Optimum light transmittance for seed germination and early seedling recruitment of Pinus koraiensis: implications for natural regeneration
iForest - Biogeosciences and Forestry, Volume 8, Issue 6, Pages 853-859 (2015)
doi: https://doi.org/10.3832/ifor1397-008
Published: May 22, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
Light transmittance regulated by canopy openness influences the microsite conditions for natural regeneration. The successful transition from seed germination to subsequent seedling recruitment (i.e., early seedling survival and growth) determines the natural regeneration potential. However, there is little information on the effect of varying light transmittance on seed germination and seedling recruitment of Pinus koraiensis Siebold & Zucc. (Korean pine). We aimed to determine the optimum light requirements for this transition process in P. koraiensis to propose practical measures for improving its natural regeneration. The transition process was studied under five light transmittance regimes (100%, 60%, 30%, 15% and 5% of full light) over two consecutive years (2010 and 2011). The highest germination percentage in both years occurred at 30% light transmittance. Generally, mean germination time (MGT) declined with increased light transmittance. Seedling survival exhibited no significant differences between treatments for 1-year-old seedlings, but was higher at 30% than at 5% light transmittance for the 2-year-old seedlings. In contrast, seedling height, root collar diameter and total biomass were highest at 60%-100% light transmittance for both 1- and 2-year-old seedlings. Furthermore, the light transmittance also influenced the growth characteristics of P. koraiensis seedlings through regulating MGT. These results suggest that growth of P. koraiensis seedling requires a higher light transmittance (60%-100%) than that required for seed germination, even though 30% light transmittance was favorable to the earlier emergence with larger specific leaf area. Silvicultural measures such as thinning are recommended to increase light irradiance in the forest understorey with the aim of improving the natural regeneration of P. koraiensis.
Keywords
Germination, Light Transmittance, Natural Regeneration, Seedling Emergence, Seedling Survival
Introduction
Korean pine (Pinus koraiensis) is mainly distributed in northeast Asia ([12]), where it covers about 500 000 km2 of mixed broad-leaved Korean pine forests (MBKPF). The MBKPF is one of the regional climax vegetation types in mountainous areas of northeast China, where P. koraiensis is mixed to a range of broad-leaved tree species such as Juglans mandshurica, Fraxinus mandshurica and Quercus mongolica. However, due to intense human disturbances and extreme natural disasters ([9], [23]), most of the original MBKPF (accounting for 52.5% of Northeast China) have been destroyed and replaced by secondary forests ([36], [31]). The productivity and ecological functions of secondary forests are much lower than those of the original MBKPF. Specifically, secondary forests are characterized by the disappearance of basic structures and intrinsic functions, including the reduction in biodiversity, stability and ecosystem productivity ([36]). Increasing the proportion of P. koraiensis in the secondary forests, and further development of these MBKPF stands is considered important to promote these ecological and productive functions. However, development of a P. koraiensis component has been severely limited due to its failure in natural regeneration.
To achieve natural regeneration, it is vital to provide abundant seeds, ensure successful seed germination, seedling emergence and survival ([37]). The successful transition from seed germination to subsequent seedling recruitment (i.e., early seedling survival and growth) determines the regeneration potential for tree species ([10]). Furthermore, these two key stages are the most sensitive to environmental conditions (e.g., light, temperature and water conditions - [11], [26]) or biotic factors (e.g., animal predation or browsing) in the regeneration processes. Due to the poor palatability of P. koraiensis seedlings, browsing pressure is not a factor limiting natural regeneration. However, the environmental requirements can change from the stages of seed germination to seedling recruitment ([7]). Therefore, determining the requirements for both these two key stages would be helpful to improve the natural regeneration of P. koraiensis.
In silviculture, adjusting the light transmittance to the forest understorey by changing the forest composition and canopy structure is crucial to improve tree regeneration ([21], [15]). Canopy openness is a characteristics widely analyzed in many studies, since it relates with the light transmission through the canopy ([1], [37], [2]). In temperate deciduous forests, canopy openings caused by natural or human disturbances produce spatial heterogeneity of light regimes in forest understorey ([1]). In addition, seasonal variation in leaf canopy also contributes greatly to the temporal variability of light conditions under temperate secondary forests ([25]). Consequently, the spatio-temporal variation of light in the understorey plays a critical role in determining patterns of tree regeneration. The opening of a canopy firstly changes the light regimes, and then brings about the variation in temperature, soil moisture, etc. The estimate of light transmittance in the understorey, therefore, represents a wide range of microsite conditions including light, temperature and soil moisture.
Many studies have been published on the response of seed germination or seedling survival/growth of Pinus spp. to different light conditions ([4]). In particular, it has been reported that light requirements may differ between seed germination and the subsequent seedling growth. For example, Parker et al. ([18]) concluded that both seedling germination and percent emergence were significantly higher under low light (13% of full sunlight) than under moderate light (47% of full sunlight) for Pinus strobus L.; however, moderate light level favored seedling growth in this species ([17]). P. pinea maintained high germination percentages in dark conditions ([8]), while seedlings required fairly high light irradiance ([6]). P. pinaster exhibited the highest germination percentage and aboveground dry weight in 25% and 100% light intensity, respectively ([26]). Pulinets ([22]) studied the development of P. koraiensis under different light intensities created by the shading of shrubs, and concluded that the light requirements varied greatly across the entire growth period. Zhang et al. ([34]) found that 30% of the full sunlight was optimum for seed germination of P. koraiensis in both shade houses and field conditions. To our knowledge, 60% of full light was the most favorable environment for the growth of 5-year-old P. koraiensis seedlings ([39]).
Despite the number of studies on the above topic, no reports based on continuous observations have been published with the aim of exploring possible differences in light requirements between seed germination and initial seedling recruitment in P. koraiensis. To quantify the light requirements of these two key stages, seeds of P. koraiensis were sowed at five light transmittances (100%, 60%, 30%, 15% and 5% of the full light), and emerged seedlings were monitored for two consecutive years. We hypothesized that the light requirements for seedling growth were different from those for seed germination in P. koraiensis.
Materials and methods
Study site
The study was conducted at the Qingyuan Forest CERN (Chinese Ecosystem Research Network), Chinese Academy of Sciences (41° 51′ 06″ N, 124°54′ 33″ E, 456-1116 m a.s.l. - Fig. 1). The climate is a continental monsoon type with a windy spring, a warm, humid summer, and a dry, cold winter. Annual average precipitation in the area is 811 mm and mean annual air temperature is 4.7 °C with a maximum 36.5 °C in July and a minimum -37.6 °C in January. The frost-free period lasts 130 days and the growing season ranges from early April to late September ([38]). The vegetation is representative of the natural secondary forest in this region, dominated by Juglans mandshurica, Phellodendron amurense, Fraxinus mandshurica, Quercus mongolica, and Fraxinus rhynchophylla.
Seed collection and pretreatment
Fresh seeds of P. koraiensis were randomly collected from at least 3 (30 × 30 m) stands with reproductively mature trees at the Qingyuan Forest CERN in late September 2009. All seeds were air-dried from September to November. Then more than 1000 seeds were mixed with wet sand and stratified at low temperatures (between -5 and zero °C) from November 2009 to April 2010 to break the deep dormancy characteristic of this species. In May 2010, the seeds were soaked in 0.5% potassium permanganate (K2MnO4) for 30 minutes to sterilize the seed surfaces. Any seeds that sunk immediately were considered as “viable” ([30]), while floating seeds were considered as “non-viable” and then removed. Viable seeds of a similar size were used for the germination experiment in this study.
Experiment design
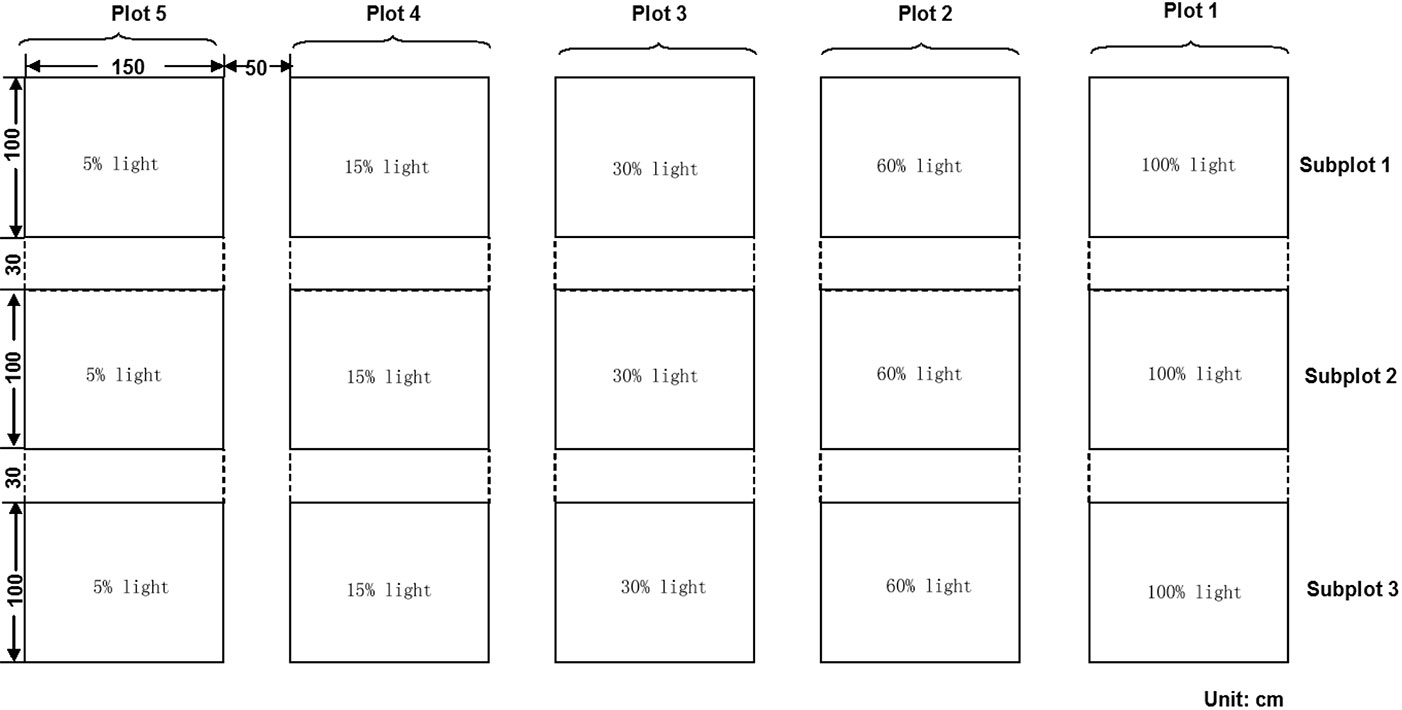
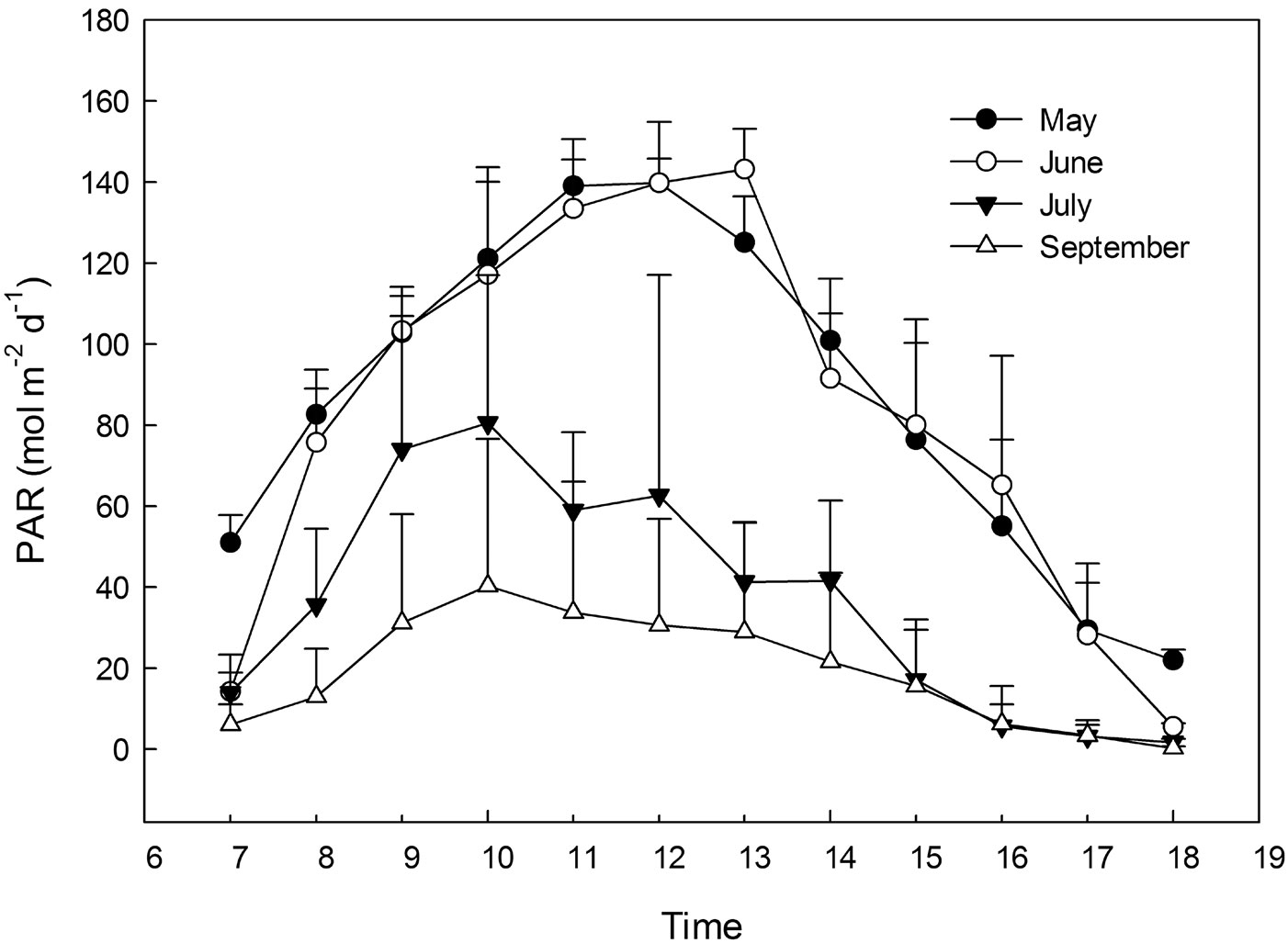
Four main seed plots were framed by wooden slats. Each plot was divided into three subplots (ca. 150 × 100 cm) with a 30 cm buffer area between the two adjacent subplots (Fig. 2). We assigned different light transmittance to each subplots by using different layers of neutral shading nets above the wooden slats. Each treatment was created by the use of independent shading nets, obtaining 3 replicates of 4 levels of light treatments (60%, 30%, 15% and 5% light transmittance), with full sunlight (100% light transmittance) as the control, which was also replicated three times. The relative light transmittance in each treatment was calculated by the specific photosynthetic photon flux density (PPFD) with respect to the full sunlight value (Fig. 3). For all the five treatments, the PPFD and topsoil (0-5 cm) temperature were measured simultaneously using quantum light sensors and temperature probes connected to a data logger (Watchdog Model 200, Spectrum Technologies, Inc., Plainfield, IL, USA). Data were logged at 15-min intervals on three uninterrupted sunny days (from 07:00 to 18:00 - [13]) monthly from May to September. Light quality was quantified by averaging the measures from 12:00 to 13:00 on one sunny day ([27]) using a different quantum sensor (Skye 110 Red/Far-Red light sensors, Skye instruments Ltd. Llandrindod Wells, Powys, UK). To improve ventilation in the shade houses, a distance of 2-3 centimeters was left between the netting edge and the ground. The relative humidity was monitored by HOBO Pro Model H08-032-08 (Onset Computer Corp., Bourne, MA, USA), and considered to be the same in the different shade houses because of the air circulation.
Fig. 3 - Instantaneous values of PAR radiation under full sun light over the diurnal period from May to September.
Fifty P. koraiensis seeds were sown at a spacing of 3-5 cm in each light treatment in May 2010. To prevent seed predation by mice or squirrels, all the seeds were sown in plastic pots (25 × 30 cm) which were protected by wire netting. The soil used in this experiment was taken from the top soil (0-5 cm) of the natural secondary forest at the Qingyuan Forest CERN. Before the experiment, soil was sieved to exclude stones, plant roots and P. koraiensis seeds, etc. No fertilizer was applied to the soil. The soil pH was 6.3 ([33]). The number of germinated seeds was recorded every 3-5 days from May to late September (the growth period) over two consecutive years (2010 and 2011), respectively. The germination experiment was terminated once no further germination occurred over a two week period (June 2011- September 2011). Germination date was recorded when the first needle sprout becoming visible ([32], [37]). Five to ten surviving seedlings in each light transmittance treatment were dug up randomly (every 5th seedling from a random start) in late September 2010 and 2011 respectively, to explore the survival rate, growth state, etc., for 1- and 2-year-old seedlings. Since we did not mark seedlings that emerged in 2010, we assumed that seedlings with relatively larger height and root collar diameter in 2011 could be classified as being two years old. After measuring the growth traits of harvested seedlings (including height, root collar diameter, number of leaves, root length and stem length), each seedling was separated into shoots and roots. These were dried at 90 °C for 30 min, then at 65 °C for 24 hours and finally the dry biomass were quantified.
Statistical analysis
Three parameters (germination percentage, coefficient of germination rate, mean germination time) were used to characterize seed germination. Seven additional parameters (survival rate, seedling height, root collar diameter, total biomass, specific leaf area, leaf area ratio, leaf mass fraction) were used to analyze the seedling survival and survival quality of P. koraiensis. The parameters not measured directly were calculated as described below. The germination percentage (GP) was calculated as (eqn. 1):
where SNM is the number of germinated seeds, and SNO is the number of total experimental seeds ([14]). The mean germination time (MGT) was obtained as (eqn. 2):
where D is the time in days from the sowing date, and N is the number of germinated seeds on a given day ([3]). The survival rate (SR) was estimates as (eqn. 3):
where SNS is the number of surviving seedlings on a given day, and SNE is the number of all emerging seedlings from the sowing day. In this study, survival rate was based on data obtained in late September in 2010 and 2011, respectively.
Leaf area (LA) was calculated using the following equation (eqn. 4):
where D represents the width measured in the middle part of each needle by calipers, and L represents the needle length. Specific leaf area (SLA) was calculated as (eqn. 5):
where LM is the leaf biomass, and LA is the leaf area, while the leaf area ratio (LAR) was obtained as (eqn. 6):
where LA is the leaf area, and TB is the total biomass. The leaf mass fraction (LMF) was estimates as follows (eqn. 7):
where LB is the leaf biomass, and TB is the total biomass.
As suggested by Zuur et al. ([40]), a transformation of the response variable should be applied when departure from normal distribution or heterogeneity of variance occur. Using normal probability plots, percentage data (i.e., germination percentage, survival rate) was tested for normality, and germination data was transformed as arcsine-square-root to meet the assumptions of ANOVA ([24], [27]). Post-hoc Tukey’s test was applied using the SPSS® v. 16 software package (SPSS Inc., Chicago, IL, USA) to ascertain the significance of differences in seed germination for the two years and seedling survival/growth across the different light transmittance regimes for 1-year-old seedlings. The non-parametric Mann-Whitney test was applied to SLA data to overcome their departure from normality. To exclude the confounding effect of morphology in the first year, a covariance analysis (ANCOVA - [29]) with light as the fixed factor and morphology values of 1-year-old seedlings as the covariate factor was conducted for 2-year-old seedlings. A backwards multiple regression was carried out to quantify the contributions of light transmittance and MGT to 1- and 2-year-old seedling growth characteristics, including seedling height, root collar diameter (RCD), total biomass, specific leaf area (SLA), leaf area ratio (LAR), and leaf mass fraction (LMF). SLA, LAR and LMF were plotted against the light transmittance, respectively. Differences obtained at a level of P≤0.05 were considered significant.
Results
Internal environmental conditions in shade houses
In all the five light transmittance regimes, the topsoil temperature varied significantly (Tab. 1). However, the topsoil temperature exhibited no significant differences from 100% to 60% light transmittance, and from 30% to 5% light transmittance. The Red/Far Red ratio and air humidity did not vary between the five treatments (Tab. 1).
Tab. 1 - Environmental conditions in the shade houses. Different letters indicate significant differences in environment conditions among light treatments.
| Shade house/site | PAR (mol m-2 d-1) |
Red/Far Red ratio |
Topsoil temperature (°C) |
Air humidity (%) |
|---|---|---|---|---|
| 100% | 60.60 ± 10.66 | 1.11 ± 0.003 a | 24.75 ± 0.53 b | 71 ± 3.8 a |
| 60% | 39.70 ± 6.76 | 1.10 ± 0.005 a | 23.17 ± 0.49 b | 62 ± 9.3 a |
| 30% | 20.15 ± 3.11 | 1.10 ± 0.002 a | 20.75 ± 0.51 a | 69 ± 10.5 a |
| 15% | 11.15 ± 2.60 | 1.09 ± 0.002 a | 20.33 ± 0.53 a | 80 ± 4.8 a |
| 5% | 5.83 ± 1.39 | 1.08 ± 0.003 a | 19.92 ± 0.24 a | 79 ± 11.0 a |
Effects of light transmittance on seed germination
The germination percentage of P. koraiensis was significantly affected by the light transmittance in the two years (ANOVA: F=9.64, P<0.01 in 2010; F=3.40, P=0.05 in 2011 - Fig. 4). The germination percentage was slightly higher in the second year than in the first year under all the light transmittance regimes. In both years, the germination percentage was the highest at 30% light transmittance, while the lowest was found at 100% (the first year) and 5% light transmittance (the second year), respectively.
Fig. 4 - Germination percentages of Pinus koraiensis under different light transmittances. Different letters indicate significant differences in germination percentage along the light transmittances (P≤0.05). The black and white column represents the cumulative germination percentage in the first year (2010) and the second year (2011), respectively.
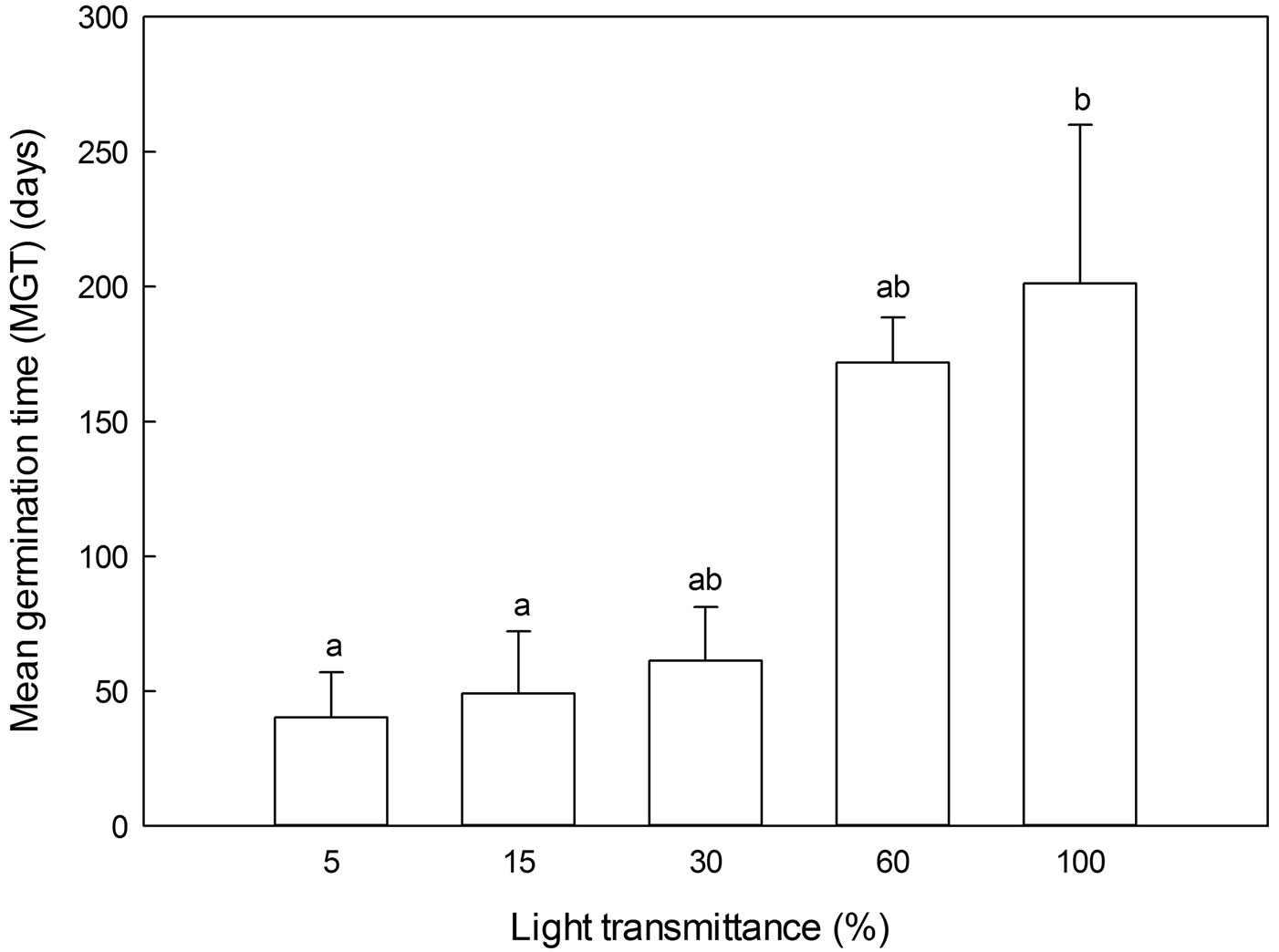
MGT was the highest in full sunlight and declined with decreasing light transmittance, with no significant differences among the four lower light transmittance regimes (Fig. 5).
Fig. 5 - Mean germination time (MGT) of Pinus koraiensis under different light transmittances for 2-year-old seedlings. Different letters indicate significant differences in MGT along the light transmittances (P≤0.05).
Effects of light transmittance on early seedling recruitment
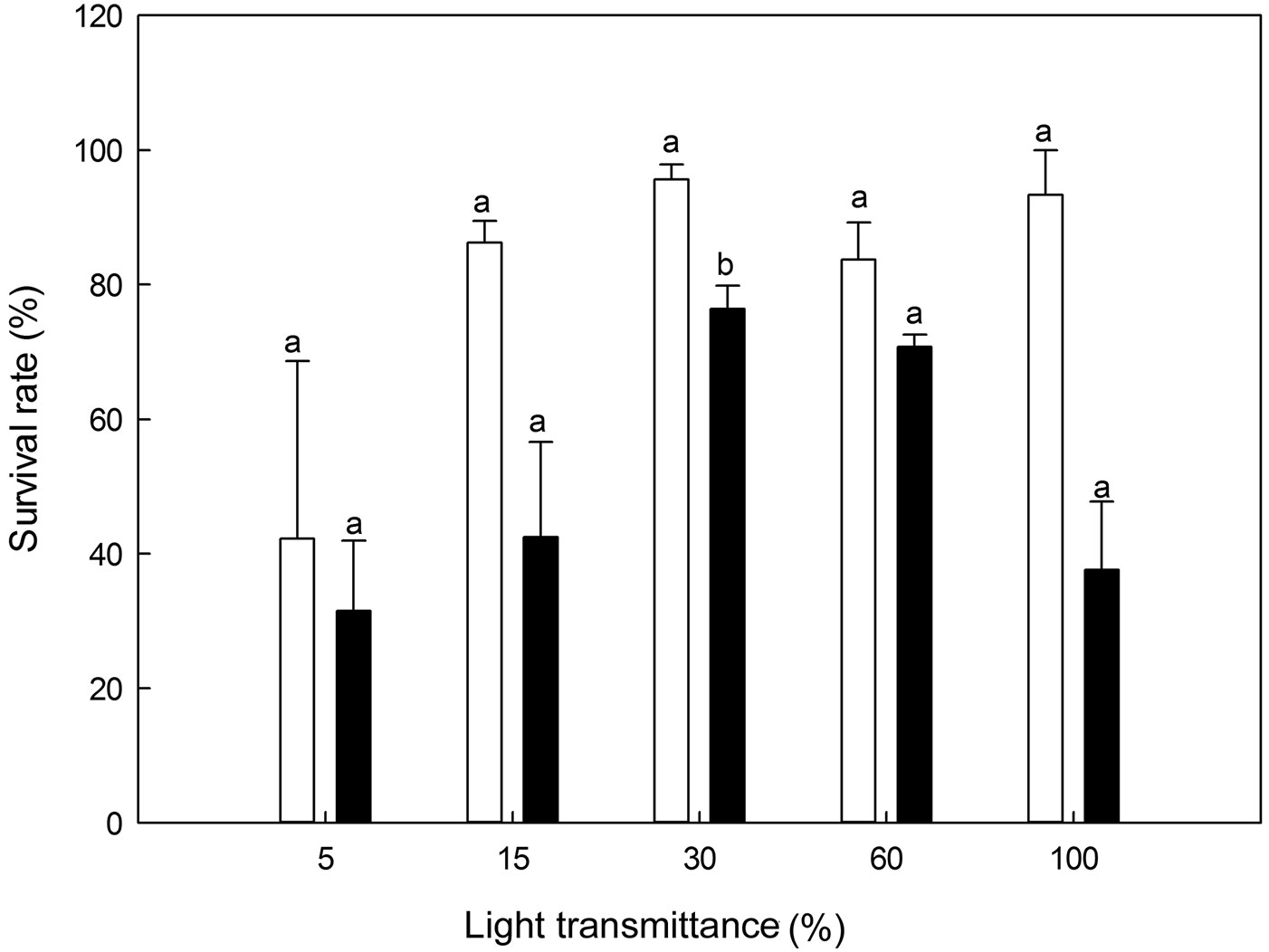
No significant differences in survival were found among the five light transmittance treatments for 1-year-old seedlings (ANOVA: F=2.91, P>0.05 - Fig. 6). However, 30% light transmittance showed significantly higher survival rates along the light levels for 2-year-old seedlings (ANCOVA: F=4.87, P=0.03 - Fig. 6). Overall, the survival rates of all the treatments were seemingly lower for 2-year-old seedlings than for 1-year-old seedlings. Compared to 1-year-old seedlings, 2-year-old seedlings exhibited significantly lower survival rates (P<0.05) at 30% and 15% light transmittance (Fig. 6).
Fig. 6 - Survival rates of Pinus koraiensis seedlings under different light transmittances. Different letters indicate significant differences in survival rate along the light transmittances (P≤0.05). The white and black column represents the survival rate for 1- and 2-year-old seedlings, respectively.
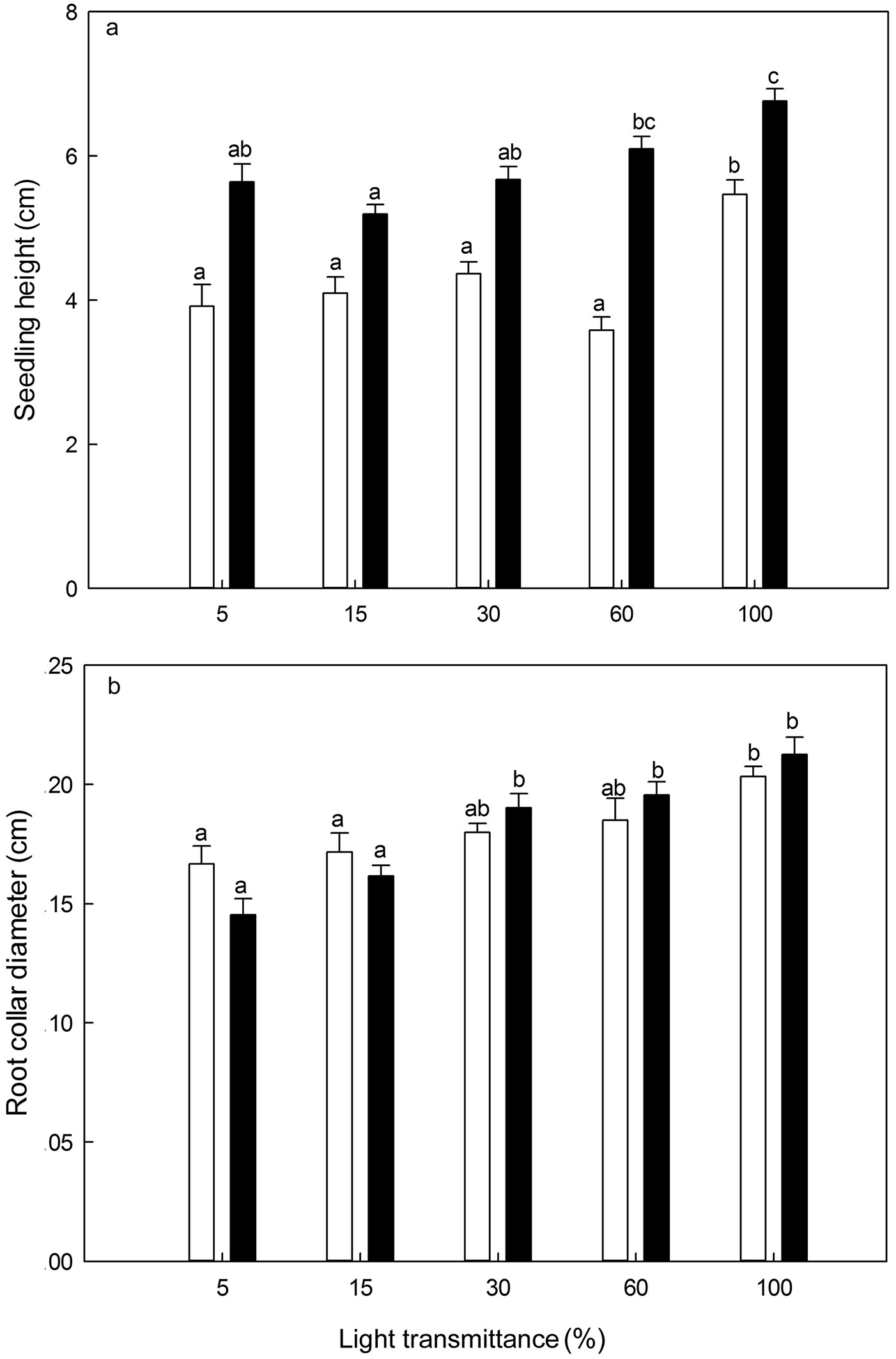
Seedling height of P. koraiensis increased with age (ANOVA: F=10.63, P<0.001 for 1-year-old seedlings; ANCOVA: F=10.82, P<0.001 for 2-year-old seedlings - Fig. 7). It was significantly higher in 100% light transmittance regime for 1-year-old seedlings and in 60-100% light transmittance regimes for 2-year-old seedlings (Fig. 7). Generally, the root collar diameter of P. koraiensis seedlings was significantly larger at 100%, 60% and 30% light transmittance, though decreased slightly at 15% and 5% light transmittance with seedling age (ANOVA: F=4.27, P<0.01 for 1-year-old seedlings; ANCOVA: F=11.28, P<0.001 for 2-year-old seedlings - Fig. 7). The largest root collar diameter was always recorded in the 100% light transmittance treatment (Fig. 7).
Fig. 7 - Seedling height (a) and root collar diameter (b) of Pinus koraiensis under different light transmittance regimes. Different letters indicate significant differences in seedling height and root collar diameter along the light transmittances (P≤0.05). The white and black column represents seedling height and root collar diameter for 1- and 2-year-old seedlings, respectively.
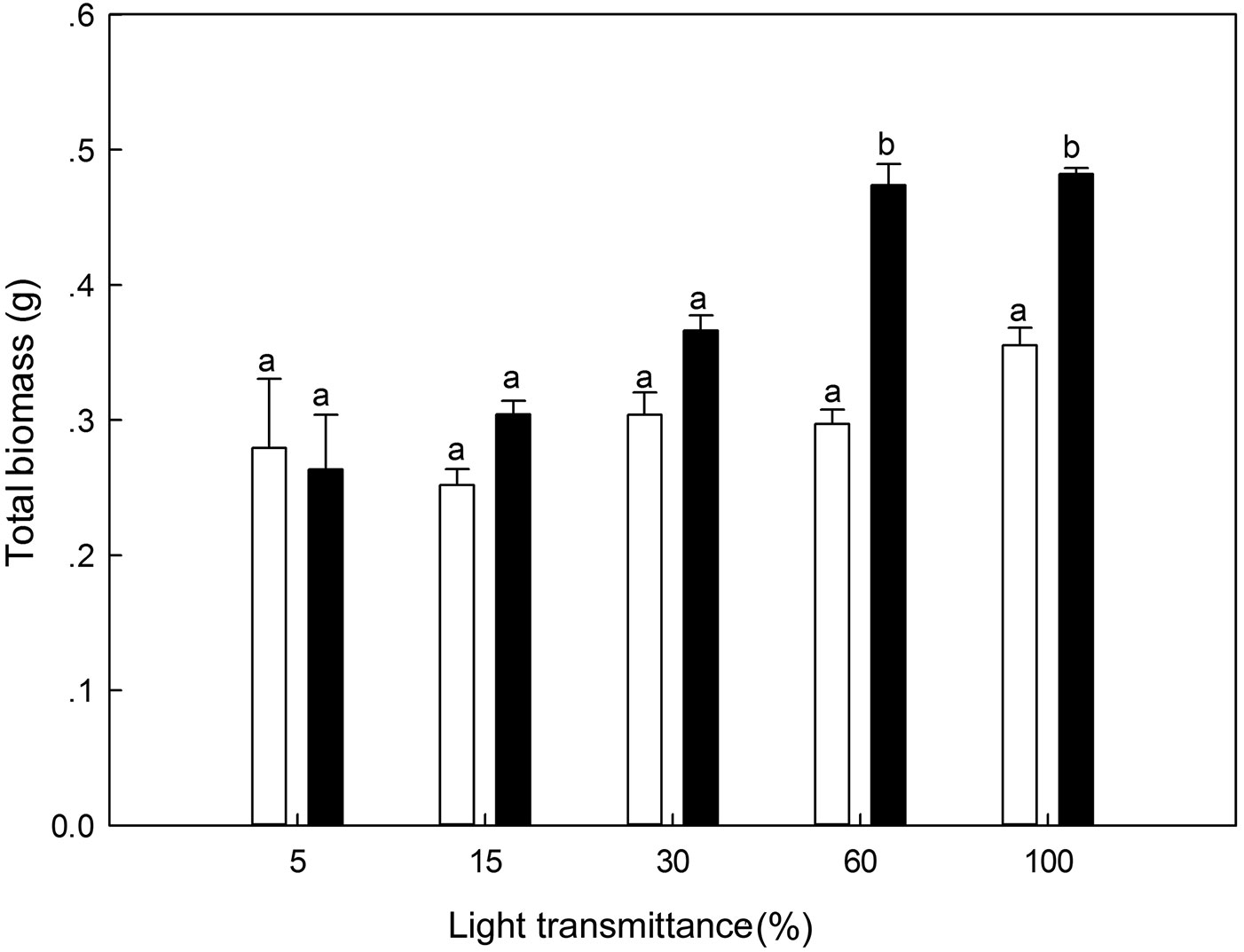
There were significant differences in biomass between 100% light transmittance and the other treatments for 1-year-old P. koraiensis seedlings (ANOVA: F=14.03, P<0.01). Light irradiance (univariate analysis: F=7.38, P=0.03) and the interaction between light and biomass in the first year (univariate analysis: F=6.91, P=0.03) jointly affected the biomass accumulation pattern in the second year. For 2-year-old seedlings, biomass at the 60% and 100% light transmittance was significantly higher as compared with the other three light regimes (Fig. 8).
Fig. 8 - Total biomass of Pinus koraiensis under different light transmittance regimes. Different letters indicate significant differences in total biomass along the light transmittances (P≤0.05). The white and black column represents the total biomass for 1- and 2-year-old seedlings, respectively.
For 1-year-old seedlings, a significant logistic relationship was determined between the SLA and the light transmittance (SLA1 = 91.14 - 6.15 · ln(Light), R2=0.854, P<0.05). A significant linear correlation was found between LAR and light transmittance for the 1-year-old seedlings (LAR1 = 2.76 - 0.01 · Light, R2 = 0.904, P<0.05), and between LMF and light transmittance for both ages of seedlings (LMF1 = 0.51 - 0.0008 · Light, R2 = 0.749, P<0.10; LMF2 = 0.499 - 0.001 · Light, R2 = 0.916, P<0.05).
Contributions of light transmittance and germination parameters on seedling performance
Except for the SLA, all the characteristics of 1- and 2-year-old seedlings can be explained by light transmittance, MGT, or a combination of them (Tab. 2). For 1-year-old plants, seedling height, root collar diameter, LAR and LMF were all significantly related to the combination of light transmittance and MGT, while total biomass was only related to light transmittance. For the 2-year-old seedlings, height, total biomass and LMF were significantly related to MGT, and RCD was significantly related to the light transmittance, while LAR was related to the combination of light transmittance and MGT. For LAR and LMF, light transmittance and MGT could explain more than 90% of their variances for 1- and 2-year-old seedlings.
Tab. 2 - Effects of light transmittance and mean germination time (MGT) on 1- and 2-year-old seedling growth characteristics. The standardized coefficients of the backwards multiple regression are shown for variables which contributed significantly to the model. Values outside and inside the brackets represented the data obtained for 1- and 2-year-old seedlings, respectively. (-): no observation; (*): P<0.05; (+): P<0.10.
| Variables | Light transmittance |
MGT
|
R 2 |
|---|---|---|---|
Seedling height (SH) |
3.08*(-) | -2.52+(0.93*) | 0.92*(0.87*) |
Root collar diameter (RCD) |
1.51*(0.92*) | -0.54*(-) | 0.99*(0.84*) |
Total biomass (TB) |
0.88*(-) | -(0.96*) | 0.78*(0.91*) |
Specific leaf area (SLA) |
- (-) | - (-) | - (-) |
Leaf area ratio (LAR) |
-1.99*(1.59*) | 1.09*(-2.42*) | 0.99*(0.99*) |
Leaf mass fraction (LMF) |
-2.50*(-) | -1.70+(-0.96*) | 0.97*(0.92*) |
Discussion
Light requirements for seed germination of P. koraiensis
The germination percentage in both years was the highest at 30% light transmittance. The results showed the same response to light transmittance in both years, demonstrating that light levels play a critical role in seed germination. According to our field observations, 30% light transmittance occurred in broadleaved secondary forest stands in the early spring prior to leaf expansion (see Fig. 1 in [34]). Thus, early spring seem a favorable period for seed germination of P. koraiensis. This result clearly confirms the findings of our previous field experiment ([34]), in which 80% of P. koraiensis seeds germinated prior to leaf expansion.
The MGTs of P. koraiensis were also affected by light transmittance. According to previous reports ([19], [28]), it is advantageous for plants to initiate germination when conditions are favorable for seedling survival. However, the optimum conditions for seedling growth might not correspond to those for seed germination and seedling survival. Indeed, our results indicated that the full sunlight favors the seedling growth, whereas it prolongs the mean germination time and slows down the germination rate. The different germination percentages in two light regimes with similar temperature and moisture conditions (Tab. 1, Fig. 4), 30% and 5% light transmittance, emphasized that there were significant effects of light on seed germination of P. koraiensis. Furthermore, compared with full sunlight conditions, shading could play a critical role in increasing germination percentage, and shortening the germination time. In summary, 30% light transmittance was the optimum light condition for seed germination in P. koraiensis, with the highest germination percentage and a relatively fast germination rate (lower values of MGT).
Light requirements for seedling survival and growth of P. koraiensis
In this study, no significant differences in survival rate were found for the 1-year-old seedlings (Fig. 6), suggesting that P. koraiensis seedlings had a wide plasticity at the earliest stage, allowing their acclimation to different light transmittance regimes. However, 30% light transmittance had the highest survival rate for the 2-year-old seedlings, indicating similar light requirements for both seedling survival and seed germination of P. koraiensis. Carbohydrates are critical for maintaining the metabolism and provide a buffer for P. koraiensis seedlings under stressful conditions ([35]). Thus, the overall lower survival rates for 2-year-old seedlings than for 1-year-old seedlings might reflect a lag effect of P. koraiensis seedlings to different light irradiance. Morphologically, 60%-100% light transmittance was the optimum level for seedling height, root collar diameter and total biomass of P. koraiensis seedlings (Fig. 7, Fig. 8). This is supported by the finding that the light-saturated photosynthetic rates in 60% and 100% treatments were higher than those in other light treatments for 5-year-old seedlings ([39]). Furthermore, as a response to shadier conditions, the largest SLA, LAR and LMF were found in 5% light transmittance to maximize the light capture.
There was a discrepancy between the best light conditions for seed germination/ seedling survival (30% light transmittance) and those for seedling growth (60%-100% light transmittance). This is due to the trade-off between carbon allocation for growth and for storage and defense ([20]). In the lowest light transmittance regime (5%), more carbon was allocated to seedling defense and storage resulting in the overall highest survival rate. However, at the 60% or 100% light transmittance levels, more carbon was allocated to seedling growth than to defense or storage.
Implications on light requirements for seed germination and early seedling growth of P. koraiensis
In this study, the interaction of light and MGT influence the growth of 1- and 2-year-old seedlings, as revealed by the regression analysis carried out (Tab. 2). Seedlings emerging from different light transmittance regimes may be influenced by both the abiotic (e.g., light) and biotic (e.g., MGT in the present study) factors ([16]). MGT was reported to have a negative relationship with seedling size, i.e., later germinating seeds produce smaller seedlings ([5]). In this study, we also found negative relationships between MGT and the height and root collar diameter of 1-year-old seedlings.
Overall, seed germination/seedling survival rate and seedling growth showed different response functions to light transmittance. Seed germination in response to light had a peak value at 30% light transmittance, while seedling height, root collar diameter and total biomass were greater with increased light. This implied that the light requirements of P. koraiensis were higher for seedling growth than for seed germination. Such uncoupled light requirements have also been reported for other tree species which can germinate under closed canopy, but need higher light at the seedling stage ([11], [6], [17], [18], [8]). Moreover, the germination of most seeds of P. koraiensis occurs in early spring before leaf expansion in the secondary forest ([34]). The subsequent reduction in light transmittance after leaf expansion would be disadvantageous to seedling growth of P. koraiensis. The distinct light requirements for seed germination and seedling growth of P. koraiensis would be a major factor hampering its natural regeneration, strongly supporting our hypothesis in the present study. Therefore, to improve the natural regeneration of P. koraiensis in the secondary forest understorey, disturbances (e.g., thinning) should be applied to allow more light reaching the understorey layer.
Conclusions
Light requirements increased from 30% to 60-100% light transmittance during the transition from seed germination to seedling recruitment in P. koraiensis. The germination characteristics (i.e., MGT) showed a coupling effect with light transmittance on the subsequent pine seedling growth. However, the highest MGT in P. koraiensis was observed at 30% light transmittance, which is not the best light regime for seedling growth. There might be a hindrance for the natural regeneration process of P. koraiensis resulting from the decreased light transmittance after leaf expansion in the growing season, contrasting with the elevated light requirement for growth. Therefore, disturbances such as gap openings or thinnings are strongly recommended as an operational measure to enlarge canopy openness in temperate secondary forests and improve the natural regeneration of P. koraiensis.
Acknowledgements
Min Zhang contributed to the experimental design, carried out the experiment, and analyzed the data; she also shared in writing the manuscript. Qiaoling Yan contributed to the design of the experiment and helped to revise the manuscript. Jiaojun Zhu contributed to the design of the experiment, helped to write the manuscript, supervised the work and coordinated the research project.
We would like to thank Dehui Zeng, Kai Yang, Lizhong Yu, Miao Wang, Jinxin Zhang and Zhanqing Hao for their constructive suggestions about this study. Thanks are also due to Qun Gang, Wenwu Ji and De’an Zheng for their field work.
This research was supported by the National Natural Science Foundation of China (contract no. 31330016 and 30830085).
References
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Qiaoling Yan
Jiaojun Zhu
State Key Laboratory of Forest and Soil Ecology, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110164 (P.R. China)
Qiaoling Yan
Jiaojun Zhu
Qingyuan Forest CERN, Chinese Academy of Sciences, Shenyang 110016 (P.R. China)
Corresponding author
Paper Info
Citation
Zhang M, Yan Q, Zhu J (2015). Optimum light transmittance for seed germination and early seedling recruitment of Pinus koraiensis: implications for natural regeneration. iForest 8: 853-859. - doi: 10.3832/ifor1397-008
Academic Editor
Gianfranco Minotta
Paper history
Received: Jul 10, 2014
Accepted: Feb 11, 2015
First online: May 22, 2015
Publication Date: Dec 01, 2015
Publication Time: 3.33 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 55553
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 46349
Abstract Page Views: 3323
PDF Downloads: 4328
Citation/Reference Downloads: 33
XML Downloads: 1520
Web Metrics
Days since publication: 3907
Overall contacts: 55553
Avg. contacts per week: 99.53
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 13
Average cites per year: 1.18
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Matching seedling size to planting conditions: interactive response with soil moisture
vol. 12, pp. 220-225 (online: 25 April 2019)
Research Articles
Effects of cultural treatments, seedling type and morphological characteristics on survival and growth of wild cherry seedlings in Turkey
vol. 5, pp. 283-289 (online: 17 December 2012)
Research Articles
Methods for predicting Sitka spruce natural regeneration presence and density in the UK
vol. 12, pp. 279-288 (online: 23 May 2019)
Research Articles
Tropical seedling performance under drought: a functional trait approach for species selection in restoration
vol. 19, pp. 9-17 (online: 10 January 2026)
Research Articles
Germination and seedling growth of holm oak (Quercus ilex L.): effects of provenance, temperature, and radicle pruning
vol. 7, pp. 103-109 (online: 18 December 2013)
Research Articles
Seed germination traits of Pinus heldreichii in two Greek populations and implications for conservation
vol. 15, pp. 331-338 (online: 24 August 2022)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Addressing post-transplant summer water stress in Pinus pinea and Quercus ilex seedlings
vol. 8, pp. 348-358 (online: 16 September 2014)
Research Articles
Regeneration dynamics in the laurel forest: changes in species richness and composition
vol. 11, pp. 308-314 (online: 13 April 2018)
Technical Reports
Nursery practices increase seedling performance on nutrient-poor soils in Swietenia humilis
vol. 8, pp. 552-557 (online: 09 December 2014)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword