Shifts in the arbuscular mycorrhizal fungal community composition of Betula alnoides along young, middle-aged plantation and adjacent natural forest
iForest - Biogeosciences and Forestry, Volume 13, Issue 5, Pages 447-455 (2020)
doi: https://doi.org/10.3832/ifor3515-013
Published: Oct 07, 2020 - Copyright © 2020 SISEF
Research Articles
Abstract
Betula alnoides is a fast-growing and native timber species prevalently planted in tropical and subtropical areas of southern China. Despite the large-scale development of B. alnoides plantations, knowledge of its association with arbuscular mycorrhizal fungi (AMF) is limited. In the present study, we chose young (3-year-old sapling, P3y) and middle-aged (12-year-old stand, P12y) B. alnoides plantations and adjacent native forest (N) in the Puwen Tropical Forest Experimental Station located in Xishuangbanna prefecture of Yunnan Province, southwestern China, as study materials and explored the change in AMF community composition in the plantation chronosequence. In addition, we combined morphological methods and Illumina MiSeq sequencing techniques to analyze rhizosphere soil AMF. The results indicated that the AMF richness and diversity indexes of B. alnoides at two ages tended to be similar to those of natural growing trees in native forest. However, the specific AM fungal compositions were distinctly different, providing evidence of the conservation value of the native forest, which harbors a unique AMF diversity. Hierarchical cluster analysis further revealed that the AMF community composition of trees in the mid-aged stand (P12y) was more similar to that of naturally growing B. alnoides (N) than that of the young-aged trees (P3y), which proved the considerable resilience of AMF to the establishment of the B. alnoides plantation. A set of at least five soil properties (available phosphorus, available nitrogen, organic matter, total nitrogen and silt content) was found to play a significant role in shaping the AMF communities. These results contribute to the understanding of the impacts of B. alnoides plantations on AMF diversity and composition. Such information is critical for the efficient planting and sustainable management of B. alnoides plantations.
Keywords
Arbuscular Mycorrhizal Fungi, Betula alnoides, Plantation, Native Forest
Introduction
Significant levels of biodiversity and ecosystem services of tropical forests are of global importance. However, due to human population growth and increasing demand for agricultural products, the conversion of natural forest to agricultural land has been increasing worldwide in recent decades. It was estimated that 68.000 km2 of tropical forest is lost annually, and this amount is increasing by 3% (>2000 km2) each year ([18]). Presently, agroforestry and human-made forests using fast-growing native species for the purposes of timber and non-timber products are the sustainable alternatives to satisfy the high increasing demand and relieve pressure on primary tropical forests ([21]).
Betula alnoides Buch. Ham. ex D. Don (a birch species) is the only species in the genus Betula that has been found naturally in the tropics. It is a valuable tree species mainly distributed in southeastern Asia and southern China with good adaptability to a wide range of soil conditions. The wood of B. alnoides has moderate density with optimal texture, is resistant to cracking and warping, and is predominantly used for flooring, in high-grade furniture and interior decoration. Artificial planting of B. alnoides in southern China began in the 1980s, and the planting areas have exceeded 150,000 ha, mainly in Yunnan and Guangdong provinces ([46]).
Along with the large-scale establishment of B. alnoides plantations in southern China, some concerns have been paid to the stability and sustainability of this artificial forest from different ecological aspects, including plant community diversity, soil physicochemical properties, carbon sequestration, and soil and water conservation ([22], [31], [25], [8]). However, the study of microorganisms in this forest is quite limited. Among the rhizosphere microbial communities, arbuscular mycorrhizal fungi (AMF) are one of the most critical components; AMF are obligate root symbionts associated with approximately 72% of vascular plants ([5]) and occur in almost every terrestrial ecosystem ([43]). AMF are known to provide a wide range of ecosystem functions, including improving plant productivity through increased nitrogen and phosphorus acquisition, enhancing drought tolerance and protecting host plants from soil pathogens ([43]). AMF not only play critical roles in determining the performance of host species ([44]) but also enhance the sustainability of ecosystems by improving soil structure ([39]). Furthermore, since they can reflect the different levels of degradation and the changes during vegetation recuperation, AMF are correlated with the vegetation type and can notably reveal the rate of soil development; therefore, the AM fungal community is considered to be a good soil quality indicator ([34]).
Previous studies reported that arbuscular mycorrhizal symbiosis significantly promoted the growth, nutrient absorption and photosynthesis of B. alnoides seedlings in greenhouse experiments ([15]). There is evidence that afforestation significantly affects the development, distribution, and function of the AM fungal community composition ([17], [41]). However, studies focusing on AM symbiosis and the composition of AMF communities in B. alnoides forests have yet to be conducted, which is critical as the first step towards the management and application of AMF communities aimed at improving the health of the soil to promote the productivity and sustainability of B. alnoides plantations.
The present study was conducted at the Puwen Tropical Forest Experimental Station located in Xishuangbanna Prefecture of Yunnan Province, southwestern China. Xishuangbanna is an Indo-Burma biodiversity hotspot and comprises 16% of the total plant diversity in China ([6]). The forests in this area have a biodiversity that is important both globally and nationally. Therefore, more studies concerning the conservation of biodiversity are needed to provide information and enhance the understanding of this specific forest ecosystem.
We selected young (3-year-old saplings, P3y) and middle-aged (12-year-old stand, P12y) B. alnoides plantations and adjacent native forest (N) at the same slope as the study targets. The primary objectives were (i) to study the AM symbiosis of B. alnoides in the forest, (ii) to assess to what extent B. alnoides plantation affects AM fungal composition and diversity by illuminating the shifts in different growing stages and comparing them with naturally growing B. alnoides trees in native forest, and (iii) to explore the soil properties affecting the shift in AMF communities after afforestation with B. alnoides.
Materials and methods
Site description and sampling
The study site is located in the Puwen Tropical Forest Experimental Station of Yunnan Academy of Forestry and Grassland, Yunnan Province, China (101° 06’ E, 22° 25’ N, 800-1354 m a.s.l.). This area belongs to the northern margin of the tropics in the Northern Hemisphere. The topology of this area is characterized by low mountains and hills, and the soil type is lateritic red soil. The climate is tropical monsoon, with a mean annual temperature of 20.2 °C and mean maximum and minimum temperatures of 23.9 °C (July and August) and 13.8 °C (January), respectively. The annual rainfall (1675.5 mm ca.) is mostly concentrated (86%) in the warm season (May-October). The zonal vegetation includes montane rainforest, monsoon evergreen broad-leaved forest, wet seasonal forest and ravine rainforest ([47]). B. alnoides plantations were initially established in this area in 1988, and the Puwen Tropical Forest Experimental Station contains stands of different ages. To reduce the effect of geographical distance on AMF, two B. alnoides plots, 3-year-old (P3y) and 12-year-old plantation (P12y), close to each other (with a distance of less than 100 m) and a plot of natural stand (N) with B. alnoides trees adjacent to the plantation were selected; the selected plots were at the same slope. Thus, the study serves as a specific case for exploring the AMF recruited by B. alnoides at different growing stages. Sampling was conducted in October 2018. Five B. alnoides trees from each plot were chosen randomly, totalling 15 selected trees. For each B. alnoides sample, the fine roots and rhizosphere soil from four directions (east, west, south and north) were collected, mixed and homogenized into a homogenous sample. To ensure identity of each sample, we followed each root to their origin and distinguished the B. alnoides roots by observing the dark red color of the velamen and the peculiar smell.
The average diameters at breast height (DBH) of sampled B. alnoides trees from P3y and P12y and N were 4.9, 18.7 and 36.6 cm, respectively, which can represent the young, mediate and mature stages of B. alnoides. There was no management in the soil of the plantation (fertilization, herbicide or tillage). Only manual weeding was conducted during the first and second years of planting.
Soil characteristics analysis
The soil samples were air-dried and homogenized by sieving through a 2-mm mesh to remove plant debris and coarse sand. Particle-size analysis was conducted with the pipette method according to the U.S. Department of Agriculture system ([42]) to examine the soil texture. Soil enzyme activities were determined using the enzyme activity assay kits (Suzhou Comin Biotechnology Co., Ltd., Jiangsu, China) according to the manufacturer’s protocol. The chemical properties of soils were analyzed using the methods described by Du et al. ([12]).
Analyses of AM fungal colonization, spore density and morphological identification
Root segments were cleared with 10% (w/v) KOH at 90 °C in a water bath for 60-90 min. After cooling to room temperature, root samples were washed with water, cut into segments approximately 1 cm in length, stained with 0.5% blue ink (Hero® 203, Shanghai, China), mounted on microscope slides and examined for AM fungal structures under a compound light microscope (Olympus BX53®, Tokyo, Japan). Fungal colonization was measured by the magnified intersection method ([29]). The percentages of root length with hyphae, vesicles, and arbuscules were quantified by examining over 200 intersections per sample.
AM fungal spore extraction was carried out by wet sieving and decanting 20 g of air-dried rhizosphere soil ([14]). Following the nomenclature of genera or species proposed by Redecker et al. ([37]), AMF species were identified according to Schenck & Pérez ([40]) and several specialized websites: International Culture Collection of Vesicular Arbuscular Mycorrhizal Fungi (⇒ http://invam.caf.wvu.edu/), Blaszkowski (⇒ http://www.zor.zut.edu.pl/Glomeromycota/), Schübler and Walker (⇒ http://schuessler.userweb.mwn.de/amphylo/).
Soil DNA extraction, PCR, Illumina MiSeq sequencing and bioinformatics analysis
Using the EZNA® Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA), genomic DNA was extracted from 0.5 g of air-dried soil sample according to the manufacturer’s protocol. The final DNA purity and concentration were determined with a NanoDrop 2000® UV-vis spectrophotometer (Thermo Scientific, Wilmington, MA, USA), and DNA quality was checked by 1% (w/v) agarose gel electrophoresis.
Nested PCR was used to improve the fragment specificity. The first PCR was performed using a combination of the AM fungal-specific primers AML1 (5’-ATCAACTTTCGATGGTAGGATAGA-3’) and AML2 (5’-GAACCCAAACACTTTGGTTTCC-3’ - [24]) with an initial denaturation at 95 °C for 3 min; 32 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s; and a final extension step of 72 °C for 10 min. The product of the first PCR amplification was used as the template DNA for the next PCR. AM fungal-specific primers AMV4.5NF (5’-AAGCTCGTAGTTGAATTTCG-3’) and AMDGR (5’-CCCAACTATCCCTATTAATCAT-3’) were added ([28]), and nested PCR was conducted with an initial denaturation at 95 °C for 3 min; 30 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s; and a final extension step of 72 °C for 10 min. Both PCRs were carried out in 20 μL of reaction mixtures containing 4 μL of 5× FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.4 μL of each primer (10 mM), 0.4 μL of FastPfu polymerase, 1 μL of template DNA (approximately 10 ng), 0.2 μL of BSA, and 10.8 μL of sterile distilled H2O. An 8-bp sequence barcode was added as a tag to distinguish the PCR products from one another.
The amplified fragments were extracted from 2% agarose gels and purified with an AxyPrep® DNA Gel Extraction Kit (Axygen, USA) following the manufacturer’s instructions and quantified using QuantiFluorTM-ST® (Promega, USA). Purified amplicons were pooled in equimolar and paired-end sequenced (2×300) on an Illumina MiSeq® platform (Illumina, San Diego, CA, USA) according to standard protocols by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
Raw fastq files were quality-filtered by Trimmomatic and assembled by FLASH. The criteria used were as follows: (i) the reads were truncated at any site with an average quality score <20 over a 50 bp sliding window; (ii) sequences with overlaps longer than 10 bp were assembled, providing their overlap had less than 2 bp mismatches; (iii) sequences of each sample were separated using barcodes (exactly matching) and primers (allowing 2 nucleotide mismatches), and reads containing ambiguous bases were deleted.
Operational taxonomic units (OTUs) were clustered with a 97% similarity cutoff using UPARSE (version 7.1 - ⇒ http://drive5.com/uparse/) with a novel “greedy” algorithm that performs chimera filtering and OTU clustering simultaneously. The taxonomy of each sequence was analyzed using the RDP Classifier algorithm (⇒ http://rdp.cme.msu.edu/) against the MaarjAM database with a confidence threshold of 70% ([33]).
Statistical analysis
Variations in soil physical, chemical and biological characteristics and AMF attributes, including AMF colonization in roots, spore density and relative abundance of morphological species identified from rhizosphere soil, and Glomeromycota OTUs in soil, were analyzed using the Kruskal-Wallis test.
To test whether the AMF composition clearly separated among different aged plantations and natural stand and to further explore the effect of soil parameters on AMF composition, we performed nonmetrical multidimensional scaling (NMDS) based on the relative abundance of morphological species and OTU matrix (OTU abundance table) using Morisita-Horn dissimilarity in the “vegan” R package ([32]). Subsequently, differences in community composition among three study stands were tested by permutational multivariate analysis of variance (PERMANOVA) with 1000 iterations performed using the package “vegan” in R. In addition, the similarity among AM fungal communities concerning the number and abundance of OTUs was determined by hierarchical cluster analysis using Spearman_approx distance. Mantel tests using Euclidean distances were conducted to understand the relationships between the AM fungal communities and environmental factors. The “ecodist” package of R version 3.2 was used ([16]).
Results
Soil characteristics
The soil physicochemical and biological parameters showed significant differences among the 3 study plots except for acid phosphatase activity and sand percentage (Tab. 1). The soil of this area was acidic. The lowest pH value was found in plot N (4.22), while the highest pH value was in plot P3y (4.64). Regarding the chemical properties, the soil fertility obviously increased with increasing age of the B. alnoides tree. P3y soil showed significantly lowest values for TN, TP, TK, AN, AP, AK and OM, whereas N soil presented the highest values for these properties except for TK. The N soil had the highest sand and silt contents, while P3y soil had the highest amount of clay. In relation to biological properties (enzyme activities), the lowest ALP, UE, CAT and SC activities were observed in P3y soil, the highest UE, CAT, SC activities were observed in P12y soil, and the ALP activities of P12y and N soils were the same (Tab. 1).
Tab. 1 - Soil properties of young and middle-aged Betula alnoides plantations and adjacent native forest located in Puwen Tropical Forest Experimental Station, Xishuangbanna prefecture of Yunnan Province, southwestern China. P3y, P12y and N represent 3-year-old saplings, 12-year-old stands and adjacent native forests, respectively. (TN): total nitrogen; (TP): total phosphorus; (TK): total potassium; (AN): available nitrogen; (AP): available phosphorus; (AK): available potassium; (OM): organic matter; (ACP): acid phosphatase activity; (ALP): alkaline phosphatase activity; (UE): urease activity; (CAT): catalase activity; (SC): sucrase activity. Data are expressed as the mean ± SE (n=5). Differences at p < 0.05 were considered statistically significant.
| Soil parameter |
Units | P3y | P12y | N | p-value |
|---|---|---|---|---|---|
| pH | - | 4.64 ± 0.04 | 4.28 ± 0.03 | 4.22 ± 0.01 | 0.006 |
| TN | g kg-1 | 0.54 ± 0.06 | 1.18 ± 0.05 | 1.48 ± 0.11 | 0.004 |
| TP | g kg-1 | 0.27 ± 0.01 | 0.37 ± 0.03 | 0.38 ± 0.01 | 0.008 |
| TK | g kg-1 | 8.81 ± 0.17 | 12.52 ± 0.77 | 10.50 ± 0.54 | 0.008 |
| AN | mg kg-1 | 44.20 ± 5.73 | 95.93 ± 12.55 | 141.90 ± 9.03 | 0.005 |
| AP | mg kg-1 | 1.97 ± 0.13 | 2.87 ± 0.23 | 3.66 ± 0.38 | 0.005 |
| AK | mg kg-1 | 54.50 ± 1.66 | 73.25 ± 6.19 | 105.25 ± 19.26 | 0.009 |
| OM | g kg-1 | 11.00 ± 1.49 | 25.50 ± 1.28 | 33.96 ± 2.40 | 0.004 |
| ACP | μmol d-1 g-1 | 12.43 ± 1.75 | 11.39 ± 0.64 | 13.18 ± 1.73 | 0.827 |
| ALP | μmol d-1 g-1 | 2.02 ± 0.34 | 12.65 ± 0.28 | 12.65 ± 0.28 | 0.009 |
| UE | μg d-1 g-1 | 538.12 ± 77.94 | 1058.85 ± 68.03 | 964.81 ± 38.75 | 0.007 |
| CAT | μmol d-1 g-1 | 4.58 ± 0.41 | 25.40 ± 1.65 | 25.33 ± 2.03 | 0.009 |
| SC | mg d-1 g-1 | 17.57 ± 2.91 | 35.27 ± 4.92 | 34.47 ± 2.00 | 0.013 |
| Sand | % | 42.40 ± 1.36 | 45.40 ± 2.71 | 47.60 ± 1.03 | 0.070 |
| Silt | % | 16.20 ± 0.37 | 24.00 ± 1.05 | 24.60 ± 1.69 | 0.009 |
| Clay | % | 41.60 ± 1.57 | 30.60 ± 3.42 | 27.80 ± 1.63 | 0.020 |
AM fungal colonization and spore density
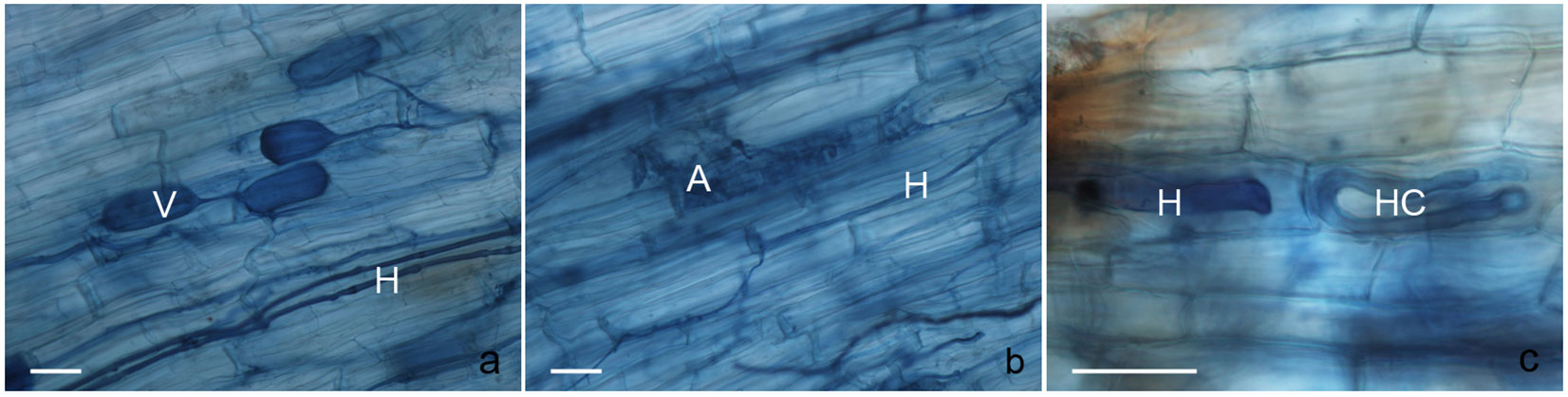
Some typical structures of AMF that colonized the roots of B. alnoides of different ages are presented in Fig. 1. Overall, the level of root AMF colonization peaked at the intermediate stage (12 years), with root length colonization percentages of 25.32%, 3.84% and 15.30% for hyphae, arbuscules and vesicles, respectively. The colonization percentages of naturally growing B. alnoides were the lowest among the three studied stands, with hyphal, arbuscule and vesicle colonization percentages of 7.54%, 0.58% and 4.16%, respectively. However, a significant difference among different stands was only detected for hyphal colonization (p = 0.044 - Tab. 2).
Fig. 1 - Structures of AMF colonizing the roots of Betula alnoides trees in different study stands. (a) Vesicles (V) and hyphae in the root apparatus of a 3-year-old Betula alnoides plant. (b) Arbuscules (A) and hyphae (H) are visible in the roots of 12-year-old Betula alnoides trees. (c) Hypha (H) and hyphal coil (HC) in the root apparatus of Betula alnoides trees growing in the native forest. Scale bar =30 μm.
Tab. 2 - AM fungal attributes of young and middle-age d Betula alnoides plantations and adjacent native forest located in Puwen Tropical Forest Experimental Station, Xishuangbanna prefecture of Yunnan Province, southwestern China. P3y, P12y and N represent 3-year-old saplings, 12-year-old stand and adjacent native forest, respectively. Data are expressed as the mean ± SE (n=5). Differences at p < 0.05 were considered statistically significant.
| Attributes | P3y | P12y | N | p-value |
|---|---|---|---|---|
| AMF hypha colonization (%) | 19.00 ± 5.61 | 25.32 ± 2.18 | 7.54 ± 2.57 | 0.044 |
| Arbuscule colonization (%) | 1.61 ± 0.67 | 3.84 ± 2.17 | 0.58 ± 0.30 | 0.170 |
| Vesicle colonization (%) | 15.97 ± 5.55 | 15.30 ± 2.13 | 4.16 ± 1.44 | 0.088 |
| Spore density (no. spore/20 g soil) | 51.00 ± 18.23 | 98.00 ± 37.18 | 225.20 ± 82.95 | 0.054 |
| Shannon index of morphospecies | 1.29 ± 0.11 | 1.59 ± 0.11 | 1.51 ± 0.08 | 0.114 |
| Simpson index of morphospecies | 0.66 ± 0.04 | 0.72 ± 0.05 | 0.70 ± 0.05 | 0.336 |
| Chao-1 of OTUs | 46.50 ± 2.54 | 56.62 ± 4.26 | 32.65 ± 6.68 | 0.026 |
| Sobs of OTUs | 42.80 ± 2.85 | 52.40 ± 4.55 | 31.60 ± 6.22 | 0.077 |
| Shannon index of OTUs | 2.74 ± 0.15 | 2.90 ± 0.07 | 2.12 ± 0.39 | 0.160 |
| Simpson index of OTUs | 0.10 ± 0.02 | 0.08 | 0.25 ± 0.10 | 0.154 |
Along the chronosequence, the AMF spore density in rhizospheric soil increased from 51 spores in P3y to 98 spores in P12y and up to 225 spores per 20 g of air-dried soil in N (Tab. 2). However, the Kruskal-Wallis test did not detect a significant difference (p = 0.054).
Morphological diversity of the AM fungal community
From the soil of the study plots, we identified 23 morphospecies belonging to 2 orders, 3 families and 4 genera, including 12 identified to the species level and 11 identified to the genus level (Tab. 3, Fig. 2). Glomus and Acaulospora were the most representative genera in the study area, with 10 and 8 species, respectively, followed by Scutellospora (4 species). The total richness of AMF from P3y, P12y and N was 12, 15 and 17 species, respectively. The fungal Shannon index and Simpson index values were highest in P21y, followed by N, and those of P3y were the lowest; however, the indexes were not significantly different (p > 0.05 - Tab. 2).
Tab. 3 - Relative abundance of AMF morphospecies in Betula alnoides rhizosphere soil of young and middle-aged plantations and adjacent native forest located in Puwen Tropical Forest Experimental Station, Xishuangbanna prefecture of Yunnan province, southwestern China. P3y, P12y and N represent 3-year-old saplings, 12-year-old stand and adjacent native forest, respectively. Data are expressed as the mean ± SE (n=5). Differences at p < 0.05 were considered statistically significant.
| Genus | Morphospecies | P3y | P12y | N | p-value |
|---|---|---|---|---|---|
| Acaulospora | Acaulospora bireticulata F.M. Rothwell & Trappe | 0 | 0.17 ± 0.17 | 0 | 0.368 |
| Acaulospora capsicula B‚aszk. | 6.73 ± 2.32 | 6.68 ± 3.58 | 20.48 ± 8.80 | 0.277 | |
| Acaulospora foveata Trappe & Janos | 3.13 ± 3.13 | 0.73 ± 0.36 | 0.48 ± 0.21 | 0.602 | |
| Acaulospora koskei B‚aszk. | 0 | 0 | 0.04 ± 0.04 | 0.368 | |
| Acaulospora laevis Gerd. & Trappe | 6.33 ± 4.98 | 7.28 ± 3.92 | 3.44 ± 1.54 | 0.871 | |
| Acaulospora sp. 1 | 0.63 ± 0.63 | 0 | 0 | 0.368 | |
| Acaulospora sp. 2 | 0 | 0 | 0.11 ± 0.11 | 0.368 | |
| Acaulospora sp. 3 | 0 | 0.17 ± 0.17 | 0 | 0.368 | |
| Gigaspora | Gigaspora sp. 1 | 0 | 0 | 0.17 ± 0.17 | 0.368 |
| Glomus | Glomus flavisporum Trappe & Gerd. | 27.08 ± 4.71 | 10.19 ± 3.72 | 4.75 ± 2.39 | 0.007 |
| Glomus fuegianum Trappe & Gerd. | 0 | 0 | 0.40 ± 0.40 | 0.368 | |
| Glomus macrocarpum Tul. & C.Tul. | 0 | 6.79 ± 3.51 | 11.71 ± 4.14 | 0.027 | |
| Glomus multicaule Gerd. & B.K. Bakshi | 0.50 ± 0.50 | 33.65 ± 10.14 | 15.96 ± 6.93 | 0.005 | |
| Glomus sp. 1 | 45.19 ± 7.83 | 14.54 ± 6.35 | 29.78 ± 9.93 | 0.065 | |
| Glomus sp. 2 | 0.63 ± 0.63 | 0.69 ± 0.33 | 0.04 ± 0.04 | 0.325 | |
| Glomus sp. 3 | 0.33 ± 0.33 | 0 | 0 | 0.368 | |
| Glomus sp. 4 | 0 | 11.50 ± 7.04 | 0.11 ± 0.11 | 0.266 | |
| Glomus sp. 5 | 0 | 0.09 ± 0.09 | 0.04 ± 0.04 | 0.581 | |
| Glomus sp. 6 | 0 | 0 | 0.04 ± 0.04 | 0.368 | |
| Sclerocystis | Sclerocystis coremioides Berk. & Broome | 0 | 1.20 ± 1.20 | 0 | 0.368 |
| Sclerocystis rubiformis Gerd. & Trappe | 6.47 ± 3.31 | 5.97 ± 1.83 | 12.25 ± 4.34 | 0.403 | |
| Sclerocystis sinuosa Gerd. & B.K. Bakshi | 0.50 ± 0.50 | 0 | 0 | 0.368 | |
| Sclerocystis sp. 1 | 2.50 ± 2.50 | 0.35 ± 0.35 | 0.20 ± 0.16 | 0.891 |
Fig. 2 - AMF spores isolated from rhizosphere soils of Betula alnoides trees and morphological identification. (a) Acaulospora foveata; (b) Acaulospora laevis; (c) Acaulospora koskei; (d) Glomus multicaule; (e)(f) Sclerocystis sinuosa; (g) Sclerocystis rubiformis; (h) Glomus sp. 6; (i) Sclerocystis sp. 1; (j) Acaulospora sp. 3; (k) Glomus sp. 2; (l) Gigaspora sp. 1. (a)-(i): scale bar = 20 μm; (j)-(l): scale bar = 50 μm.
In accordance with the NMDS permutation test on the relative abundance of different morphological taxa, there was no clear separation of the AM fungal community composition of P3y, P12y and N. Only Glomus flavisporum (p = 0.007), G. macrocarpum (p = 0.027) and G. multicaule (p = 0.005) showed different abundances of AMF spores among the studied stands (Tab. 3).
Nine taxa were recorded in all three plots: Acaulospora capsicula, A. foveata, A. laevis, G. flavisporum, G.multicaule, Glomus sp. 1, Glomus sp. 2, Sclerocystis rubiformis and Sclerocystis sp. 1. Three taxa, namely, Acaulospora sp. 1, Glomus sp. 3 and S. sinuosa, occurred only in P3y; three taxa, namely, A. bireticulata, Acaulospora sp. 3 and S. coremioides occurred only in P12y; and five taxa, namely, A. koskei, Acaulospora sp. 2, Glomus fuegianum, Glomus sp. 6 and Gigaspora sp. 1, occurred only in N.
AM fungal community composition assessed by Illumina MiSeq sequencing
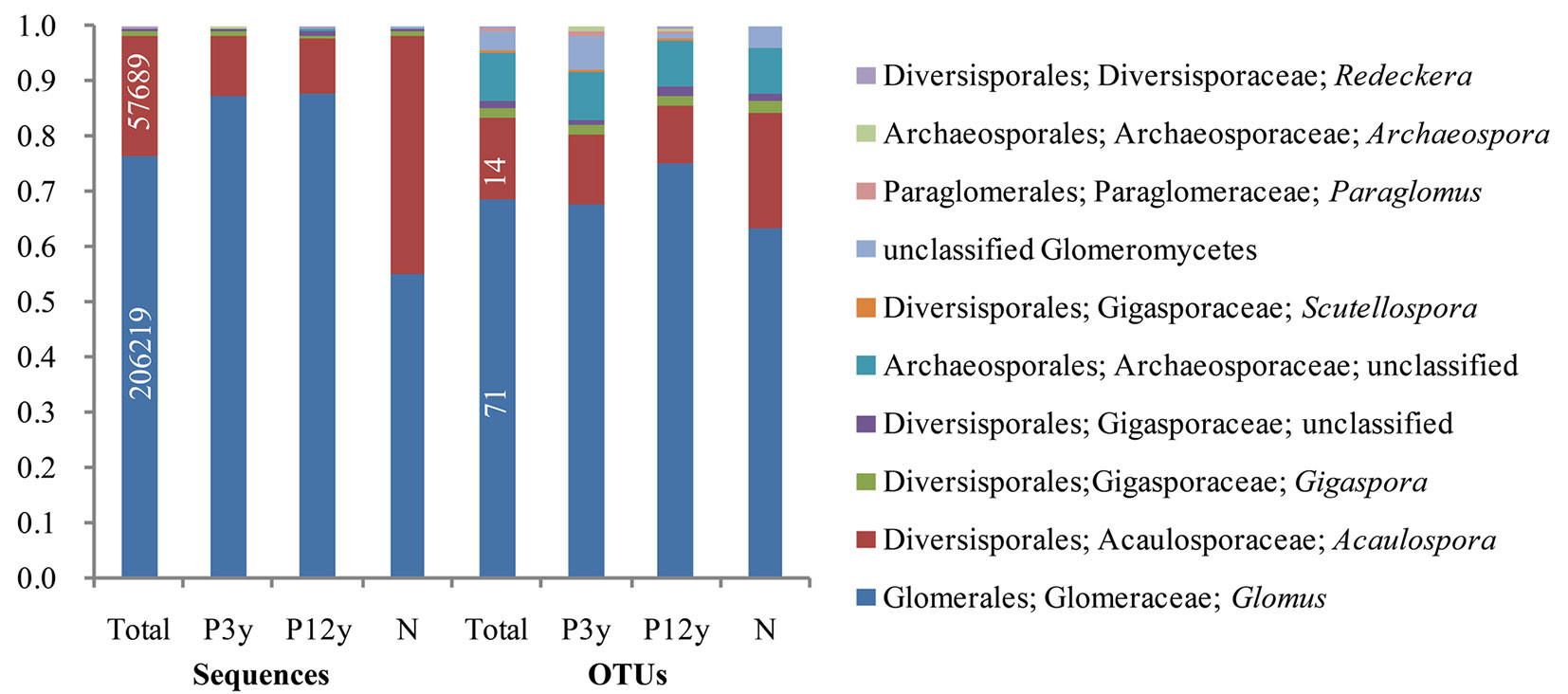
A total of 269.130 sequences were obtained from all 15 samples from Illumina MiSeq® sequencing after quality control, and a total of 119 OTUs were detected based on 97% similarity (Tab. S1 in Supplementary material). All sequences belonged to the phylum Glomeromycota, including ten genera, namely, Archaeospora, unclassified Archaeosporaceae, Acaulospora, Redeckera, Scutellospora, unclassified Gigasporaceae, Gigaspora, Glomus, Paraglomus and unclassified Glomeromycetes. With increasing read numbers, the species accumulation curves (Fig. S1 in Supplementary material) tended to reach a saturation plateau, indicating that the sampling intensity was sufficient.
Nine, 10, and 6 genera were detected from P3y, P12y and N, respectively (Tab. S2 in Supplementary material). Based on Illumina MiSeq® sequencing, the diversity indexes of Chao-1, Sobs and Shannon index peaked in P12y, followed by P3y, and those of N were the lowest. Nevertheless, only the difference in Chao-1 was significant (p = 0.026 - Tab. 2).
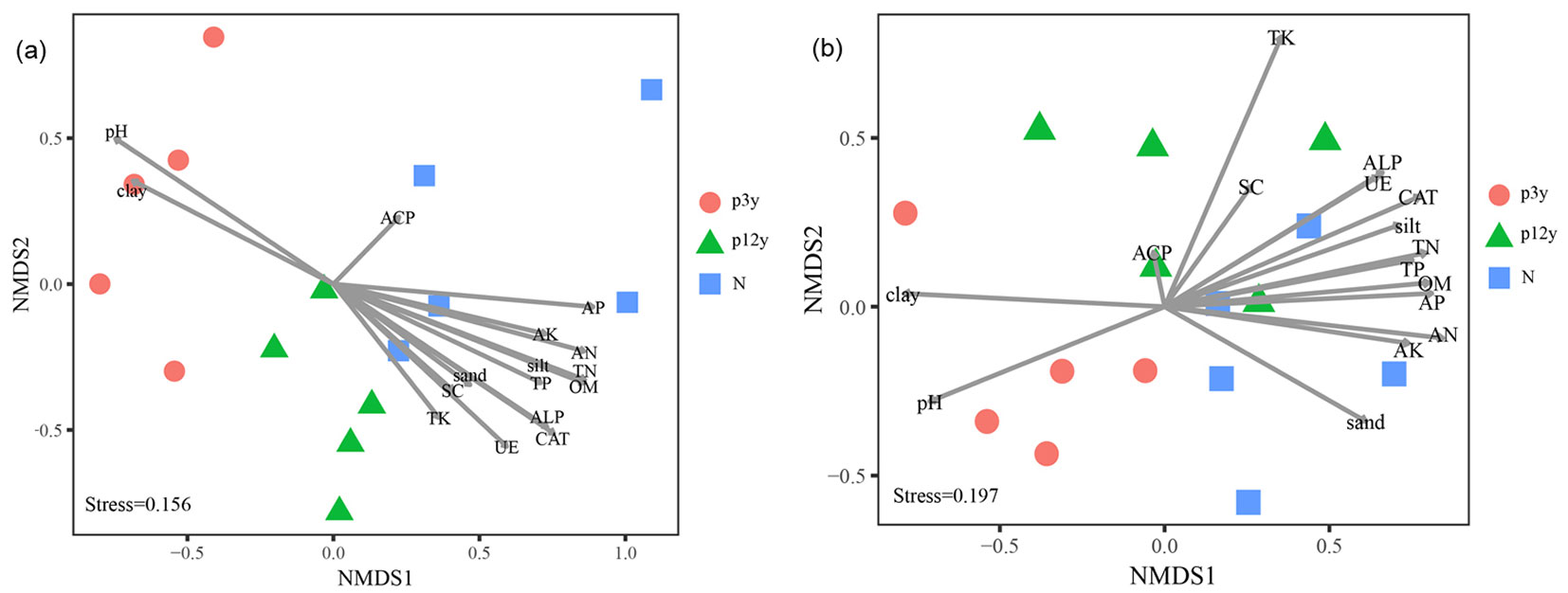
The NMDS permutation test resulted in a clear separation of the AM fungal community composition of P3y, P12y and N (Fig. 3a). This observation was further confirmed by the PERMANOVA test (F=7.2688, p=0.001 - Tab. S3 in Supplementary material). The 3 stands shared Glomus, Acaulospora, Gigaspora, unclassified Gigasporaceae, unclassified Archaeosporaceae and unclassified Glomeromycetes. Glomus was the most dominant genus in all 3 stands, with sequence proportions of 87.31%, 87.56% and 55.00% in P3y, P12y and N, followed by Acaulospora, with proportions of 11.01%, 10.17% and 43.12% in P3y, P12y and N, respectively; Redeckera was detected only in P12y. Three taxa, namely, Archaeospora, Scutellospora and Paraglomus, were shared by P3y and P12y and were not found in N (Fig. 4 - Tab. S2 in Supplementary material).
Fig. 3 - Non-metric multidimensional scaling (NMDS) plots showing the correlations between AM fungal community and soil characteristics. NMDS permutation test on OTU abundance (a) and on the relative abundance of different morphological taxa (b). Physicochemical correlations are shown with lines. The length of lines indicates the relative importance of that variable in explaining the variation in AM fungal community composition, while the angle between arrows indicates the degree to which they are correlated.
Fig. 4 - Proportional distributions of the sequences and operational taxonomic units (OTUs) in the phylum Glomeromycota among the total, P3y, P12y and N samples. P3y, P12y and N represent 3-year-old sapling, 12-year-old stand and adjacent native forest, respectively.
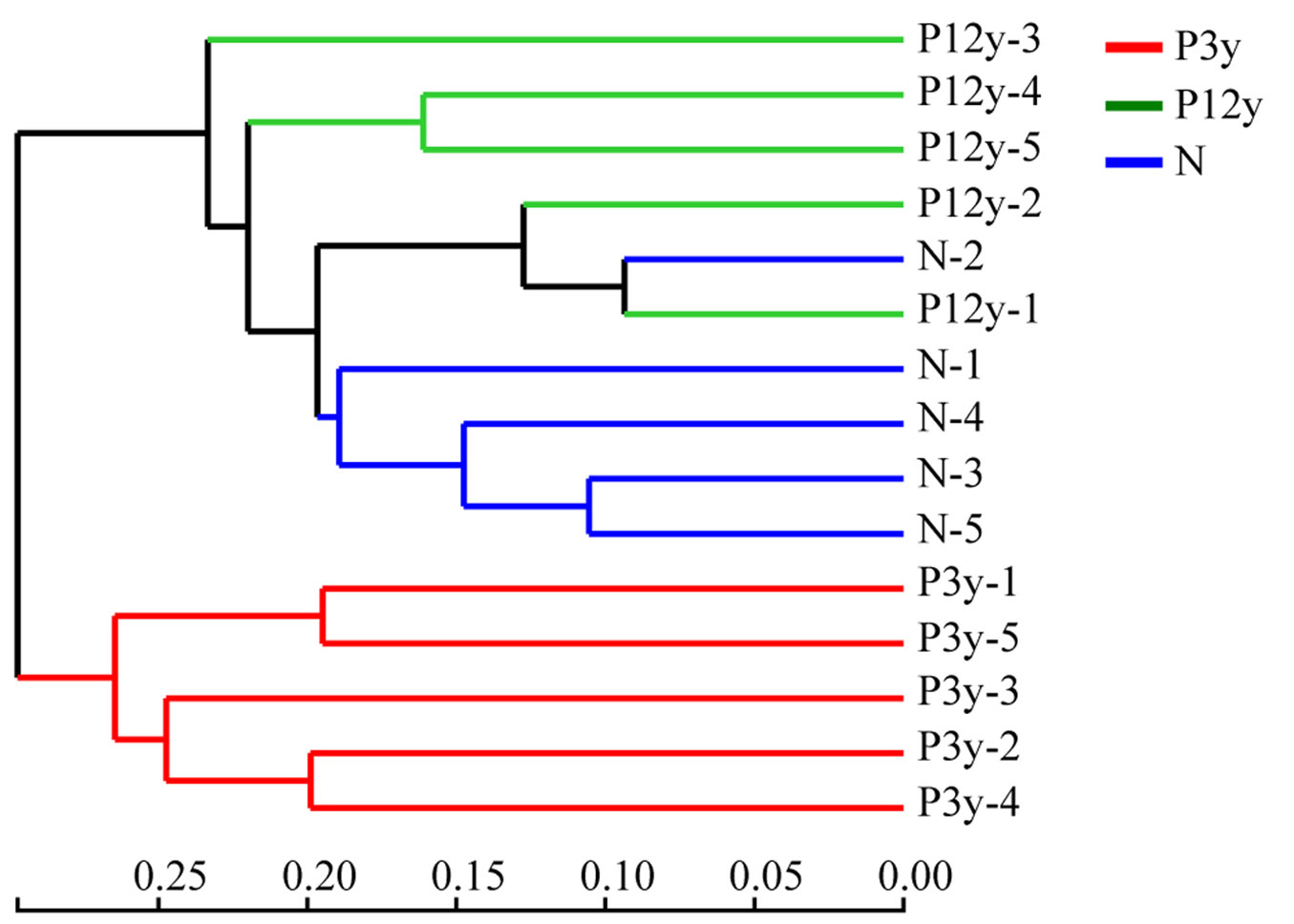
Hierarchical cluster analysis concerning the number and abundance of OTUs showed that the AMF communities of 15 samples were divided into two distinct clusters, one cluster contained only the five samples of P3y. In contrast, the other cluster included the five samples of P12y and five samples of N, showing a high degree of similarity of AMF communities from these two study stands (Fig. 5).
Fig. 5 - Hierarchical cluster analysis based on the OTU similarity of AM fungal communities from P3y, P12y and N. P3y, P12y and N represent 3-year-old saplings, 12-year-old stand and adjacent native forest, respectively.
AM fungal attributes and community composition in relation to soil characteristics
Considering the AM fungal attributes, including AM colonization, spore density and diversity indexes, including morphological and molecular, the Kruskal-Wallis test only found significant differences among different study stands on two attributes, namely, colonization percentage of AMF hyphae (p = 0.044) and Chao-1 index of OTUs in the molecular analysis (p = 0.026 - Tab. 2).
Neat clustering was not apparent in the morphological analysis of AM fungal communities on the relative abundance of different taxa (Fig. 3b), and significant dissimilarity between different study stands was not observed (PERMANOVA test r2=0.1320, F=1.9775, p=0.1480 - Tab. S3 in Supplementary material).
The variation in AM fungal community composition based on OTUs was positively correlated with a series of parameters, including soil AP, AK, AN, TN, TP, TK, OM, silt, sand, ALP, CAT, UE and SC along axis NMDS1, whereas pH and clay were positively correlated with variation along axis NMDS2 (Fig. 3a). Furthermore, to compare the effects of variables on the AM fungal community composition, Mantel tests were conducted. AP had the most substantial effect on the community (p=0.001), and AN, OM, TN, and silt content also significantly influenced the AM fungal community composition (Tab. 4).
Tab. 4 - Mantel test of relationships of AM fungal community composition of Betula alnoides stands with soil characteristics. (TN): total nitrogen; (TP): total phosphorus; (TK): total potassium; (AN): available nitrogen; (AP): available phosphorus; (AK): available potassium; (OM): organic matter; (ACP): acid phosphatase activity; (ALP): alkaline phosphatase activity; (UE): urease activity; (CAT): catalase activity; (SC): sucrase activity. Values in italics indicate significant correlations (p < 0.05).
| Variable | AM fungal community | |
|---|---|---|
| Mantel r | p value | |
| pH | 0.1536 | 0.2150 |
| TN | 0.3259 | 0.0170 |
| TP | 0.1342 | 0.3490 |
| TK | -0.0520 | 0.7650 |
| AN | 0.3076 | 0.0040 |
| AP | 0.5774 | 0.0010 |
| AK | 0.3069 | 0.0800 |
| OM | 0.3326 | 0.0060 |
| ACP | 0.1032 | 0.4100 |
| ALP | 0.1045 | 0.3670 |
| UE | 0.0780 | 0.6620 |
| CAT | 0.1465 | 0.1910 |
| SC | -0.0995 | 0.5700 |
| sand | 0.0061 | 0.9840 |
| silt | 0.2426 | 0.0220 |
| clay | 0.2029 | 0.1310 |
Discussion
The present study was the first to explore the AM symbiosis of B. alnoides in both plantations and natural stand and to compare AM fungal community composition among young sapling, middle-aged and mature natural growing B. alnoides in tropical ecosystems of Xishuangbanna, Yunnan Province, southwestern China. Thus, it is the first step in understanding the ecological aspects of AM fungal communities in B. alnoides plantations and the first step towards the management of AM fungal communities aiming at improving the plantation soil quality and health as a whole.
Primary status of AM of B. alnoides stands at different ages
The results indicated that among the AM fungal attributes studied, hyphal colonization in roots and the Chao-1 index of AMF OTUs in rhizosphere soil differed among the three studied stands; those in P12y were the highest, followed by P3y, and the lowest was N. This result could be explained considering the nutrient-rich conditions of the natural stand; sufficient mineral nutrients may be taken up by plants from the soil without the help of AMF, leading to a gradual reduction in the dependency of plants on AMF ([27]). The higher root AMF colonization of middle-aged B. alnoides trees in our study was similar to that reported by Sheng et al. ([41]) of black locust (Robinia pseudoacacia) plantations. Sheng et al. found that the AMF colonization of black locust tree roots in plantations along a chronosequence of 11, 23, 35 and 46 years of age showed a hump-shaped variation, peaking at an intermediate stage of 35 years. Moreover, AMF have different life strategies ([20]); for instance, Glomus species are known as competitive root colonizers since they can colonize roots from spores ([20]). The experiment on Plantago lanceolata showed that Claroideoglomus species could colonize roots profusely and form numerous arbuscules and hyphal coils ([4]). In this study, the B. alnoides trees at different ages harbored distinct AMF groups, which might partly explain the divergence of AMF colonization in roots.
AMF spore density in rhizosphere soil
AMF spore densities recorded from 51 spores/20 g air-dried soil to 225 spores/20 g air-dried soil in this study were comparable to those observed from the tropics ([3]). AMF spores are resting structures that are involved in “long-term” survival, and their abundance varies with the sporulation rates of different species and has also been found to be closely related to host plants ([11], [2]).
In our study, the spore density increased over a chronosequence from 3 years old to 12 years old and in natural stand with long growing time and denser vegetation cover. These results appear to contradict those of Birhane et al. ([3]), who observed a low spore density in dense vegetation cover and significantly higher spore density in poor vegetation cover but agree with the findings of Sheng et al. ([41]), who found a linear increase in spore abundance in black locust (Robinia pseudoacacia) plantations over a chronosequence from 11 to 46 years.
In our study, the low AMF spore density in 3-year-old plantation could be attributed to disturbances in the soil due to vegetation removal during land preparation before planting B. alnoides seedlings, as AMF availability and activity can be affected by tillage or soil preparation, which can result in a significant decrease in spore density ([10]).
AM fungal community diversity and composition
A total of 9, 10 and 6 AM fungal genera were identified in P3y, P12y and N by Illumina MiSeq sequencing, respectively. However, based on spore morphological identification, 12, 15 and 17 species belonging to four genera, namely, Acaulospora, Glomus, Gigaspora and Sclerocystis, were detected. Variation in the sporulation rate of different AMF species might explain the different findings between the morphological and molecular analyses since morphology depended on spore surveys in rhizosphere soil. In contrast, the molecular method could be used to detect all the AMF propagules, including spores, hyphae and AMF-infected rootlets of the host plant. Nevertheless, to more thoroughly assess AMF community diversity, it is necessary to associate classical taxonomic evaluations with molecular biological techniques ([23]). An accurate depiction of the AM fungal community could be obtained by Illumina MiSeq® sequencing since a tremendous amount of amplicon data can be collected with this technique ([27]). In contrast, morphological studies can accumulate propagules, which are necessary for studying the application of AMF in the following step.
AMF species richness was high in plantations, pointing to a broader range of niches and opportunities in these study plots. Similar results were obtained by Reyes et al. ([38]), who compared the morphospecies of AMF in 3-4-year-old and 6-8-year-old degraded secondary forests with mature rainforests and found that both the species richness and Shannon index of degraded secondary forest regrowth were significantly higher than those of mature rainforests. They thus concluded that the resilience of secondary forests was high and excellent, and this kind of resilience was also confirmed by the study of reforestation plots on degraded pastures in South Ecuador ([19]). Likewise, in our study, B. alnoides plantations did not significantly reduce the AM fungal community diversity, and resilience was not low in B. alnoides plantations compared with neighboring natural forest. Moreover, with increasing stand age, the AMF fungal communities showed a noticeable development trend towards the natural stand. However, the distinct composition of the natural stand itself revealed the considerable potential for AMF diversity conservation, suggesting the crucial importance of protecting pristine tropical forests.
The results of our study, including both the morphological identification of spores and molecular analysis, showed that Glomus and Acaulospora were the dominant taxa. These two genera have a high prevalence in most ecosystems ([9], [10], [36]), which is possibly due to the high adaptability of these AM fungal groups to different plant hosts, soil types, climatic conditions and other environmental characteristics ([45]).
Regarding the proportion of specific genera, the results of Illumina MiSeq® sequencing revealed that the proportions of Glomus sequences in P3y and P12y were 87.31% and 87.56%, respectively, showing the absolute position. In contrast, in the natural stand, Glomus sequences had a proportion of 55.00%, which was not as significantly dominant as that in plantations. Glomus taxa are thought to be resistant to many kinds of disturbances ([7]), which might partly explain the higher proportion of this group in plantations.
Soil characteristics driving AM fungal communities
The influences of climate, soil and plant communities on AMF communities are well documented ([1], [41], [30]). Although many researchers have reported that soil impacts AMF diversity, there is no consistent conclusion. Some authors have reported that soil texture has a more significant influence on the AMF community than soil chemical properties ([9], [13]). However, other studies have found that while soil chemistry does modify the AMF community, soil texture does not appear to do so ([35]).
In the present study, soil pH, AP, AK, AN, TN, TP, OM, silt, sand, clay, ALP, CAT, UE, SC and TK differed significantly among the three studied stands. These factors are vital in shaping AM fungal communities in different natural ecosystems ([26], [48], [38]). However, which factors principally contribute to the differences in AM fungal communities in different stands is of critical concern. We revealed that AP was the main driving factor of the AM fungal communities among different stands. Phosphorus uptake is considered the primary role of AM fungal symbionts. However, high phosphorus availability can reduce the dependency of plants on AMF ([26]), which may decrease the carbohydrate supply from plants for AMF in roots and lead to a decline in the fungal community ([27]). In addition to available phosphorus, the Mantel test revealed that TN, AN, OM and silt content were the explanatory variables that significantly shaped the AMF communities in our study.
Conclusions
The results of the present study provide a general picture of the composition of the AM fungal community at different growth stages of B. alnoides stands in Xishuangbanna, a tropical area in Yunnan Province, China. The roots of Betula alnoides trees at different ages were typically colonized by AMF. In the rhizosphere soil of plantation stands, we detected diverse but distinct AM fungal communities compared with that of neighboring natural stand. AMF showed considerable resilience to the establishment of the B. alnoides plantation, wherein the 12-year-old plantation harbored AM fungal communities more similar to those in the natural stand than those in the 3-year-old plantation. This implies that along with the growth of plantation, which is driven by the improvement in environmental factors such as soil parameters, especially available phosphorus, available nitrogen, organic matter, total nitrogen and silt content, the AM fungal diversity and community composition could develop towards and close to the state of the natural forest. The importance of natural forest conservation is considerable since it harbored unique AMF composition and diversity. Evaluation of the mycorrhizal status and variation of AM fungal communities is only a first step for providing some information to improve the management of B. alnoides plantations; ideally, future research should be aimed at screening for suitable AMF species and practical technology, which are crucial for efficient planting and sustainable management of B. alnoides plantations.
Acknowledgments
The authors thank Liqun Yang, Feng’e Zhang and Shuming Zhang for their assistance in samples collection and some laboratory work. This work was financially supported by Technological Innovation Talents Raising Foundation of Yunnan Province (2012HB053); Young Academic and Technical Leader Raising Foundation of Yunnan Province (2011CI027); and Tropical and Subtropical Precious Timber Species Innovation Research Team Construction Project of Yunnan Province (2017HC024).
Author contribution
Yuebo Jing and Tao Li contributed equally to this work.
References
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Tao Li 0000-0001-8121-0111
Lin Chen
Zhiwei Zhao 0000-0001-9451-9847
Laboratory of Conservation and Utilization for Bioresources and Key Laboratory of Microbial Diversity in Southwest China, Ministry of Education, Yunnan University, Kunming, 650091 Yunnan (China)
Yunnan Reascend Tobacoo Technology (group) Co., Ltd., Kunming, 650106 Yunnan (China)
College of Food Science and Technology, Yunnan Agricultural University, Kunming, 650201 Yunnan (China)
Department of Plant and Environmental Sciences, Agricultural Science Center, New Mexico State University, Farmington, NM 87401 (USA)
Corresponding author
Paper Info
Citation
Jing Y, Li T, Cui H, Li L, Allen Samuel C, Chen L, Li Y, Zhao Z (2020). Shifts in the arbuscular mycorrhizal fungal community composition of Betula alnoides along young, middle-aged plantation and adjacent natural forest. iForest 13: 447-455. - doi: 10.3832/ifor3515-013
Academic Editor
Werther Guidi Nissim
Paper history
Received: May 15, 2020
Accepted: Aug 04, 2020
First online: Oct 07, 2020
Publication Date: Oct 31, 2020
Publication Time: 2.13 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 37816
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 31528
Abstract Page Views: 3075
PDF Downloads: 2525
Citation/Reference Downloads: 0
XML Downloads: 688
Web Metrics
Days since publication: 1940
Overall contacts: 37816
Avg. contacts per week: 136.45
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 8
Average cites per year: 1.33
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effects of arbuscular mycorrhizal fungi on microbial activity and nutrient release are sensitive to acid deposition during litter decomposition in a subtropical Cinnamomum camphora forest
vol. 16, pp. 314-324 (online: 13 November 2023)
Research Articles
Arbuscular mycorrhizal colonization in black poplar roots after defoliation by a non-native and a native insect
vol. 9, pp. 868-874 (online: 29 August 2016)
Research Articles
Effect of plant species on P cycle-related microorganisms associated with litter decomposition and P soil availability: implications for agroforestry management
vol. 9, pp. 294-302 (online: 05 October 2015)
Review Papers
Arbuscular mycorrhizal fungi as a tool to ameliorate the phytoremediation potential of poplar: biochemical and molecular aspects
vol. 7, pp. 333-341 (online: 17 April 2014)
Review Papers
Soil fungal communities across land use types
vol. 13, pp. 548-558 (online: 23 November 2020)
Research Articles
Arbuscular mycorrhizal fungal symbiosis with Sorbus torminalis does not vary with soil nutrients and enzyme activities across different sites
vol. 8, pp. 308-313 (online: 03 September 2014)
Research Articles
Potential spread of forest soil-borne fungi through earthworm consumption and casting
vol. 8, pp. 295-301 (online: 26 August 2014)
Research Articles
Fungal and bacterial communities in a forest relict of Pinus pseudostrobus var. coatepecensis
vol. 16, pp. 299-306 (online: 09 November 2023)
Research Articles
Soil of the parent plant and AMF mix improve Cerrado’s seedlings growth in forest nurseries
vol. 15, pp. 197-205 (online: 28 May 2022)
Short Communications
Culturable fungi associated with wood decay of Picea abies in subalpine forest soils: a field-mesocosm case study
vol. 11, pp. 781-785 (online: 28 November 2018)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword