Methods of soil seed bank estimation: a literature review proposing further work in Africa
iForest - Biogeosciences and Forestry, Volume 15, Issue 2, Pages 121-127 (2022)
doi: https://doi.org/10.3832/ifor3850-015
Published: Mar 26, 2022 - Copyright © 2022 SISEF
Review Papers
Abstract
A number of methods are used to assess the soil seed banks of a range of plant species in various habitats around the world, with approaches that differ between countries and continents. An understanding of the differing techniques emphasises the need for further research, especially in Africa. We reviewed 97 articles on soil seed bank estimation, published between 2010 and 2020, and only 13.41% of these were from Africa. Soil sample collection in Africa was based mainly on stratified random sampling, systematic sampling, random sampling or cluster sampling carried out at the end of each region’s rainy season. Random and cluster sampling were more widely used in savannas, while stratified random and systematic samplings were more common in forests. The shape of the samples was either circular or quadrilateral (square and rectangular) or they were measured by soil mass or volume. The soil sampler cores most often applied were: circular diameter of 5 cm; square sizes of 10 × 10 cm, 20 × 20 cm and 25 × 25 cm; and rectangular sizes of 20 × 25 cm and 20 × 10 cm. The most-used soil core depths were 5 cm and 10 cm. No specific sample shape was linked with either forest or savanna ecosystems, although the number of samples depended on the land use and land cover. Soil seed bank densities and species composition were mainly assessed with direct greenhouse germination over trial duration depending on the plant species’ functional traits. In analysing soil seed bank data, non-parametric statistics were more frequently used than parametric statistics because of the skews in the data. This review will contribute to future soil seed bank studies in Africa.
Keywords
Soil Seed Bank, Sampling Methods, Greenhouse Germination, Literature Review
Introduction
Seeds from a broad range of plant species occur in soil seed banks in various habitats and may be peculiarly important in restoration projects, where preferred species have been lost from the vegetation but survive in the seed bank ([9]). Large amounts of seeds can remain dormant in soil for many years, and can germinate when conditions become favourable ([74]). Soil seed banks are therefore important in understanding vegetation history as the vegetation composition in terms of plant species is influenced by seeds production, dispersal and longevity of seeds as well as soil depth ([66]). Significant role can be attributed to seed banks as the determinant of future vegetation, especially after a disturbance ([74]).
In assessing soil seed banks, basic approaches (for example wet sieving and flotation, both of which are followed by identification of seeds under a stereoscopic microscope) are emphasised ([66]). Other approaches include the cultivation of soil samples and subsequent identification of the emergent seedlings ([4]). The advantages and disadvantages of each of these methods have been demonstrated independently by various researchers and have been subject to extensive discussion ([29], [43]). Moreover, the flotation approach was criticised for its inaccuracy ([30]), and therefore the two remaining approaches (cultivation of soil samples approach and wet sieving approach) would have been more widely used in recent soil seed bank analyses.
Although soil seed bank studies have been conducted in many parts of the world, the literature shows a large range of methods from sampling stage to the estimation of species diversity and density and sometime with methodological biases along the study process ([11]). Thus, no adequacy of soil seed bank assessment methods has been reported yet. For example, in the attempts to minimize sampling method biases, many research dealt with the tedious compensable process of huge amount of soil samples without sufficient guarantee ([9]).
In addition, soil seed bank assessment in different ecosystems was conducted with time and labour investment because of the technicalities of the method procedures ([5]). It is therefore important to document the soil seed bank assessment method in relation to the ecosystems for further research on the technical and method procedures.
Many African ecosystems are degraded due to multiple factors such as fire, intensive logging, grazing and climatic change ([56]). Thus, there is a need for ecosystems restoration and conservation in Africa. The soil seed bank of theses ecosystems may be of interest for ecological restoration due to the presence of seeds from the above vegetation in the soil ([55]). Moreover, it might be acknowledged that few studies concern this topic in Africa. Despites the few studies on soil seed bank assessment in African ecosystems (Tab. S1 in Supplementary material), there is a lack of reference method for trials experiment, data collection and analysis. The method bias is an issue for seed bank analyses and discussion of the results ([10]). To make soil seed bank analysis more useful and especially in Africa, it should be important to integrate data from various databases. Combined environmental data (soils, vegetation and climate) would allow modelling of plant species distribution and/or ecological characteristics of stand vegetation ([21], [64]) to aid landscape restoration. The challenge of this literature review was to find which seed bank assessment methods would be preferentially adopted in soil seed bank assessment in Africa. This paper was then based on soil seed bank literature in relation to vegetation patterns (grassland or savannas and forests) and aims both to highlight the relevant literature on recent methods used in seed bank studies and to emphasise the need for further research within this area in Africa.
Methods of literature search
The Web of Sciences® database was consulted for the papers published in the period of 2010-2020. The keywords used to search the papers included “soil seed bank”, “seed bank and methods”, “seed bank and soil sample”, “soil seed bank and Africa”, “seed bank and grassland”, “seed bank and savannas”, “seed bank and forest”, “seed bank and herbaceous”, “seed bank and tree species”. The papers that did not clearly provide the methods used in soil seed bank assessment were discarded as well as the review papers that did not focus on understanding the seed bank assessment efficiency and/or the accuracy of a method or the comparison of methods. A total of 97 papers were finally considered for this review. Data were analyzed with regard to the objectives of the study, the soil sampling methods, soil sample size, number of samples, seed bank estimation methods, above-ground vegetation analysis methods, soil analysis methods, duration of trial, type of data collected during trial, data analysis methods, plant species studied (herbaceous, trees or both), vegetation type (savanna, grassland, forest), country and continent. Frequency, tables and charts were used to present the findings.
Review of seed bank literature
The main objectives of the studies examining soil seed banks were: (1) to assess the effects and intensity of earlier disturbance on aboveground vegetation ([69], [39], [48], [54]); (2) to evaluate restoration methods ([36], [32], [42]); and (3) to understand habitat resilience to threat ([14], [75], [22]). Others studies have focused on the comparison of ecological habitats in terms of plant species, diversity variation in seed banks ([1], [18], [57], [19]) and dynamics of soil seed banks in relation to aboveground vegetation ([25]).
Studies concerning the methods of soil seed banks assessment were mostly related to the composition and structure of the above-vegetation in relation to the soil seed bank ([3], [29], [60], [53], [49]). Other studies addressed how to reduce bias in greenhouse seed bank data by using post-disturbance gap emergence trials ([49]) and considered whether a large number of small-sized samples are important in forest soil seed bank characterisation ([60]). In addition, these methodologies were widely tested in different ecosystems in North and South America, Asia and Europe. However, few studies addressed the methods of soil seed banks assessment in Africa with diverse ecosystems. Two categories of research questions were addressed in the studies conducted in Africa, such as: (i) how land use or land disturbance affect seed bank richness, density and distribution ([20], [1], [67], [69], [26], [2], [54]); and (ii) the relationship between the soil seed bank and aboveground vegetation and the impact of forest management on seed bank ([13], [27], [28], [55], [65], [19]). This review is therefore an important step to guide future soil seed bank study in Africa.
Seed bank sampling methods
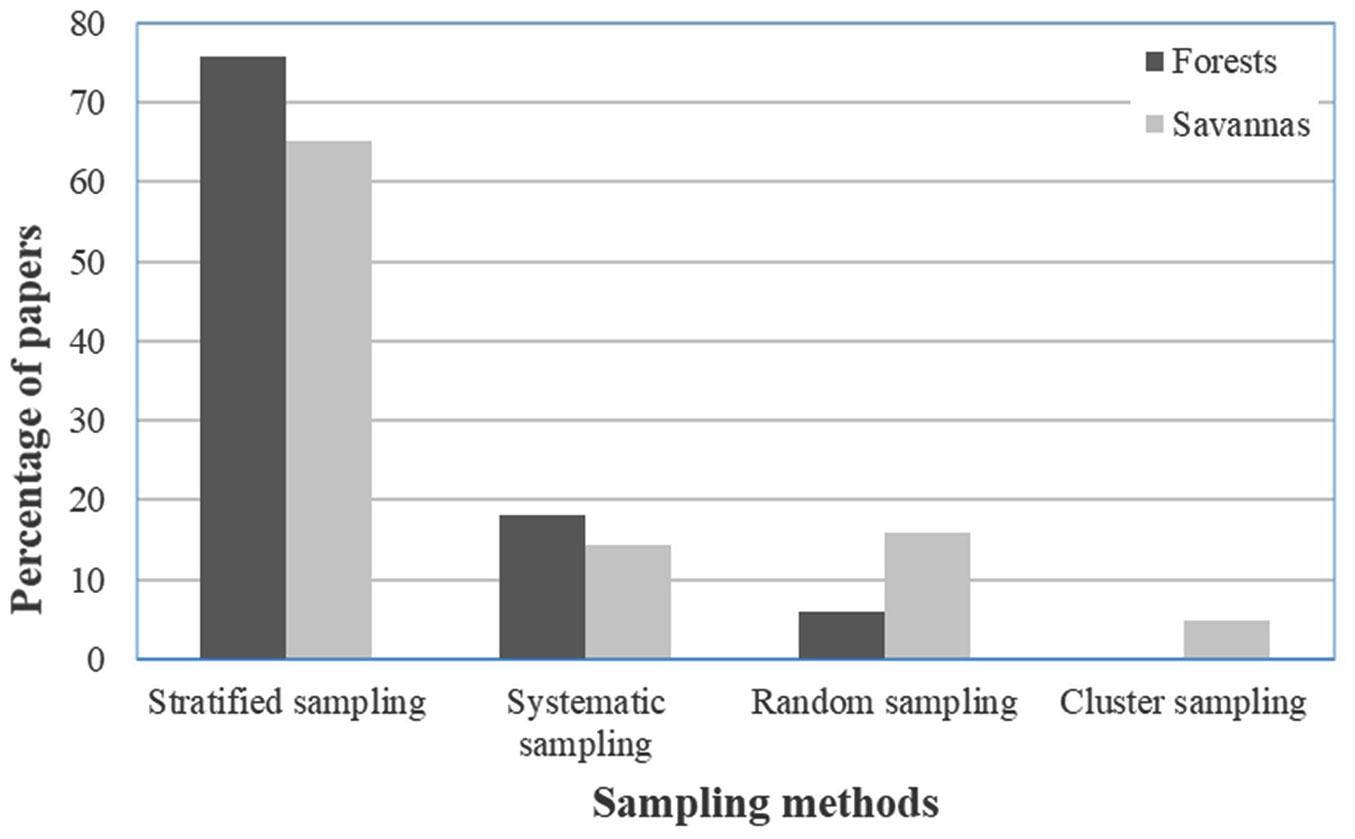
The choice of soil sampling technique in seed bank assessment is as important as the number and dimensions of the sample ([5], [45]). A total of 75% of the papers considered used stratified random sampling techniques for sample collection, while 14% used systematic sampling, 10% used random sampling and 1% used cluster sampling. The chosen sampling method did not depend on the study location and the objectives of the study, but rather on the homogeneity of the aboveground vegetation of the study area ([33], [66]), the slopes of the vegetation site surveyed ([60], [49]) or the intensity of land use and disturbance ([20], [63], [44], [17], [59]). Thus, all the sampling methods can be used in Africa ecosystems. Up to now, two sampling methods (systematic and stratified random samplings) were used in the studies carried out in Africa. The systematic sampling concerned three studies in forest ecosystems ([13], [19]), savanna and grassland ecosystems ([54]). The stratified random concerned 10 studies in woodland and savanna ecosystems ([20], [69], [55], [2]), desert ecosystems ([27], [28]), farmland ([1]) and tree plantations ([67], [65], [26]).
With the systematic sampling method, a complete description of the units (or individuals) and their arrangement in the population is required. The first unit is drawn at random from the population, and every n-th unit is selected until the desired sample size has been obtained. With stratified random sampling, a population is first divided into subpopulations or strata, which may or may not be of equal size. Within each stratum, a sample is selected randomly and independently. With cluster sampling, groups of units are selected randomly from the population. These groups can also be called clusters or primary units and are composed of secondary units. With cluster sampling, all secondary units are sampled. Simple random sampling is a method where each possible sampling unit has an equal (or known) probability of being selected, and the random selection of such units ensures unbiased estimates of population means and sampling variance.
The systematic and stratified random methods were used for trees and herbaceous plants in forest, savanna and grass vegetation patterns. The cluster and random methods were used with herbaceous plants in savannas and grasslands. The stratified random sampling technique was mostly applied in the forest and savanna vegetation (Fig. 1). The choice of method can be due to the heterogeneity in land cover within these ecosystems, to reduce bias ([17], [59]). In Africa, stratified random design method was mostly used due to the spatial heterogeneity within each ecosystem (physical, biological, or environmental characteristics - [43]). Thus, this method can be the most appropriate within Africa ecosystems when heterogeneity has to be taken into consideration.
Soil sample shape and dimension
The samples taken in soil seed bank studies were circular, square or rectangular. The most common sample shape was the circular method with 47% of studies, followed by quadrilateral at 37%, of which squares made up 58.74% and rectangles 41.26%. There was no specific sample shape linked with either forest or savanna ecosystems. This is due to the land cover heterogeneity within each ecosystem of forest or savanna ([74]). Thus, all the sample shapes can be used in Africa ecosystems. The shapes used in Africa included circular plots in savanna, grassland and tree plantations ecosystems ([20], [2]), square plots in savanna and woodland ecosystems ([54], [55]) and rectangular plots in desert ecosystems ([27], [28]). More evidences are needed on the relevant shape to consider within each ecosystem in Africa, as the shape used to assess vegetation pattern varied according to the ecosystem ([52]).
The diameter of the circular-shaped plot varied from 1.8 cm to 40 cm, although 5 cm was most commonly applied (55.3%), followed by 1.8 cm (13.03%), 2 cm (10.31%), 2.5 cm (7.19%), 9 cm (6.38%), 12.5 cm (4.51%), 20 cm (2.94%) and 40 cm (0.34%). No specific diameter was attributed to a study area, or to a country or continent. Thus, the different diameters can be used in Africa ecosystems. The diameters used in Africa included 5 and 8.5 cm in savanna and grassland ecosystems, respectively ([20], [2]), 5 cm in pine plantations and 6 cm in Acacia plantations ([26], [65]). More research is needed to provide evidence on the specific diameter to consider within each ecosystem in Africa.
With the quadrilateral shape, the square was more widely used than the rectangle. The most common sizes were 10 × 10 cm (48.25%), 20 × 20 cm (25.75%) and 25 × 25 cm (22.59%), with others (15 × 15 cm, 30 × 30 cm, etc.) rarely considered (3.41%). The most-used rectangular shapes were 20 × 25 cm (62.36%) and 20 × 10 cm (23.01%), followed by 15 × 8 cm (8.21%), 25 × 39 cm (3.33%) and others (37 × 27 cm; 30 × 10 cm; 135 × 50 cm, etc.) at 3.09%. Soil volume or soil mass ([1], [7], [23], [35]) and sampling area ([71], [72], [41]) were rarely used in soil seed bank assessment studies. The size mostly used in Africa included 15 × 15 cm in savanna and woodland ([55], [54]) and 20 × 25 cm in desert ecosystem ([28]). More research is needed to provide evidence on the specific size to consider within each ecosystem in Africa.
Soil depth also influences soil seed bank estimation ([11]). The most frequently applied depths of soil cores were 5 cm (53.27%) and 10 cm (40.64%). Other studies assessed the variation in the soil core depths from 0 to 20 cm at intervals of 5 cm (0-5 cm, 5-10 cm, 10-15 cm and 15-20 cm) and its influence on soil seed banks ([16], [61], [40]). There was no relationship between soil core depth and study area or geographic location. However, soil core depth was linked to the plant species’ seed behaviour (seeds mass and shape) or habitat (soil and vegetation type - [74], [47], [54]). While the different soil depth can be considered in Africa ecosystems, further studies are needed to address the relevant soil depth within each ecosystem. The soil depths actually considered in studies conducted in Africa included simple and multi layers. The simple layers included 0-4 cm in savanna and grassland ecosystems ([20]), 0-5 cm in desert ecosystems ([27], [28]) and forest ecosystems ([13]), 0-10 cm ([26]) and 0-15 cm ([65]) in tree plantations. The multi layers included 0-3, 3-6, 6-9 in savanna and woodland ecosystems ([55]), 0-5, 5-10, 10-15 cm in savanna and grassland ecosystems ([2], [54]), 0-5, 5-10, 10-20 cm in forest ecosystems ([19]).
Sample number
In assessing soil seed banks, the number of soil samples taken is crucial not only to promote the accuracy of the study and the relevance of its results ([45]) but is also key to considerations of both time and labour intensity ([5], [3]) and hence to cost-benefit ([46]). The challenge of this review is to find which sample number should be preferentially adopted in soil seed bank evaluation, especially in Africa. Regarding this matter, it has been stated that the sampling method can influence the sample number in any given ecosystem ([3]). For example, the sample number can be less from systematic sampling than from random sampling without losing relevance in results while using the same sample dimension. However, regardless of sampling method, accuracy in soil seed bank estimation can be improved by ensuring a sufficient number of samples ([6]). This is because the precision in gauging seed densities may be under- or overestimated when the number of samples is small ([74]). Therefore, the number of soil samples should be more than 50 to provide a reasonable estimate of the seed density ([6]). However, this number can be less in areas with high density of seed banks ([45]).
Among examples of soil seed bank studies in Africa, Sanou et al. ([54]) used 720 soil samples when comparing the aboveground vegetation and soil seed bank composition related to different grazing intensities in Burkina Faso. Tessema et al. ([69]) used 544 soil samples to assess changes in grass plant populations and temporal soil seed bank dynamics in a semi-arid African savanna. Gomaa ([28]) considered 450 soil samples in a desert ecosystem when reporting on the variation between soil seed banks and stand vegetation in Egypt. In other papers focused on Africa, the number of soil samples depended on the volume of soil available to use ([19]).
Period of soil sampling
The timing of soil sample collection is of great importance in soil seed bank assessment studies ([70]). Several papers highlighted the period of field soil sample collection, at least in reference to the seasonal climate of the study area. The end of the rainy season was most cited for soil sampling in tropical regions, particularly those in Africa ([8], [55], [65], [54], [62]). During this period, it is easier to investigate the composition, density and vertical distribution of the viable soil seed bank. Seed dispersal in rainy season could attain the peak and the persisting seed from the previous season could still germinate ([55], [65], [54]). The earlier germination of the transient seeds can also justify the choice of the end of the rainy season. Moreover, the end of the rainy season can allow collecting information on total soil seed bank, because seed dispersal ended in this period and most transient seeds may already emerge ([43]).
Other studies generally matched sampling to the period between earlier seed bank germination and when the new seeds had matured and spread ([69]), which may correspond to the rainy season when there is abundant seed availability in the soil ([61], [62]). Moreover, the particular research goal can often lead the timing of soil sampling for vegetation evaluation ([11]). In these cases, phenological processes of the stand vegetation or of the given species would be important in seed bank composition. Evidently, the life duration of seeds would also be important in setting the time period of sampling ([51]). More literature focusing on the study object would therefore be helpful in further establishing the timing of soil sampling in seed bank characterisation.
Seed bank estimation methods
Many methods have been used in the literature to estimate seed numbers in soil samples. Warr et al. ([74]) highlighted the separation of seeds from soil by using water (washing or flotation); this method was not appropriate in several cases because of the non-distinction between viable and unviable seeds and the underestimation of species numbers due to the similarity of different seeds. Also, the risk of washing out of very small seeds makes the method very uncertain. Thus, alternative methods of seed numbering using germination were developed to improve the accuracy in soil seed bank estimation.
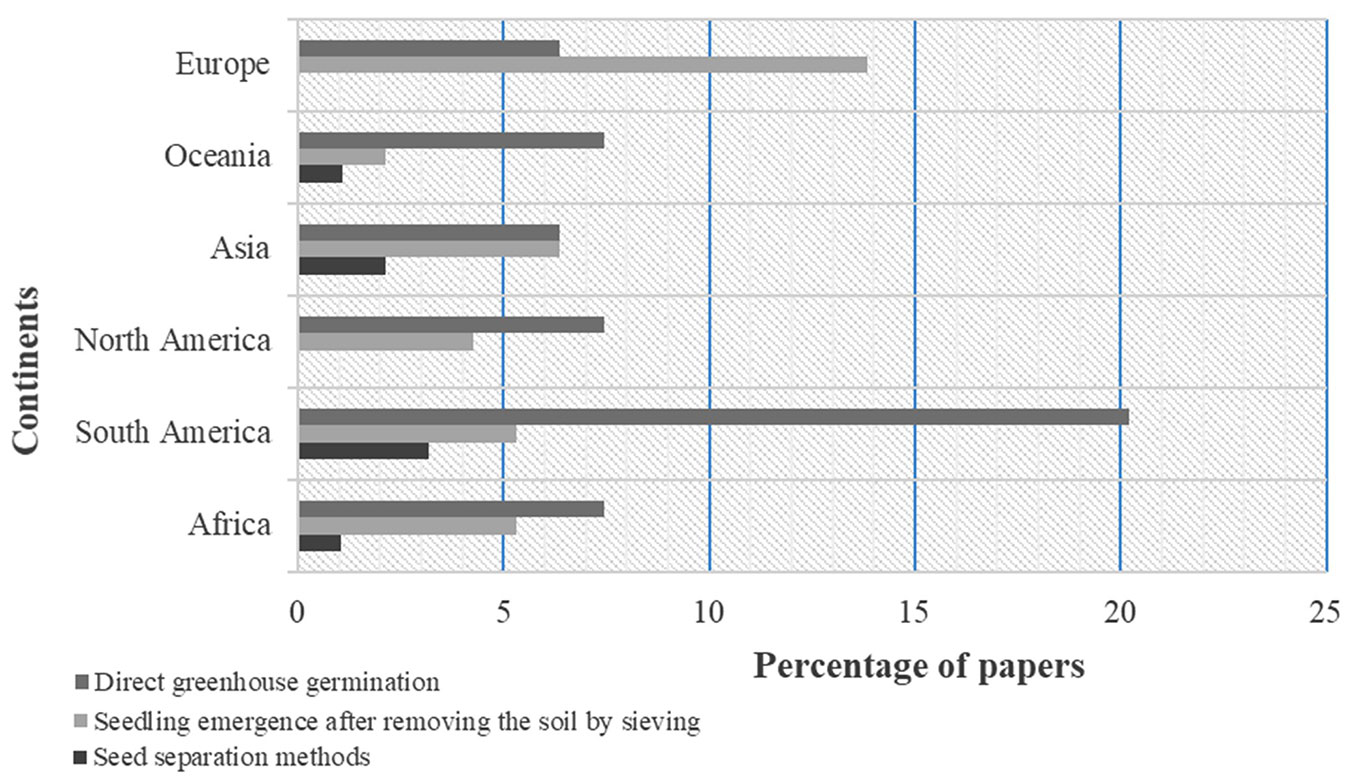
Three methods of seed numbering by germination have been used to assess seed banks in soil samples. Direct greenhouse germination was most frequently used to assess soil seed bank densities and species composition (60.22% of the examined studies). This method of quantifying seeds in soil samples was most practised in South America, Oceania, North America, Asia and Africa (Fig. 2). The second method most favoured worldwide was the use of sieving before seed germination (23.86%). Soil seed bank estimation in Europe used this method more than the others. The third approach was the seed separation method (15.92 %), which was less used in all the regions (Fig. 2). Combinations of methods includng the seed concentration method ([68]) or sieving ([50]) before seed germination were used in the estimation of soil seed bank (40% of papers). Therefore, the germination of soil seed (greenhouse trial) can be applied after sieving seeds, for example (seed concentration). The combination of methods can often be used to test when seed already identified is also viable or to confirm the plant species.
Each method has its advantages and disadvantages ([74]). Many authors highlighted that methods in combination could increase precision in seed density estimation ([71]). However, the methods can also be used separately, not only to determine seed densities in soil layers, but also to test seed viability via germination ([65]) or to compare the efficacy of different methods ([29]). Of the entire above, no specificity was found for the methods used and habitat type ([43]). The same method singly or in combination that is applied in soil seed assessment for forest ecosystem can also be used for savanna ecosystems. Thus, all the methods can be used in Africa ecosystems. Direct greenhouse germination was mostly used in Africa (62%) with samples from savannas ecosystems ([1], [69], [55]), savanna and grassland ecosystems ([2], [54]) and tree plantation ([67], [26], [65]). The combination of the direct greenhouse germination method with either the seed concentration method of Ter Heerdt et al. ([68]) or sieving of Roberts ([50]) was also used (38%) in seed bank assessment in Africa ecosystems, including forest ([13], [19]), savanna and grassland ecosystems ([20]) and desert ecosystems ([27], [28]). More research is needed to provide evidence on the appropriate methods for each ecosystem in Africa.
Data collection and seed bank analysis methods
In the greenhouse, the frequency of data collection on seed germination, seedling growth and radicle elongation was daily, weekly or monthly, depending on plant species biology. More than 83% of the examined papers showed that data on the emerged seedlings were collected during the growth trial in the greenhouse. Many authors noted that the trays needed to be checked at regular intervals for new emergent seedlings ([47], [18], [55], [54]). Each germinated seed was counted, recorded and removed. When seedling identification was not easy, it was transplanted elsewhere and grown until species identification was possible. Therefore, the trial duration depended on the plant species under study and could vary from two weeks to two years. The study area or habitat had no link with the duration of the trial for species identification. This variation can also be applied in Africa ecosystems where the actual duration of trial germination varied from 3 weeks ([1], [2]) to 9 months ([69], [26]) for samples collected from savanna ecosystems ([1], [69], [55]) or grassland ecosystems ([2], [54]).
Seed density or diversity indices (stand vegetation and seedling) were mostly calculated in the published papers regarding soil seed bank assessment. Thereby, in order to compare diversity between areas of seed banks, the coefficients of similarity were often used ([74]). Therefore, the Sørensen’s similarity index between seed bank and aboveground vegetation was calculated using presence-absence data ([58], [24], [59]). Other statistical methods were also used to compare the seed bank density with aboveground vegetation. In this case, non-parametric statistics were found to be more relevant than parametric statistics because of the skews in seed bank data (even after data transformation) in order to meet the statistical requirements ([74]). Several studies used the Kruskal-Wallis test or the Mann-Whitney U test to compare density of emerged seedlings, floristic composition, richness and diversity of species ([12], [15], [25], [38], [44], [34], [57], [61]). However, parametric statistics were still used either with or without data transformation to compare seed banks with standing vegetation. Analysis of variance (ANOVA) followed by post-hoc Tukey’s test were applied to structural differences by Kunz & Martins ([37]), Shang et al. ([58]), Galloway et al. ([26]), Luo et al. ([42]), Forte et al. ([23]) and Sharma et al. ([59]). Data can also be analysed by performing a generalised linear mixed model to highlight the relationship between seed density and species composition ([31], [65], [22], [48], [73]).
Conclusion
This study reviewed the existing literature on soil seed bank assessment and the methodologies used from sampling to data analysis. Of the 97 scientific papers reviewed, only 13.40% were from Africa. The stratified random sampling method was the most applied for soil sampling due to heterogeneity in the land cover within the ecosystems. The circular sample with 5 cm diameter and 5 cm depth was most widely used to sample the soil. For soil seed bank estimation, the greenhouse germination method was the most adopted. Data on seed germinated, seedling growth and radicle elongation were collected at daily, weekly or monthly intervals based on species behaviour. For data analysis, floristic data were generally analysed with the Sørensen’s similarity index, while ANOVA or the Kruskal-Wallis test were used for density data. Generalized linear models were used to show the relationship between seed density and species composition. The methods used in soil seed bank assessment are not specific to a region and can be transferred in all ecosystems in Africa for ecological restoration. This review is an important step in furthering soil seed bank estimation in Africa for ecosystems restoration.
Acknowledgements
This work was supported by the ARES development cooperation. The authors thank Dr. Kasso Daïnou, Dr. Félicien Tosso and Prof. Jean Louis Doucet from the tropical and subtropical forestry laboratory, University Faculty of Agricultural Sciences, Gembloux (FUSAGx), Belgium.
References
Online | Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Bernard Tchigossou
Bruno Djossa 0000-0002-4033-5126
School of Tropical Forestry, National University of Agriculture, BP 43 Kétou (Benin)
Bokon A Akakpo
Bruno Djossa 0000-0002-4033-5126
Laboratory of Applied Ecology, Faculty of Agronomic Sciences, University of Abomey-Calavi, 01 BP 526, Cotonou (Benin)
WASCAL, Climate Change and Human Habitat, Federal University of Technology, Minna PMB 65, Niger State (Nigeria)
Corresponding author
Paper Info
Citation
Padonou EA, Akakpo BA, Tchigossou B, Djossa B (2022). Methods of soil seed bank estimation: a literature review proposing further work in Africa. iForest 15: 121-127. - doi: 10.3832/ifor3850-015
Academic Editor
Michele Carbognani
Paper history
Received: Apr 20, 2021
Accepted: Jan 21, 2022
First online: Mar 26, 2022
Publication Date: Apr 30, 2022
Publication Time: 2.13 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2022
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 38639
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 31638
Abstract Page Views: 3127
PDF Downloads: 3404
Citation/Reference Downloads: 14
XML Downloads: 456
Web Metrics
Days since publication: 1403
Overall contacts: 38639
Avg. contacts per week: 192.78
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2022): 10
Average cites per year: 2.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Review Papers
Soil seed banks of pioneer tree species in European temperate forests: a review
vol. 11, pp. 48-57 (online: 25 January 2018)
Research Articles
Is there an effect of storage depth on the persistence of silver birch (Betula pendula Roth) and rowan (Sorbus aucuparia L.) seeds? A seed burial experiment
vol. 14, pp. 224-230 (online: 06 May 2021)
Research Articles
Influence of mother plant and scarification agents on seed germination rate and vigor in Retama sphaerocarpa L. (Boissier)
vol. 7, pp. 306-312 (online: 08 April 2014)
Research Articles
Use of brassinosteroids to overcome unfavourable climatic effects on seed germination in Pinus nigra J. F. Arnold
vol. 17, pp. 1-9 (online: 02 February 2024)
Research Articles
Seed germination traits of Pinus heldreichii in two Greek populations and implications for conservation
vol. 15, pp. 331-338 (online: 24 August 2022)
Short Communications
Evidence of Alectoris chukar (Aves, Galliformes) as seed dispersal and germinating agent for Pistacia khinjuk in Balochistan, Pakistan
vol. 14, pp. 378-382 (online: 22 August 2021)
Research Articles
Effects of brassinosteroid application on seed germination of Norway spruce, Scots pine, Douglas fir and English oak
vol. 10, pp. 121-127 (online: 02 October 2016)
Research Articles
Optimum light transmittance for seed germination and early seedling recruitment of Pinus koraiensis: implications for natural regeneration
vol. 8, pp. 853-859 (online: 22 May 2015)
Research Articles
The effect of seed size on seed fate in a subtropical forest, southwest of China
vol. 9, pp. 652-657 (online: 04 April 2016)
Research Articles
Role of serotiny on Pinus pinaster Aiton germination and its relation to mother plant age and fire severity
vol. 12, pp. 491-497 (online: 02 November 2019)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword