulp had begun to decompose (Fig. 1b). Rowan fruits buried at depths of 5 cm and 10 cm exhibited no morphological changes after the first winter, whereas at 2 cm the pulp was soft and had started decomposing. By the autumn 2016, no fruits were detected at 2 cm while in the deeper layer the pericarps were still hard and intact. In the second autumn (2017), no fruits were found in any layer. The model results revealed no significant influence of fixed effects on germination within soil.
Discussion
Seed persistence in soil
The results revealed a markedly longer duration of seed storability in soil for smaller birch seeds than for larger rowan seeds, either with or without pulp, which confirmed our first hypothesis. It is a well-established hypothesis that seed longevity is strongly correlated to seed size, mass and shape ([4], [5], [36]). Smaller, spherical seeds with a hard seed coat tend to have longer seed longevity than large, elongated and flattened seeds ([5], [45]). Bakker et al. ([5]) mentioned that small and low-mass seeds may better penetrate into deeper soil layers.
The model finding a birch seed persistence of 13 years in soil showed a clear trend of birch seed longevity in the soil. But the own data corresponded not to the longevity determined by Skoglund & Verwijst ([46]), who derived a theoretical seed half-life of 13 years for B. pubescens seeds. A buried seed experiment by Granström ([23]) revealed viability of at least five years for B. pendula and B. pubescens seeds. Bakker et al. ([4]) categorised birch as having a short-term to long-term persistent soil seed bank (seeds are viable >1-5 years in soil - [49]). No germination of birch seeds within the soil was observed in our study. The absence of seed germination within the soil ensures the accumulation of birch seeds in the soil as a seed reserve. We conclude that birch belongs to at least the short-term persistent type and that a replenishment of the soil seed bank with fresh seeds is required every few years.
Most of the buried rowan seed sets in our study, with and without pulp, exhibited very low percentage of germination (3-13%) after two years and produced a predicted maximum seed persistence of 3.5-4.5 years. Similar forecasts about rowan seed longevity were made by Hill ([25]) and Erlbeck ([17]). Granström ([23]) found 60% of rowan seeds to be viable after three years, but only one seed germinated after five years. The declining viability in Granström’s study largely reflected our findings. Therefore, the classification of rowan as transient (viable <1 year) by Grime et al. 1988 (cited in [39]) and Dölle & Schmidt ([16]) appears incorrect and suggests rowan seeds should be re-classified as short-term persistent, although the seeds are more short-lived than birch seeds.
The hard-coated seeds of rowan are smaller and lighter than the long-term persistent Robinia pseudoacacia seeds ([26]), which have a mean seed length of 4.9 mm and a mean seed mass of 18.7 mg ([14]). Therefore, rowan seeds may more readily drift into the soil than Robinia pseudoacacia seeds and would also have to form a long-term soil seed bank, according to Bakker et al. ([4], [5]) and Plue et al. ([36]).
The low storability of rowan seeds in the artificial burial experiment can be explained by early seed germination within the soil before excavation of the seed bags. Schafer & Chilcote ([43]) and Bradbeer ([11]) described the phenomenon of early seed germination within soil in other species, indicating that it is not specific to rowan.
We observed early germination within the soil of rowan seeds without pulp in each spring, and germination of the rowan seeds with pulp only after the second and third winter, in accordance with Granström ([23]). Under natural stratification conditions (varying moisture and temperature conditions), the dormancy of rowan seeds without pulp will be broken after 24-28 weeks ([6], [1]), which corresponded to our findings. To overcome dormancy, rowan seeds in fruits required longer periods of cold treatment because the pericarp must first decompose before the fruit-induced dormancy could be broken (Devillez 1979, cited in [39], [6], [8]). After breaking dormancy, rowan seeds usually germinate within soil even without the occurrence of a disturbance event ([23]), because the seeds have the ability to germinate in darkness (Devillez 1979, cited in [39]). This leads to the loss of the buried seed reserve ([43]). There is no sufficient accumulation of rowan seeds in the soil to serve as a seed reserve until the prevailing environmental conditions change. Therefore, the rowan soil seed bank does not meet the definition of a classical soil seed bank as a seed reserve ([49], [10]). Unlike birch, rowan requires a constant supply of seeds to maintain a seed reserve within the soil.
Influence of pulp on rowan seed persistence in soil
The results showed that the pulp surrounding rowan seeds offers no benefits in terms of either persistence or preventing early germination in soil, refuting our second hypothesis. The model estimates about one year longer storability of rowan seed with pulp in soil, compared to rowan seed without pulp, and this is due to the need to overcome fruit-induced, embryo and seed coat dormancies ([8], [39]). Observations made during the excavations of the net bags from the soil revealed that the decomposition of the rowan pulp starts after the first winter period in the upper soil layer, where decay proceeds faster than in the deeper mineral soil layers. Granström ([23]) documented the decay of rowan fruits in soil over two years, with the first germination of seeds within the soil after the second winter. This corresponded with our results.
In deeper soil layers, decomposition of the pulp was found to start later and proceed more slowly. Sometimes the exocarps were not destroyed in the lower soil layer, with the pulp having become dry and hard, and remaining wrapped around the seeds. The dry mesocarp means that there was not enough water in the deeper layer to leach away the inhibitors in the mesocarp and break dormancy. As a result, swelling and breathing of the seeds was inhibited and the seeds could not germinate. As was the case for rowan seed without pulp, seed with pulp does not store in the soil over longer periods.
Influence of burial depth on seed persistence
There is solid evidence of the importance of seed protection and seed viability provided by litter and humus cover ([52], [53]). Litter protects seeds against, for example, predation and drought, and prevents early germination in light-demanding seeds ([24], [35], [3]). However, the burial depth had no influence on either seed longevity or on the quantity of germinated seeds within soil, nor was there an effect in interaction with storage time. As a consequence, we could confirm our third hypothesis.
Rowan seeds germinated in the soil regardless of depth or pulp because they do not have a light requirement for germination (Devillez 1979, cited in [39]). As a consequence, no effect of soil depth was evident in the model. However, a comparison of different birch burial experiments revealed that birch seeds sown on bare ground remained viable over a period of up to three years ([33]), whereas birch seeds buried under a litter layer to a depth of 10 cm in the mineral soil remained viable for 5 and more than 13 years, respectively ([23], [46]). The reasons underlying the lack of any effect of burial depth on birch seed longevity in our study may have been: (1) that the effect only occurs between the humus and the mineral soil layer, but not within mineral soil layers: (2) only long-term persistent species, like Potentilla norvegica L., exhibit higher seed viability in deeper soil layers ([52]). Maybe short-term persistent seeds possess the same seed longevity in deeper soil layers as in the upper soil layer; (3) it is also possible that the duration of the study was not long enough to detect such significant differences within the mineral soil. It was only after 10 years that Toole & Brown ([52]) observed significantly higher seed viability percentages in the deepest soil layer for the Rosaceae Potentilla norvegica.
Conclusions
The artificial seed burial experiment proved the ability of birch and rowan seeds to form a short-term persistent soil seed bank, with no influence of the storage depth in the soil. The findings of our study indicate that birch seed may in theory persist in the mineral soil for a maximum of 13 years, independent of the burial depth. Silver birch is able to build up a short-term to long-term soil seed bank until the prevailing environmental conditions change to allow for successful germination. In contrast, rowan seeds with and without pulp germinated within the soil after the breaking of dormancy, independent of the soil depth. This significantly limited the seed reserve in the soil over time. The duration of the persistence of rowan seeds in the mineral soil is a maximum of 3.5 years without pulp and 4.5 years with pulp. The pulp offers no benefits for seed persistence; rather, it would appear to act as a physiological inhibitor of germination and ultimately leads to reduced seed viability. A continuous, almost annual input of rowan seeds to the soil seed bank is necessary for successful regeneration, whereas birch seed input every few years seems sufficient.
Only the soil seed bank of birch might be exploited to make a significant contribution to the regeneration of disturbed forest sites or succession sites. Any contribution by the soil seed bank of rowan will be accidental and insufficient.
Acknowledgements
The work carried in this study was supported financially by a scholarship granted to KT by the foundation “Deutsche Bundesstiftung Umwelt” (DBU) and the “Graduiertenakademie” (GA) of the TU Dresden. We would like to thank Antje Karge, Angelika Otto and Katja Skibbe for assistance in the greenhouse and Kathrin Tiebel for sewing the seed bags. We thank David Butler Manning for proofreading the text and Jan Plue for helpful tips.
This paper is a part of the KT’s dissertation (Tiebel K, 2020. The ability of pioneer tree species to mitigate the effects of site disturbance by fast and effective natural regeneration. PhD thesis, Dresden University of Technology, Germany, pp. 187), which is available at ⇒ https://nbn-resolving.org/urn:nbn:de:bsz:14-qucosa2-724321.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Franka Huth
Sven Wagner 0000-0003-3796-3444
TU Dresden, Institute of Silviculture and Forest Protection, Chair of Silviculture, Pienner Str. 8, 01737 Tharandt (Germany)
Corresponding author
Paper Info
Citation
Tiebel K, Huth F, Wagner S (2021). Is there an effect of storage depth on the persistence of silver birch (Betula pendula Roth) and rowan (Sorbus aucuparia L.) seeds? A seed burial experiment. iForest 14: 224-230. - doi: 10.3832/ifor3685-014
Academic Editor
Emilia Allevato
Paper history
Received: Oct 28, 2020
Accepted: Mar 21, 2021
First online: May 06, 2021
Publication Date: Jun 30, 2021
Publication Time: 1.53 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2021
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 36409
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 30453
Abstract Page Views: 2682
PDF Downloads: 2626
Citation/Reference Downloads: 4
XML Downloads: 644
Web Metrics
Days since publication: 1758
Overall contacts: 36409
Avg. contacts per week: 144.97
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2021): 8
Average cites per year: 1.60
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Review Papers
Soil seed banks of pioneer tree species in European temperate forests: a review
vol. 11, pp. 48-57 (online: 25 January 2018)
Review Papers
Methods of soil seed bank estimation: a literature review proposing further work in Africa
vol. 15, pp. 121-127 (online: 26 March 2022)
Research Articles
Physiological dormancy and dormancy release of Sassafras tzumu, a colored-leaf tree species with high landscape and economic value
vol. 15, pp. 349-355 (online: 01 September 2022)
Research Articles
Influence of mother plant and scarification agents on seed germination rate and vigor in Retama sphaerocarpa L. (Boissier)
vol. 7, pp. 306-312 (online: 08 April 2014)
Short Communications
Evidence of Alectoris chukar (Aves, Galliformes) as seed dispersal and germinating agent for Pistacia khinjuk in Balochistan, Pakistan
vol. 14, pp. 378-382 (online: 22 August 2021)
Research Articles
Use of brassinosteroids to overcome unfavourable climatic effects on seed germination in Pinus nigra J. F. Arnold
vol. 17, pp. 1-9 (online: 02 February 2024)
Research Articles
Seed germination traits of Pinus heldreichii in two Greek populations and implications for conservation
vol. 15, pp. 331-338 (online: 24 August 2022)
Research Articles
Effects of brassinosteroid application on seed germination of Norway spruce, Scots pine, Douglas fir and English oak
vol. 10, pp. 121-127 (online: 02 October 2016)
Research Articles
Soil stoichiometry modulates effects of shrub encroachment on soil carbon concentration and stock in a subalpine grassland
vol. 13, pp. 65-72 (online: 07 February 2020)
Research Articles
Soil fauna communities and microbial activities response to litter and soil properties under degraded and restored forests of Hyrcania
vol. 14, pp. 490-498 (online: 11 November 2021)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword