Soil seed banks of pioneer tree species in European temperate forests: a review
iForest - Biogeosciences and Forestry, Volume 11, Issue 1, Pages 48-57 (2018)
doi: https://doi.org/10.3832/ifor2400-011
Published: Jan 25, 2018 - Copyright © 2018 SISEF
Review Papers
Abstract
The ability of short-lived tree species such as birch, alder, willow, poplar and rowan to form even a short-term soil seed bank is discussed controversially in the literature. Soil seed banks are an important component of succession and regeneration in ecosystems. Following disturbance, buried viable seeds germinate and the seedlings that establish cover the disturbed, exposed soil surfaces. The objective of this study was to undertake a literature review of soil seed bank research carried out in central and north-west European temperate forests to provide an overview of the ability of pioneer tree species to form a viable seed bank. The review of 33 publications revealed that birch is the only pioneer tree species of temperate forests with longer-lived seeds, persisting in the soil for 1 - 5 years. Birch seeds remain viable in deeper soil layers (5 - 10 cm), so birch may be assigned to the short-term persistent soil seed bank type. The seeds of alder, willow and poplar would appear to be short-lived. Maximum seed densities of all tree species were found in the upper soil layers. With increasing soil depth, seed density declined. Viable seeds of rowan were not detected in any of the soil seed bank studies, although seed trees were present. We found that in spite of the capacity for long seed dispersal distances, high densities of birch, alder and willow seeds were only observed in close proximity to seed trees. The higher the numbers of seed trees, the higher the seed densities in soils. Maximum seed densities were recorded during and shortly after seed rains had occurred. Our results reveal that a birch seed bank may compensate for years with lower levels of seed production. However, as the seed bank is only short-term persistent, it must be supplemented by fresh seeds from surrounding seed trees as often as possible to guarantee a continuous capacity for regeneration.
Keywords
Betula, Buried Seeds, Propagule Bank, Seed Density, Viable Seeds, Germination
Introduction
Soil seed banks are an important component in the succession and regeneration of ecosystems. Soil seed banks are buried seed reserves, which are viable and able to germinate under changing environmental conditions ([33], [98], [11]). The formation of a soil seed bank is a strategy developed by plants to prevent germination under unfavorable soil and climate conditions ([18], [67], [86]). In disturbed areas of forest, seeds of different species are granted an opportunity to germinate and cover the open soil surface, even though these species may not have been represented in this area for a long time ([33], [15]). Soil seed banks could contribute significantly to the reforestation of disturbed woodlands. They may also compensate for a recent absence of seed sources within or around a damaged area.
Soil seed banks of forests generally exhibit lower species diversity and seed densities than those present in other ecosystems ([59], [50], [17]). Deciduous, young or managed forests are characterized by larger seed numbers and greater species richness than coniferous, older or unmanaged forests ([27], [15], [36], [29], [83]). The seed bank compositions of northern and western European forests differ from those of eastern European forests ([16]). The composition of seed banks and ground flora in forests also differ from each other ([15], [16], [107]). In central European temperate forests, soil seed banks predominantly contain herbaceous plant species of early or middle successional stages. The seed banks are refreshed by seeds of species that emerge in the event of disturbance in forest ecosystems. Species of early or middle successional stages are light demanding species, adapted to disturbances, and able to form a persistent soil seed bank ([27], [16], [36]). Hopfensperger ([50]) suggested that pioneer species, present in early successional stages, can form a persistent seed bank at the beginning of succession to woodland. Seeds of ancient, shade tolerant forest species, shrubs and tree species in general, are not well represented in the soil seed bank, because the seeds of these species do not remain viable for long ([27], [15]). However, pioneer tree species are also regarded as light demanding species. In Europe, Betula spp., Salix spp., Populus spp., Alnus spp. and Sorbus aucuparia L. represent deciduous pioneer tree species. These tree species are short-lived species, which produce large quantities of seeds, have large seed dispersal distances and exhibit fast juvenile growth ([81], [84], [105]). Pioneer tree species are very common in early successional stages and in disturbed woodlands in central Europe ([105]). With climate change, and the associated increase in the frequency and intensity of disturbances (e.g., storm events) ([90]), pioneer tree species are of growing importance for natural reforestation; and so too soil seed banks. The pioneer tree species can regenerate rapidly and successfully colonize large areas in years in which high quantities of seed are produced ([81], [68], [84], [3]). As a consequence, pioneer tree species can mitigate negative consequences associated with disturbed areas, for example, soil erosion and the loss of nutrients (see [8], [89], [3], [106], [34]). However, pioneer tree species exhibit irregular seed production patterns (mast years) ([87], [13], [49], [80], [93], [53]). A question that arises is whether pioneer tree species have the potential to regenerate from a soil seed bank in non-mast years, like Hopfensperger ([50]) found this for pioneer species. Currently little is known about the capacity of pioneer tree species in European temperate forests to establish seed banks, or how long their seeds persist in soil. Some burial experiments showed that rowan and birch remain viable in soil for more than 5 years ([74], [42], [40], [92]) and sometimes viable birch and willow seeds were detected in soil samples collected from deeper mineral soil layers ([48], [95], [6], [59], [26]). However, the ability of pioneer tree species to form at least a short-term seed bank is discussed controversially in the literature. The short viability period of pioneer tree seeds after dispersal is often mentioned and many authors espoused the opinion that pioneer tree species do not generally form a seed bank ([48], [1], [21], [29], [45]). By contrast, Granström & Fries ([42]), Osumi & Sakurai ([80]), Erlbeck ([31]), Rydgren et al. ([85]) and Decocq et al. ([25]) suggested that birch, alder and rowan may make up part of the forest seed bank. If pioneer tree species have the capacity to establish a seed bank, years with low levels of fructification can be compensated for and the colonization of open areas, for example, would not depend exclusively on annual seed rain ([80]).
In this review, available data pertaining to densities of birch, alder, willow, poplar and rowan in soil seed banks in central and north-west European temperate forests are documented based on a survey of the literature. The aim was to summarize the general findings and to identify knowledge gaps concerning the soil seed bank with respect to these short-lived tree species. This species-specific information will be discussed in the context of the meaning of the soil seed bank in relation to disturbance regimes, succession and reproductive ecology.
Methods of literature search
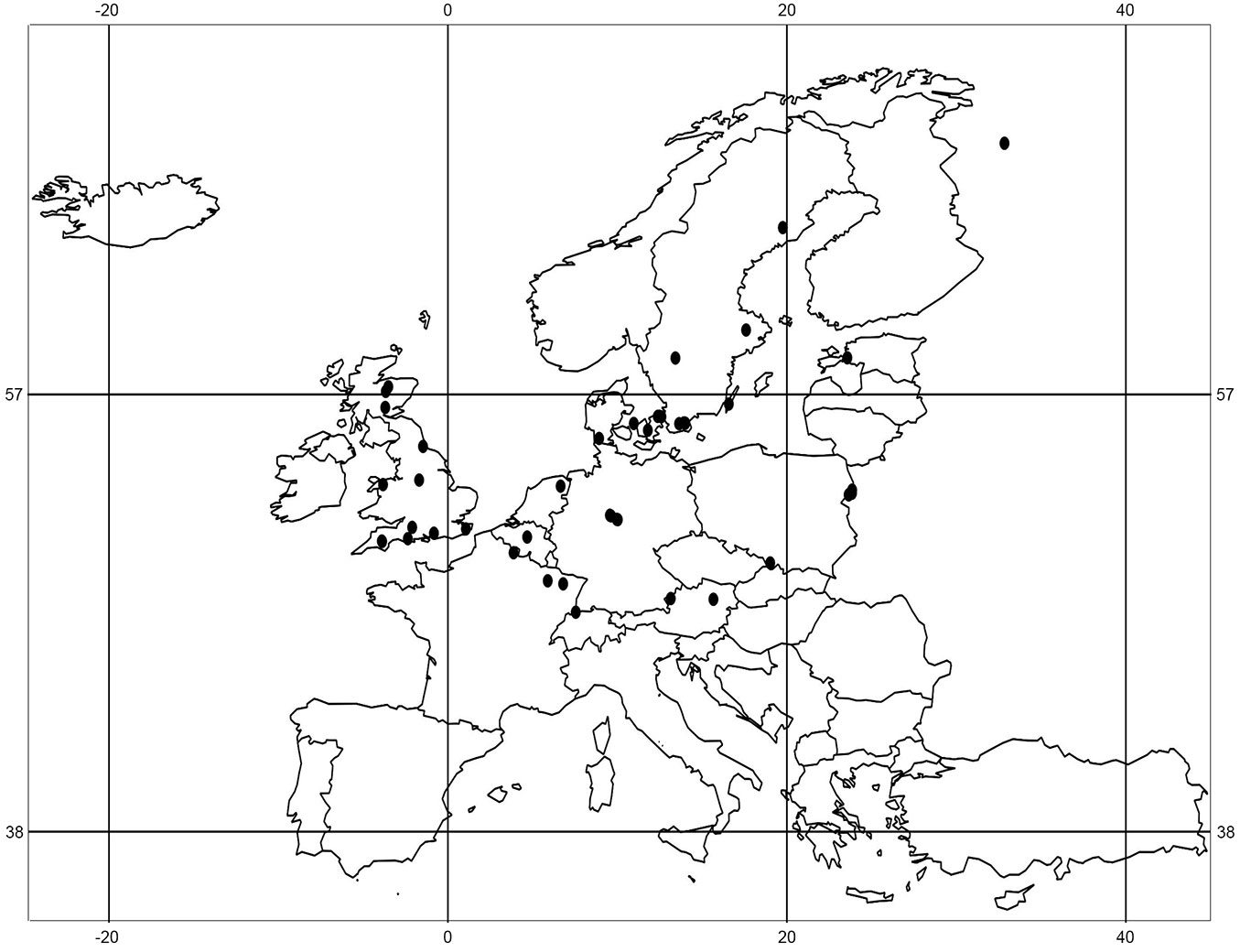
Our review is based on studies carried out in central and north-west European temperate forests published in the period 1979 - 2013 (Fig. 1). The keywords “seed bank,’ “propagule bank’ and “buried seeds’ were used in combination with either “forest” or “woodland.’ An article was selected when the seed density per m² could be calculated to make the results comparable with those of other studies. A total of 33 studies from 14 countries matched the criteria. Most of papers were found by searching the “Web of Science’ database, meaning the papers had to be published in international peer reviewed journals with an impact factor. Only 3 papers included in the review were published in non-peer reviewed journals, 2 of which were written in English ([29], [45], [57]). These papers were found through citations within other international soil seed bank papers. The forests presented in all of the chosen studies were considered to be distinct sample plots wherever the authors classified the study sites and their sample plots as independent (e.g., [95], [28], [26]). In this way, 136 sample plots were recorded, which differed in their histories, forest types, stand ages, canopy densities and management strategies (Tab. S1 in Supplementary material). The mean seed density per m² of birch (Betula spp.), alder (Alnus glutinosa (L.) Gaertn.), aspen (Populus tremula L.) and willow (Salix spp.) was calculated for each plot. The soil samples differed in their depths and in terms of the soil layers. Authors took samples from humus and mineral soil, or only from the mineral soil layer. In some cases no information about whether litter and humus were removed prior to sampling was provided (Tab. 1). In this paper the term “birch’ is used to represent Betula pendula and B. pubescens, with “Salix spp.’ used to indicate all willow species detected in soils. This corresponds to the approaches used by the authors of the identified studies.
Tab. 1 - Summary of the 33 selected seed bank studies in central and north-west European temperate forests and the information about seed densities provided. (‡) Depth of core: (?) not clear whether the humus layer was tested or not; (-) humus layer not tested; (+) humus layer tested separately; (++) humus and mineral soil layer tested together; (5) soil sample depth of 0-5 cm in the mineral soil. (†) Temperature: (1) cold stratification of soil samples before seedling-emergence treatment; (2) cold stratification of soil samples integrated within the seedling-emergence treatment. (§) Species: (?) the species or genus with individually defined small numbers in the soil was excluded from the presentation; (+) species or genus detected; (-) species or genus not detected; (-/-) tree species excluded, which germinated in sterile control trays.
| Author(s) & Year | Date of soil sampling | Depth of core (‡) (cm) | Seedling-emergence method | Seed extraction method |
Study duration (months) |
Species (§) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp (†) (range orday/night) |
Artificial light (day/night) | Betula spp. |
Alnus glutinosa | Salix spp. |
Populus tremula | Sorbus aucuparia | |||||
| [2] | Jan | (-) 10 | 16 °C | 16/8 h | - | 5 | - | - | - | - | - |
| [5] | Feb | (-) 5 | nursery and closed shade house | - | - | 7 | + | - | -/- | - | - |
| [6] | Apr | (-) 10 | 25 - 45/15 °C | - | + | 4.5 | + | - | + | - | - |
| [10] | Mar | (?) 10 | 25/15 °C | 12/12 h | + | 3 | + | + | + | - | - |
| [11] | Feb, Mar, Jun, Sep | (+) 35 | glasshouse | 6-9 pm | - | 3-4 | + | - | + | - | - |
| [16] | Mar, Sep | (-) 20 | 14 - 25 °C | 16/8 h | - | 5 | + | - | - | - | - |
| [20] | Apr | (-) 15 | glasshouse (1) | - | - | 24 | + | - | + | - | - |
| [21] | Nov-Feb | (?) 10 | unheated polythene tunnel | - | - | 6-9 | + | - | - | - | - |
| [25] | Jun | (-) 20 | 20/16 °C | 12/12 h | - | 6 (2) | + | + | - | - | - |
| [26] | Mar | (-) 30 | unheated glasshouse | - | - | 12 | + | - | + | - | - |
| [27] | May | (?) 7 | unheated glasshouse | - | - | 3 | + | - | - | - | - |
| [28] | Apr | (-) 10 | polythene tunnel/glasshouse | - | - | 4 | + | - | - | - | - |
| [29] | Mar | (+) 10 | unheated glasshouse | - | - | 10 | + | - | - | - | - |
| [32] | Mar/Apr, Sep/Oct | (?) 3 | 18 - 22 °C | - | + | 36 | + | + | + | - | - |
| [38] | Jul | (+) 15 | 22/5 °C 1) | 16/8 h | - | 16 (2) | + | ? | ? | ? | ? |
| [39] | Jul | (+) 5 | 22/12 °C (1) | 18/6 h | + | 6 | + | - | - | - | - |
| [41] | Apr | (+) 6 | 22/12 °C (1) | 18/6 h | - | 63 (2) | + | - | + | - | - |
| [45] | Mar | (+) 20 | unheated glasshouse | - | - | 12 | + | ? | -/- | + | ? |
| [46] | May | (++) 5 | glasshouse | - | - | 12 | + | - | - | - | - |
| [48] | Apr | (+) 10 | unheated glasshouse | - | - | 8 | + | - | - | - | - |
| [54] | Mar | (?) 5 | unheated glasshouse | - | - | 43 | + | - | - | - | - |
| [55] | early spring | (-) 10 | unheated glasshouse | - | - | 8 | + | - | - | - | - |
| [56] | Jun | (-) 10 | unheated glasshouse | - | - | 25 | + | ? | ? | ? | ? |
| [57] | Oct, Nov | (?) 10 | 18 - 24 °C | 12/12 h | - | 5 | + | - | - | - | - |
| [59] | May | (-) 10 | unheated glasshouse | - | - | > 4 | + | - | - | - | - |
| [63] | Mar, Apr | (++) 17.5 | 22/12 °C (1) | 16/8 h | - | 4 | + | + | - | - | - |
| [64] | Jun | (++) 10 | 25 °C | - | + | > 10 (2) | + | - | - | - | - |
| [73] | May | (?) 8 | 20/8 °C (1) | 16/8 h | - | 12 (2) | + | ? | ? | ? | ? |
| [75] | Jul, Aug | (++) 5 | 6 - 25 °C (1) | 12/12 h | - | 12.5 | + | - | - | - | - |
| [76] | Feb | (?) 6.3 | polyethylene tunnel | - | - | 15 (2) | + | - | - | - | - |
| [95] | Apr | (+) 5 | unheated glasshouse (10 - 30 °C) | - | - | 3 | + | - | + | - | - |
| [97] | Oct-Oct (every 6 weeks) | (++) 3 | 20/15 °C | 16/8 h | - | 1.25 | - | - | - | - | - |
| [102] | May, Jun | (?) 15 | shade tunnel | - | - | 10-12 | + | - | - | - | - |
Species-specific reproductive ecology determining the potential of soil seed banks
It is often assumed by practitioners that bountiful fructification of pioneer tree species recurs annually. However, like intermediate and climax tree species, short-lived species exhibit irregular seed production patterns, influenced by soil and climate conditions, and the individual fitness of seed trees ([87], [13], [49], [80], [93], [53]). The germination percentage of the seeds also varies from year to year, with mast years usually characterized by the highest germination rates ([87], [13], [49], [80], [93], [84], [53]). However, pioneer tree species exhibit seed morphologies, seed dispersal distances and requirements for germination and seedling establishment that are different to those of intermediate and late-successional species (see [71], [4], [65], [103], [84]). Despite differences between their fruits and seeds, birch and alder (winged nuts), willow and poplar (catkins, seeds with pappus) and rowan (small seeds within a red fleshy fruit) can be analyzed together as each group possesses morphological similarities ([72], [81], [103], [84]).
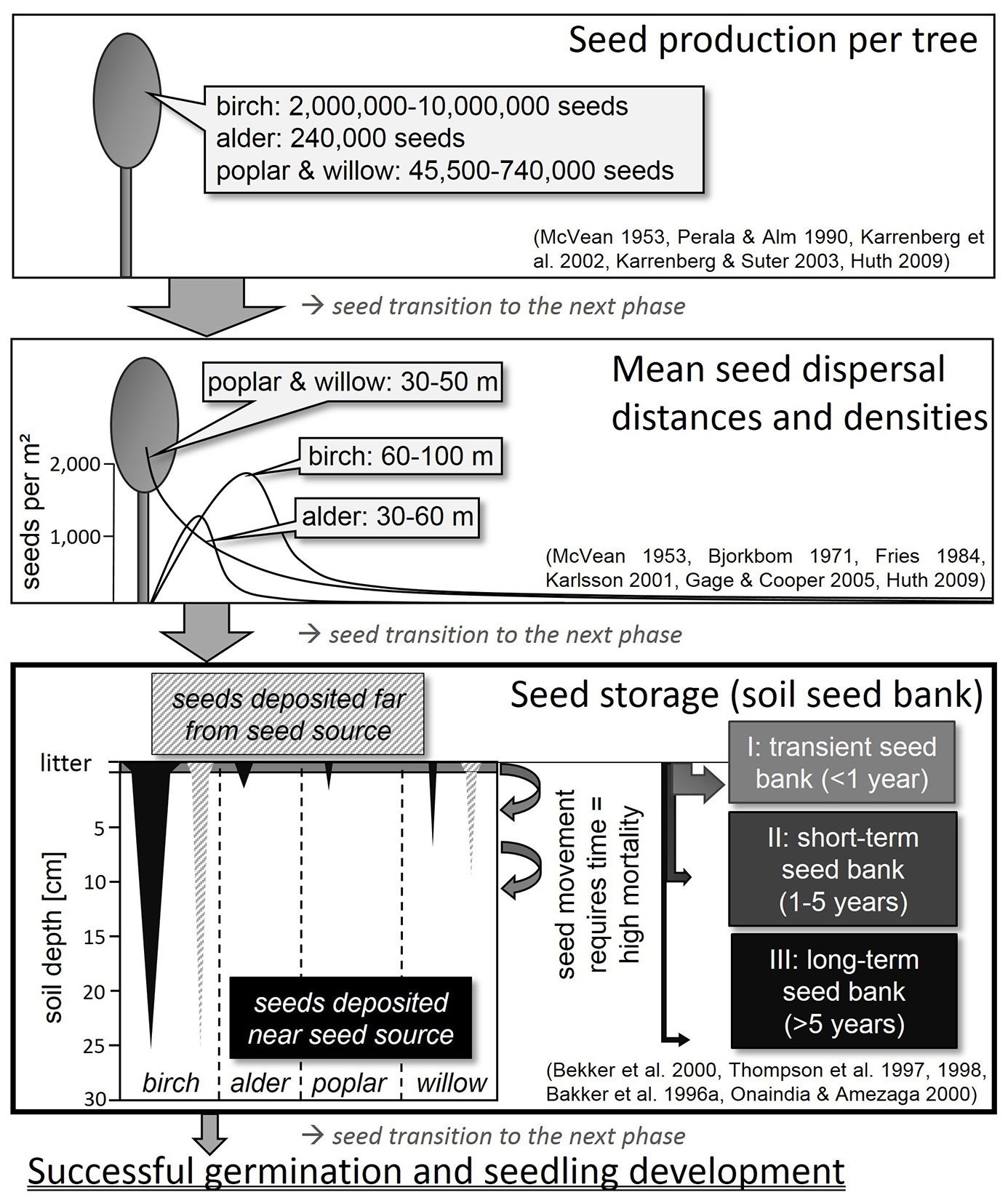
Birches can produce 2 - 10 million winged seeds per tree ([81], [52]), which are 1.5 - 2.0 mm in size without the wings ([19]). Seed rain takes place mainly from June to November ([81], [52]). From November until the end of the following June, the seed rain falls to less than 100 seeds per m² ([52]). Mean dispersal distances by wind vary between 60 - 100 m (Fries 1984, cited in [81], [60], [52]), but the highest seed densities are deposited within distances of 25 - 50 m ([14], Fries 1984, cited in [81]) (Fig. 2). Maximum propagation distances amount to 550 - 800 m ([52]). Most seeds germinate in spring after dispersal ([81]). Alder seed trees generally produce lower seed numbers (240.000 seeds per tree) than birch. The diaspores of alder have smaller wings and larger seed nuts (2.0 - 2.5 mm), and their mean and maximum dispersal distances from seed trees are 30 m and 60 m, respectively ([71], [19]). High seed densities were found within distances of less than 10 m ([71], [72], [60]). Seeds of alder trees are mature in November, but most are only released in February and March, and, like birch, germinate predominantly after dispersal in spring ([82]). Seeds of birch and alder do not exhibit dormancy ([71], [4]). The spatial distribution of deposited seeds on soil depends on the position of the seed trees and on the dispersal agents ([7]). The distribution of wind-dispersed seeds tends to be nonrandom ([43]). This applies especially to birch and alder but also for willow and poplar. Deposited seeds of these species often occur in aggregations because seeds stay together in dense infructescences ([48], [63], [28]).
Fig. 2 - Schematic diagram showing the relation between seed production, seed dispersal and seed storage in soil of birch, alder, poplar and willow.
Willows and poplars can produce between 45.500 and 740.000 seeds per tree ([61], [62]). The wind-dispersed (anemochorous) willow and poplar seeds possess hairs to facilitate flight and range in size between 0.8 - 1.5 mm respectively 1.0 - 2.5 mm ([19]). Maximum willow seed dispersal distances of 2 - 3 km are much longer than for birch and alder ([88]). For this reason, Gage & Cooper ([35]) always adopted a background level (i.e., “noise”) of 10 - 30 seeds per m². Nevertheless, the largest densities of deposited seeds were measured at distances of between 30 - 50 m from willow seed trees ([35]) (Fig. 2). The small seeds of both genera are short-lived ([103], [9], [104]). For early flowering (spring) willow species, like Salix caprea L., and poplars, seed rain takes place from April to June ([23]). Seeds lying on soil that do not germinate immediately after dispersal lose their viability after 1 - 6 weeks ([58], [77], [68], [103], [62]). In contrast, seeds of late flowering (summer and autumn) willow species remain viable until the next spring ([23]), which means a lifespan of about half a year.
The fruits of rowan ripen between August and October ([84]). Fruits and seeds are dispersed endozoochorously, by birds and small mammals. The seeds of rowan exhibit embryo and seed coat dormancy. If rowan fruits are not eaten by animals, the seeds germinate in the second year after maturation, when embryo and seed coat dormancy is broken under natural conditions ([84]). Sometimes the seeds undergo a second period of dormancy when temperatures rise above 10 °C after winter or cold storage ([94]). In such cases, seeds can remain viable for up to 5 years in the soil ([31]). Birds, as the main consumer of rowan fruits, have a significant influence on the spatial patterns of seed distribution. Bird droppings were mostly found under trees in more or less closed forests, and only very rarely in large gaps or open areas lacking structural complexity ([109]). Structural elements are used as perches by birds ([70]). Most frequently, birds drop seeds up to 40 m from seed trees ([108]). In the case of endozoochory, the density patterns of rowan seeds in the soil are clumped rather than randomly distributed ([24], [108]).
Characterization and classification of soil seed banks
Simpson et al. ([91]) emphasized that, “all viable seeds present on or in soil or associated litter constitute the soil seed bank.” Seeds buried in the upper soil layer (litter and humus layer) have in most cases only been part of the seed bank for a short time. These seeds are probably part of the last seed rain, and so the character of the seed bank in the upper soil is prevailingly transient ([37], [6], [7], [80], [51], [45]). Given the species-specific timing of seed rain, the time of sampling represents an important piece of information for the interpretation of seed densities in transient seed banks. Seeds found in deeper mineral soil layers are older and have persisted in the soil for a longer period ([63], [7], [98]). Therefore, information on seed depth can be used as an indirect method to determine the seed longevity in the soil of a particular tree species. Buried seed experiments have contributed to a better understanding of the duration of seed viability in deeper soil layers. The experiments demonstrated what might happen to seeds in relation to viability, decomposition and mortality over time during storage in soil ([40], [92]).
Litter cover and litter thickness are also two very important factors. Thick litter protects deposited seeds against movement, drought, predation and early germination, and so may help to maintain higher viability ([42], [30]). Almost no viable seeds were found on sites without this litter protection. Thin litter, including seeds, for example, will be carried away by wind and accumulated at other places ([30]). Independent of the species, the number of viable seeds on and in peatland increased with litter thickness. The thickness of the humus layer determines the period of time required by seeds to penetrate litter and humus. During this time seeds are subject to mortality ([87], [101], [49]). Small, light and dry seeds without pulp are less prone to predation than larger seeds ([66]). Findings of glass bead experiments (the size and weight of the beads corresponded to rowan fruits) revealed that 30 - 40 % of all glass beads moved 1 cm in the soil over a 6-month period, whereas 4 % were transported 2 - 5 cm ([101], [22]). Small seeds pass through litter faster, reaching the humus and mineral soil layer in a shorter time than larger seeds or beads ([30]). This is an advantage for pioneer tree species with regard to their strategy of fast colonization of disturbed areas: the small, light and dry seeds without pulp ([66]) achieve quicker contact with the mineral soil and can germinate successfully.
Soil seed banks can generally be classified according to the seed longevity of the species; that is, the period of time for which a seed stays viable and capable of germination. The classification most widely applied is that described by Thompson et al. ([98]) and Thompson et al. ([99]), who differentiated four types. The first type (I) includes all species with transient seed banks with a persistence in the soil of less than 1 year. Species with short-term persistent seeds (1 - 5 years) are assigned to type II. Type III is as a long-term persistent seed bank and includes species with seeds that persist in the soil for at least 4 - 5 years. Species that cannot be assigned to any seed bank type are combined in type IV.
Soil seed bank of Betula spp.
Houle ([51]) found that birch seeds are viable for less than 2 - 3 years under field conditions, due to multiple causes of mortality. Mortality rose to 99 % under certain climate conditions ([51]). However, 50 - 80 % of birch seeds buried artificially in soil were still viable after 3 - 5 years of storage ([40], [92]). After 5 years stored birch seeds were partly degraded, but 50 - 60 % remained viable ([40]). It is assumed that birch seeds decompose within a period of 5 - 7 years ([87]). Skoglund & Verwijst ([92]) concluded that, at a depth of 10 cm, birch seeds buried in the soil have a theoretical half-life in forest soil of roughly 13 years, but less than 1 year in the soil of wet meadows. In wet soils, early germination and fungal attacks lead to higher mortality rates (Harper 1955, cited in [69]). Birch seeds sown on bare ground germinated in the first year ([74]). Therefore, seeds found in deeper mineral soil probably did not originate from the previous seed rain and so were part of the soil seed bank.
In most of the publications analyzed, birch was the only tree species exhibiting a high degree of consistency in soil seed banks in central and north-west European temperate forests (83 % of sample plots). Often birch was the second most abundant species of all, including herbaceous plant species. However, some authors found the extent of birch seed in the soil to be negligible ([48], [95], [21]). Betula species were present in all kinds of forest type, but seed densities depended on the presence or absence of seed sources. Large numbers of seeds can be found in the soil in the vicinity of seed trees ([15]) (Fig. 2). Birch seed density ranged from 1 - 1100 seeds per m² in coniferous stands, mostly spruce and pine forests ([39], [64], [102], [28], [5], [75], [11], [45], [56]). In deciduous forests 7 - 3850 viable seeds per m² were detected ([95], [63], [102], [28], [55], [5], [16], [75], [25], [57]). The seed density on succession sites ranged from 6 seeds per m² in a 4-year old Norway spruce clear cut ([45]) to a maximum of 3120 seeds per m² in a long-term overgrown grassland ([59]). Highest densities of 70 - 3760 seeds per m² were found in pure or birch-dominated stands ([46], [63], [102], [54], [59], [76], [32], [75], [26]). However, 0 - 144 viable seeds per m² were detected in soils of deciduous and coniferous forests without any mature trees or seedlings in the proximity ([39], [95], [2]). Hill & Stevens ([48]) detected more viable birch seeds in a 4-year old clear cut of a former Douglas fir plantation than seedlings in the vegetation layer, an indication that birch seeds remain viable in the soil for a longer time than is frequently assumed.
The birch seed density in different soil layers was reported for only a few samples. Various authors mentioned that birch seeds are mostly found in the humus or uppermost soil layers ([47], [41], [51], [96]). Houle ([51]) concluded that less than 2 % of birch seed rain reaches the persistent seed bank. The numbers of birch seeds found at different soil depths ranged from 1 - 188 seeds per m² in the litter and humus layer ([39], [95]) to 1 - 80 seeds per m² at a depth of 0 - 5 cm in mineral soil, independent of seed source presence or absence ([39], [95], [5], [56]). In samples taken at a depth of 5 - 10 cm in deciduous and coniferous forests 3 and 71 birch seeds per m² were detected ([56], [57]). Independent of the occurrence of seed trees, an average of 33 seeds per m² were present in the mineral soil down to a depth of 10 - 20 cm ([16]), which may lead one to assume that birch seeds live longer in the soil than is often assumed (Fig. 2). With increasing soil depth, the number of viable birch seeds declined, but remained high enough for reforestation. On succession sites, where many seed sources are available for seed supply, 324 seeds per m² were recorded in the humus and litter layer ([48]). At depths of 0 - 5 cm and 5 - 10 cm in the mineral soils of these sites, densities reached 79 - 2880 seeds per m², and 20 - 880 seeds per m², respectively ([48], [6], [59]).
Irrespective of the timing during the year of soil sample collection, high densities of viable birch seeds could always be found. Highest seed densities of 20 - 3850 viable birch seeds per m² occur in the period from May to June ([102]). The results of the studies showed that seed densities were more dependent on the presence or absence of seed sources than on the timing of soil sampling or on the forest community of the sampling site ([63], [51], [15]).
The different studies, and the different assessments of birch seed longevity, explained the varying assignments of birch to the contrasting soil seed bank types by researchers, which ranged from purely transient ([10]), through transient/short-term persistent ([98]) to short-term/long-term persistent ([6]). The assumption made by Olmsted & Curtis ([78]), Bakker et al. ([7]) and Graber & Thompson ([37]) that seed rain from outside of a stand is necessary for the regeneration of the species where birch seed trees are not present on a site, due to an insufficient seed bank, cannot be supported without new research.
Soil seed bank of Alnus glutinosa (L.) Gaertn.
Viable alder seeds were detected in the soil seed bank less often than birch seeds (10 % of sample plots). This is probably due to the lower frequency of alder trees than birch in European managed forests. Seeds were only found in soils where there were seed sources close by ([63], [32], [25]) (Fig. 2). Alder seeds were mainly detected on meadow and hayfield succession sites aged between 0 - 25 years. The seed density ranged between 8 - 216 seeds per m2. The maximum was found in a 20-year old dry hayfield. In contrast to this, the maximum number recorded on a wet hayfield succession site was only 80 seeds per m² ([10]). In 40 - 175-year old deciduous forests 2 - 7 alder seeds per m² were observed ([63], [25]). The highest alder seed density recorded in humus and mineral soil was 354 seeds per m², which was obtained from a mixed lime-alder-birch forest ([63]). No alder seeds were detected in coniferous or mixed stands. The studies presenting the findings from such stands provided no information about the presence of alder seed trees or woodlands, in contrast to soil seed bank studies undertaken in deciduous stands or on succession sites. Apart from the study by Decocq et al. ([25]), no viable alder seeds were found in soil samples taken between May and December; not even from samples taken next to alder seed trees ([59], [102]). This is a clear indication of a transient seed bank.
The number of alder seeds transported vertically in the soil, and the depth of transport, could not be derived in any detail from the studies evaluated. Kalamees & Zobel ([59]), who collected samples without litter from a pioneer forest with alder and birch, detected high numbers of viable birch seeds but no viable alder seeds. Only in one case were very low densities of 2.4 and 3.2 viable alder seeds per m² confirmed in two mineral soil samples taken close to seed trees in June ([25]). However, studies providing the occurrence of alder seeds in the soil indicated that they tend to be more prevalent in the upper soil and in the humus layer than in the lower soil layers (Fig. 2). Kjellsson ([63]) concluded, therefore, that alder seeds are short-lived and that large seed numbers in the soil were probably due to recent seed rain.
The buried seed experiment by Granström ([40]) indicated a shorter lifespan of alder seeds than for birch. Early germination in the field before sampling could not be ruled out but after 1.5 years of seed storage in soil the proportion of viable seeds was about 60 %, and only 2 % after 5 years. Interestingly, the pericarp and wings of buried alder seeds were still intact, while parts of birch seeds had begun to decompose ([40]). It seems unlikely that after a long period of vertical drift many alder seeds reach the deeper soil layers in a condition allowing for germination. Decocq et al. ([25]) claimed that alder can establish a more persistent seed bank, whereas Thompson et al. ([98]), Bekker et al. ([10]) and Onaindia & Amezaga ([79]) assigned alder to the transient seed bank type. Considering the lack of available data and literature, a reliable statement on the alder soil seed bank type is not possible. Some results suggested a transient seed bank, but this seems to have been influenced in part by peculiarities of the sites in question. In future research, typical alder sites such as floodplains should be included in sampling.
Soil seed banks of Salix spp. and Populus tremula L.
The results provided on Salix spp. were often no more specific than a mere reference to the genus “willow.’ Therefore, it is not possible to discuss different willow species in detail. Despite the common assumption that willows have short-lived seeds ([58], [77], [9], [104]), the genus was the second most abundant pioneer tree species in the papers analyzed, occurring in 17 % of all sample plots. Poplar seeds, morphologically similar to willow seeds, were almost always absent in soil seed banks (1 % of sample plots). 3 European aspen seeds per m² were observed only once by Heinrichs ([45]) on a succession site, which indicates a rapid loss of poplar seed viability in soil ([103], [9]).
Viable willow seeds germinated predominantly in soil samples from succession sites, where seed trees were present. The highest recorded number of willow seeds was 350 seeds per m2 on a 15-year old meadow succession site dominated by willow ([32]). Summarizing all succession studies, seed density ranged from 6-350 seeds per m2 ([6], [32], [10], [26]). A few willow seeds were also present in some soil samples from beech forests, with 7 and 28 seeds per m² ([95]), in Norway spruce forests with 11 and 104 seeds per m² ([41], [11]), and in 65-year old mixed beech-spruce forest with 156 S. caprea seeds per m² ([11]). All of these authors studied the humus and mineral soil layers. In one study, S. alba L. grew at high frequencies in the vegetation (7 - 24 %), but no viable seeds were identified in the soil ([12]). Gurnell et al. ([44]) also detected willow species in the vegetation along a newly created riverbank but not in the seed bank. The authors explained the results by assuming transience of the seeds and immediate germination after ground contact.
Information on willow seed densities in different soil layers and at different depths was rare in the evaluated studies. Staaf et al. ([95]) observed 7-14 willow seeds per m2 in the humus layer and in the mineral soil at a depth of 0-5 cm in a beech forest. Bakker et al. ([6]), by contrast, recorded 80 goat willow seeds per m2 in some 0-5 cm soil samples taken from 20 to 80-year old Juniperus shrubland, whereas 80 seeds per m² were detected in a 5-10 cm soil sample in annually grazed Juniperus shrubland. Six seeds of S. caprea were present only once in mineral soil sampled from a succession site ([26]). All authors reported the absence of willow seed trees, which highlights long willow seed dispersal distances ([88]) and the possibility of formation of a willow soil seed bank (Fig. 2). However, viable willow and poplar seeds were only derived from samples collected in March and April ([95], [41], [6], [32], [10], [11], [26]). Samples taken near willow and European aspen seed trees in May and June contained no viable seeds of either genus ([32], [25]) due to a rapid loss of germination ability after deposition on soil ([9]). This also provides a strong indication of a transient seed bank for both genera.
Information about willow seeds buried in the soil artificially could not be found in the literature. Thompson et al. ([99]) concluded that willows do not have a persistent seed bank. Certain aspen and Salix spp., especially S. caprea, an early flowering species, were assigned to the transient seed bank type ([98], [99], [10]), whereas S. alba, a late flowering willow species, was assigned to the long-term persistent seed bank category ([11]). Perhaps the high numbers of viable willow seeds recorded in the soil had all fallen into cracks or were from late flowering willow species. It also seems possible that willow seeds protected by soil are viable for a longer time than those on the ground surface or humus layer, or that the movement of the smaller and lighter seeds to deeper soil layers proceeds rapidly, as documented by Van Tooren ([101]) and Burmeier et al. ([22]).
Soil seed bank of Sorbus aucuparia L.
In contrast to all the other pioneer tree species mentioned, rowan was not found in any of the soil seed bank studies, although seed trees were present in some of the study areas ([39], [25], [26], [45], [57]). Often the only indication of successful reproduction was the presence of young rowan trees in the herb and shrub layer, for example, in conifer forests ([39], [45]). With secondary dormancy, the seeds can be part of the soil seed bank for at least 1 - 2 years ([68]). However, the findings of this review indicate that rowan seeds are always absent from the soil seed bank. Grime et al. (1988 cited in [84]) and Dölle & Schmidt ([26]) assigned rowan to the transient seed bank type, because seeds persist in soil for less than 1 year. In contrast, Hill ([47]), Leder ([68]) and Erlbeck ([31]) agreed that rowan seeds can remain viable in the soil for long time, up to 5 years. Based on the above, it would appear possible that rowan has a short-term persistent seed bank. This assumption would seem to have been confirmed by an experiment with buried pomes, which showed that more than 80 - 90 % of the seeds remain viable after 2 years storage in the soil. During the third year the ability to germinate decreased to 30 - 50 %, but some rowan seeds remained viable after 5 years of storage ([40]). Up to 9 % of fresh, stratified rowan seeds exposed under field conditions germinated in the second or third year after sowing ([74]). Due to the clumped distribution of rowan seeds by birds ([70]), future studies of the occurrence of rowan in soil seed banks should take into consideration the structural elements used as perches by birds ([100]).
Conclusions
Pioneer tree species are short-lived, light demanding species, which are very important for the successful colonization and reforestation of large disturbed woodlands in central and north-west Europe. Soil seed banks can drive reforestation in the absence of seed rain. Soil seed banks in woodlands play an important role in succession and in the regeneration of disturbed areas in European temperate forests.
This review showed that pioneer tree species do not possess the kind of long-term seed banks that certain herbaceous species can have. The findings suggest that birch is the only pioneer tree species of temperate forests in central and north-west Europe with a longer-lived soil seed bank. Often birch was the second most abundant species in soil and the only pioneer tree species with a high degree of consistency in soil seed banks. In medium to deeper soil layers (5 - 10 cm) birch seeds seem to have at least a short-term persistent seed bank. Alder seeds are poorly represented in forest soils compared to birch, so a reliable statement on alder soil seed bank type is not possible. The few results available suggest a transient seed bank. The studies for willow and poplar seeds partly confirmed the assumption of very short-lived seeds, although willow was the second most abundant pioneer tree species in soil seed banks and also found in mineral soil (0 - 5 cm). Surprisingly in the case of rowan, the only fleshy-fruited pioneer tree species with proven seed dormancy, a transient seed bank must be assumed due to the absence of rowan seeds in the soil. Buried seed experiments showed, however, that rowan seeds can build up a short-term persistent seed bank due to dormancy.
Statements on the seed densities of pioneer tree species in the soils of different coniferous and deciduous forest types cannot be given. The reason for this is that these seed densities are primarily influenced by the number of and the distance from seed sources, and the seasons of seed production and seed dispersal. Our review revealed that the successful regeneration of birch, alder and willow depends mainly on the proximity of seed trees. Therefore, the proximity of trees is important for the regeneration of species with short-lived seeds. The findings of the review also indicate a dependence between seed density in the soil and the season in which soil sampling occurs. Maximum seed densities of birch, alder and willow were detected during and shortly after seed rain. No statement can be made in this regard in relation to rowan and poplar.
This review revealed a number of open questions concerning the capacity of all European pioneer tree species to establish seed banks. These issues are connected to (a) the seed viability under different soil conditions and litter thickness, (b) the speed of seed movement into deeper soil layers, and (c) the direct correlation between the proximity of seed trees and the resultant number of seeds in the soil. At present, it can be concluded that birch, representative of pioneer tree species in temperate forests of central and north-west Europe, has the capacity to establish a seed bank of a duration of 1 - 5 years, sufficient to compensate for years with lower levels of seed production and to regenerate successfully after disturbance. However, the soil seed bank must be supplemented by fresh seeds from surrounding seed trees as often as possible in order to guarantee continuous regeneration.
Acknowledgements
This review was supported financially by a scholarship provided from the Deutsche Bundesstiftung Umwelt (DBU) fund to promote young scientists. We thank the reviewers for their helpful comments and David Butler Manning for proofreading the text.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Franka Huth
Sven Wagner
Institute of Silviculture and Forest Protection, TU Dresden, Pienner Str. 8, 01737 Tharandt (Germany)
Corresponding author
Paper Info
Citation
Tiebel K, Huth F, Wagner S (2018). Soil seed banks of pioneer tree species in European temperate forests: a review. iForest 11: 48-57. - doi: 10.3832/ifor2400-011
Academic Editor
Michele Carbognani
Paper history
Received: Feb 09, 2017
Accepted: Jan 12, 2018
First online: Jan 25, 2018
Publication Date: Feb 28, 2018
Publication Time: 0.43 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 56887
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 44122
Abstract Page Views: 5495
PDF Downloads: 6227
Citation/Reference Downloads: 38
XML Downloads: 1005
Web Metrics
Days since publication: 2960
Overall contacts: 56887
Avg. contacts per week: 134.53
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 35
Average cites per year: 4.38
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Is there an effect of storage depth on the persistence of silver birch (Betula pendula Roth) and rowan (Sorbus aucuparia L.) seeds? A seed burial experiment
vol. 14, pp. 224-230 (online: 06 May 2021)
Review Papers
Methods of soil seed bank estimation: a literature review proposing further work in Africa
vol. 15, pp. 121-127 (online: 26 March 2022)
Short Communications
Evidence of Alectoris chukar (Aves, Galliformes) as seed dispersal and germinating agent for Pistacia khinjuk in Balochistan, Pakistan
vol. 14, pp. 378-382 (online: 22 August 2021)
Research Articles
Dispersal and hoarding of sympatric forest seeds by rodents in a temperate forest from northern China
vol. 7, pp. 70-74 (online: 18 November 2013)
Research Articles
Use of brassinosteroids to overcome unfavourable climatic effects on seed germination in Pinus nigra J. F. Arnold
vol. 17, pp. 1-9 (online: 02 February 2024)
Research Articles
Influence of mother plant and scarification agents on seed germination rate and vigor in Retama sphaerocarpa L. (Boissier)
vol. 7, pp. 306-312 (online: 08 April 2014)
Research Articles
Effects of brassinosteroid application on seed germination of Norway spruce, Scots pine, Douglas fir and English oak
vol. 10, pp. 121-127 (online: 02 October 2016)
Research Articles
Seed trait and rodent species determine seed dispersal and predation: evidences from semi-natural enclosures
vol. 8, pp. 207-213 (online: 28 August 2014)
Research Articles
The effectiveness of short-term microwave irradiation on the process of seed extraction from Scots pine cones (Pinus sylvestris L.)
vol. 13, pp. 73-79 (online: 13 February 2020)
Research Articles
The effect of seed size on seed fate in a subtropical forest, southwest of China
vol. 9, pp. 652-657 (online: 04 April 2016)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword