Effects of arbuscular mycorrhizal fungi on microbial activity and nutrient release are sensitive to acid deposition during litter decomposition in a subtropical Cinnamomum camphora forest
iForest - Biogeosciences and Forestry, Volume 16, Issue 6, Pages 314-324 (2023)
doi: https://doi.org/10.3832/ifor4324-016
Published: Nov 13, 2023 - Copyright © 2023 SISEF
Research Articles
Abstract
Arbuscular mycorrhizal fungi (AMF) play an important role on litter decomposition, which is increasing suffering the negative impact of acid deposition. In this study, we investigated the AMF effects on litter decomposition via suppressing AMF and simulating acid deposition in a subtropical Cinnamomum camphora forest. The results showed that acid deposition and AMF suppression decelerated C. camphora leaf litter decomposition, especially at late decomposition stage; soil water content was the main factor restricting early-stage decomposition. The inhibiting effect of acid deposition was enhanced with acid intensity increase and AMF suppression aggravated the negative effect of acid stress on decomposition. Nitrogen-cycling enzymatic activity was significantly higher in later than in early decomposition stage, and acid deposition and AMF suppression significantly decreased microbial activity. Despite the seasonal effect was overwhelming, we still detected the effects of acid deposition and AMF suppression on litter nutrient release. Without or under low acid deposition, AMF suppression significantly increased organic matter and decreased alkali-hydrolyzable nitrogen content of detritusphere soil. Acid deposition significantly reduced soil organic matter content, while high acid deposition intensity increased alkali-hydrolyzable nitrogen content after 2- and 12-month decomposition, and decreased it at other months. Both acid deposition and AMF suppression decreased available phosphorus content, but did not affect phosphatase activity. AMF effects on invertase and nitrogen-release enzyme activities, and alkali-hydrolyzable nitrogen and available phosphorus contents of detritusphere soil were highly sensitive to acid deposition. Our results revealed that AMF effects on microbial activity and nutrient release during litter decomposition are sensitive to acid deposition.
Keywords
Litter Decomposition, Arbuscular Mycorrhizal Fungi, Acid Deposition, Extracellular Enzyme Activity, Detritusphere Soil Nutrients
Introduction
With the development of industry, global climate and environmental problems such as nitrogen deposition and climate warming are becoming more and more serious, and the increasingly serious acid deposition has been one of the major environmental problems ([38]). Although environmental conditions are improving in China in recent years due to some control measures, China has become the third major acid rain area in the world after North America and Europe, and has been experiencing acid rain pollution since the 1980s ([46]). Acid deposition is harmful to terrestrial ecosystems by damaging vegetation and acidifying soil and surface water ([46]), which subsequently cause the degradation of forest ecosystems by directly damaging soil organisms, and reducing microbial activity and soil nutrient availability ([49]). Therefore, there is a pressing need to determine the impacts of changes in acid deposition properties (e.g., level of acidity) on forest ecosystem processes, such as litter decomposition.
Litter decomposition is essentially a complex soil biogeochemical process driven by biotic and abiotic factors ([42]). Previous studies have reported that acid deposition have positive, negative, or no effect on soil microbial communities, depending on specific contexts, for instance, level of acidity, composition of acid rain, composition and/or acid-resistance of microbial communities ([22]). Soil microbial decomposers participate in litter decomposition mainly by exuding various functional enzymes ([2]). These extracellular enzyme activities can be used to predict microbial functions since they process organic matter and release resources such as carbon (C), nitrogen (N), and phosphorus (P) ([15]). The simulated acid rain of different chemical composition and intensities consistently reduced soil microbial enzyme activities such as cellulase and urease that are involved in C- and N-release, but it stimulated acid and alkaline phosphatase activities in two subtropical forest soils ([24]). Thus, in-depth research is needed regarding to the effect of acidity level on soil microbial enzyme activity and the subsequent nutrient release.
Arbuscular mycorrhizal fungi (AMF) are ubiquitous and abundant mutualistic root symbionts that associate with 72% of terrestrial plants, and form extensive mycelia that increase the exploited soil volume ([36]). Colonization by AMF on decomposing leaves can accelerates organic material decomposition and transfer litter N to host plants ([12]). As much as two-thirds of tree-derived N in maize leaves was attributed to AMF-mediated N uptake from faidherbia leaf litter beyond the maize rooting zone ([4]). Unlike ectomycorrhizal and ericoid mycorrhizal fungi that can directly secrete extracellular enzymes, AMF lacks the saprophytic ability and cannot mineralize organic matter when they exist alone ([28]). However, AMF mycelia can accelerate the decomposition of plant litter and increase litter C and N release by enhancing the activity of surrounding soil saprophytic fungi ([1], [6]). Besides, AMF can improve the relative abundance of basidiomycetes and promote decomposition at the early stage of litter decomposition ([9]). Nevertheless, the AMF effects on litter decomposition are environment dependent, for example, depending on host requirement on soil nutrients ([15]), species composition of AMF community, as well as soil physical and chemical properties ([44]).
In recent years, soil pH is reported to be one of important abiotic variables affecting AMF relative abundance in local scale and functions in ecosystems ([3]). Several studies have also shown that soil pH remarkably influences the community composition of AMF ([39]). In soil of neutral pH and low phosphorus content, AMF had the highest effects on the activity of most soil enzymes, and the greatest effects on N releasing enzymes ([31]). Soil acidification induced by acid and nitrogen deposition seriously reduces AMF colonization, spore production and growth of extraradical hyphae ([33]). Thus, in addition to directly affecting microbial decomposers, acid deposition can further affect litter decomposition by affecting AMF functions ([22]). Under acid deposition circumstance, AMF effects on litter decomposition would be constrained and are rarely reported.
Central China region has the highest acid rain intensity in China and sulfuric acid is the main component ([37], [46]). In this study, the leaf litter of Cinnamomum camphora L., a representative dominant tree species of artificial forests in the subtropical region of Hunan Province, was investigated. Litter decomposition, soil microbial activity and litter nutrient release were evaluated to study the effects of AMF on litter decomposition via suppressing AMF with benomyl in field under acid deposition. We hypothesized the following: (i) despite AMF promoting microbial activity, single acid deposition would decrease microbial activity, and acid deposition induced low pH likewise inhibits AMF activity; (ii) both acid deposition and AMF suppression would decelerate the decomposition of C. camphora leaf litter, and nutrient release would decrease as microbial activity was inhibited by acid deposition and AMF suppression.
Materials and methods
Study site description
The experiment was carried out in an artificial Cinnamomum camphora forest (110° 27′ 28.80″ E, 29° 08′ 37.59″ N) in the back hill of Zhangjiajie campus of Jishou University, Hunan Province. C. camphora is the dominant tree species, and the ground layer is rich in shrub and herb plants. The elevation of the sample site is about 250 m a.s.l., which belongs to prototype monsoon humid climate of subtropical mountains. The rainfall is abundant, with an average annual precipitation of about 1500 mm and an average annual temperature of about 16 °C. The average temperature in July, the hottest month, is about 27 °C, and the average temperature in January, the coldest month, is about 4 °C. The ultisol soil with pH 5.98 ± 0.02 ([37]).
Experimental design
As the dominant species of subtropical evergreen broad-leaved forests for ecological greening, C. camphora experiences two large defoliation periods in spring and autumn equinoxes. Before the experiment, a nylon network was set up in the C. camphora forest to collect the fallen leaves in March 2021. The fallen leaves were taken back to the laboratory for sub packaging, and 10 g fresh fallen leaves were put into a 20 × 20 cm nylon litterbag (1 mm mesh size). The mesh could exclude soil macro- and meso-fauna that have strong gnawing effect.
We conducted a factorial experiment of AMF suppression × acid deposition (pH4.8 and pH4.0 sulfuric acid for two acidity levels) to determine their impacts on litter decomposition. In order to effectively suppress AMF without affecting other fungi, the AMF suppression groups received benomyl solution ([41]). This resulted in a total of 6 different treatment combinations: (1) deionized water as positive control (CK); (2) benomyl application for suppressing AMF (B); (3) pH4.8 sulfuric acid (pH4.8); (4) pH4.8 sulfuric acid and benomyl (pH4.8+B); (5) pH4.0 sulfuric acid (pH4.0); and (6) pH4.0 sulfuric acid and benomyl (pH4.0+B). In a 20 × 20 m C. camphora forest plot, 5 subplots of 5 × 5 m were set (5 replicates). In each subplot, 6 homogeneous areas (blocks) of 1 × 1 m were set, with at least 1 m apart between adjacent blocks to ensure that the blocks do not interfere with each other. In order to obtain enough detritusphere soil, 2 litterbags were put in each block for every sampling time. Thus, each block had 12 litterbags for 6 sampling times, resulting in totally 360 litterbags. In March 2021, litterbags were placed on the surface of mineral soil under the canopy of conspecific trees. In order to avoid exogenous interference, subpackaging and placing of leaf litter were completed within two days after collection. Part of leaf litter was oven dried at 60 °C for one week to determine litter water content for calculating the initial dry mass of C. camphora leaf litter.
During the one-year decomposition period, 1 L sulfuric acid solution was sprayed every 20 days to simulate the acid deposition environment, and the non-acid groups received 1 L deionized water. As benomyl is easy degraded under strong acid environment, an interval of 10 days between the two spraying treatments was set. Thus, after 10 days, 5 L benomyl solution was sprayed with 1.2 g L-1 active ingredient every 40 days, and the non-benomyl groups received 5 L deionized water. Every two months, two litterbags were randomly retrieved from each block and composited to get one replicate; soil samples attached and/or around the litter or litterbags (detritusphere soil) were brushed, collected and composited to get one replicate. Detritusphere soil samples were sieved through a 2-mm mesh and stored in a 4 °C refrigerator.
Determination of litter dry mass and AMF colonization
When harvested, leaf litter after brushed was cleaned with deionized water and oven-dried to constant weight under 60 °C; the dry mass was then determined. The rate of mycorrhizal colonization on C. camphora leaf litter was calculated using the gridline intersect method after staining with trypan blue. Dark- to light-blue stained aseptate hyphae with characteristic unilateral angular projections (“elbows and coils”) were considered mycorrhizal, whereas non blue stained or blue stained hyphae with regular septation or straight growth were considered non-mycorrhizal ([1]). Very short or deteriorated pieces were excluded from the analysis.
Determination of soil microbial activity
Extracellular enzyme activities involved in C-release (invertase and catalase), N-release (urease and protease) and P-release (phosphatase) were determined. Soil invertase activity (EC.3.2.1.26) was determined by the 3.5-dinitrosalicylic acid colorimetric method, which was expressed as the number of milligrams of glucose produced in 1 g of dry soil after 24 h ([20]). Catalase (EC 1.11.1.6) activity was measured by back-titrating residual H2O2 with KMnO4. Two g soil sample was added to 40 mL distilled water with 5 mL of 0.3% hydrogen peroxide solution. The mixture was shaken for 20 min and then 5 mL 1.5 mol L-1 H2SO4 was added. Afterwards the solution was filtered and titrated using 0.02 mol L-1 KMnO4. The reacted amount of 0.02 mol L-1 KMnO4, calculated per gram of dry soil, was used to express the activity of catalase. Urease activity (E.C. 3.5.1.5) was determined using 10% urea solution as substrate with incubation at 37 °C for 24 h under pH 6.7; NH4+-N concentration was determined with a spectrophotometer at a wavelength of 578 nm. Protease activity (EC 3.4) was determined according to Kandeler et al. ([14]). Soil samples were incubated for 24 h in a buffered casein solution (pH 8.1) at 50 °C. The aromatic amino acids released were extracted with trichloroacetic acid (0.92 M) and measured colorimetrically after adding the Folin-Ciocalteu reagent. Acid phosphatase activity (EC 3.1.3.2) was determined using 0.5% disodium phenyl phosphate solution as substrate with incubation at 37 °C for 24 h under pH 5.0; phenol concentration was determined with a spectrophotometer at 570 nm ([14]). All enzyme assays were started within 48 h of sample collection.
Determination of detritusphere soil nutrient contents
In order to determine litter nutrient release, detritusphere soil nutrient contents were measured. Soil water content was determined by drying method. Soil organic matter (SOM) content was determined by potassium dichromate oxidation method ([6]). A 0.2-g sample was digested in 10 mL 0.136 mol L-1 potassium dichromate sulfuric acid solution, cooled and titrated with 0.2 mol L-1 ferrous sulfate standard solution. Alkali-hydrolyzable N (AN) content was quantified by the method of Roberts et al. ([32]). Briefly, 2-g soil were distilled with 2 mol L-1 NaOH for 5 h and then with 10 mol L-1 NaOH for 7 min. Boric acid (40 g L-1) was used to absorb the liberated NH3 using the method of direct steam distillation. Soil AN content was quantified by conductometric titration. Available P (AP) was extracted with 0.5 mol L-1 NaHCO3 (pH 8.5) and determined according to the Olsen method ([29]).
Data analysis
Assuming exponential decay, we calculated the decomposition rate (k) of C. camphora leaf litter by linear regression of the Ln transformed negative exponential model ([30]): k = - ln(x0/xt) / t, where k is the litter decomposition coefficient (month-1), x0 is the original dry mass of leaf litter, xt is the amount of litter remaining at time t (in month). The quantity xt/x0×100 was calculated as litter mass remaining at time t. Based on the negative exponential model, time required for decomposing 50% and 95% of C. camphora litter was predicted.
The effect size of AMF on enzymatic activity and nutrient contents were calculated by dividing the measured variable without AMF suppression (AMF+) by the measured variable under AMF suppression (AMF-), where effect size greater than 1 indicates positive AMF effect, while less than 1 indicates no or negative AMF effect.
Statistical analyses and graphs drawn were performed in R software and Adobe illustrator software. Data were checked for deviations from normality and homogeneity of variance before analysis. Analysis of variance (ANOVA) and significant differences among treatments were compared through post hoc Tukey’s honest significant difference (HSD) at P < 0.05. Least significant difference test, paired t-tests, and (nonparametric) Mann-Whitney U tests were also used when necessary. We ran repeated-measures ANOVA to determine the effects of sampling time (T, repeated factor), main factors, and their interactions on mass remaining, enzymatic activity, and SOM. To evaluate and visualize their relationships, principal component analysis (PCA) was performed by “vegan” package in R and heatmap of Spearman’s correlation was also drawn. After 6-month decomposition, nearly half of the litter was decomposed, and the later 6 months (later decomposition stage) displayed contrasting decomposition trend with the first 6 months (early decomposition stage), we thus analyzed some parameters of these two stages separately.
Results
Effects of acid deposition and AMF on litter mass remaining
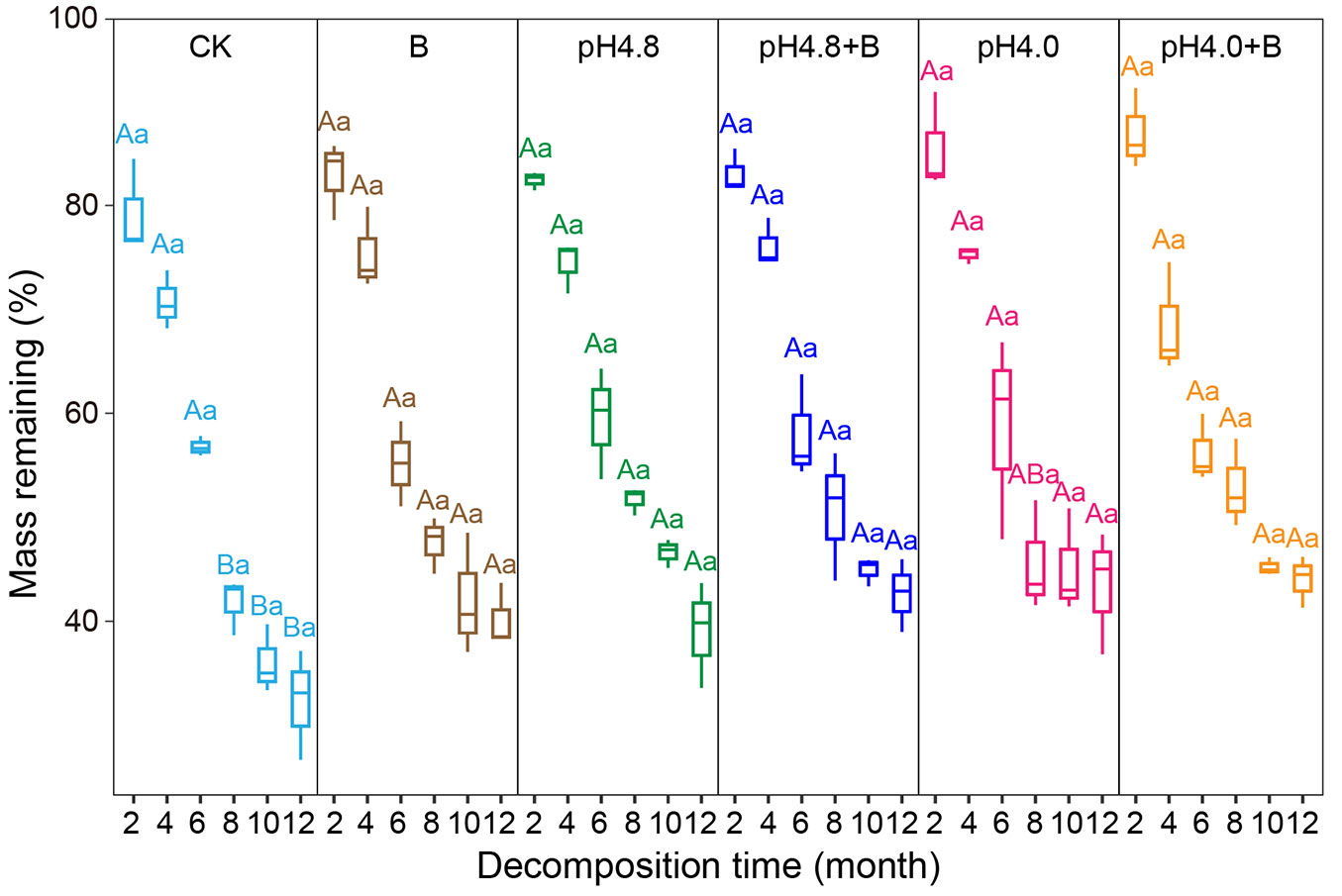
At the early decomposition stage, C. camphora litter decomposed rapidly and mass remaining decreased dramatically with time (Fig. 1). At the later decomposition stage, except for CK, the decomposition speed slowed down rapidly, and especially in pH4.0 treatment which had no significant decomposition afterward. Repeated measures ANOVA showed that sampling time, acid deposition, and AMF suppression had significant effects on mass remaining at the later decomposition stage, while at the early decomposition stage only the effect of sampling time was significant (P < 0.005 - Tab. 1). After 6-month decomposition, litter mass remaining were 56.80% (CK), 63.14% (pH4.8), 58.70% (pH4.0), 55.17% (B), 61.04% (pH4.8+B) and 56.24% (pH4.0 +B). The monthly decomposition amount of C. camphora leaf litter was 7.20% (CK), 6.14% (pH4.8), 6.88% (pH4.0), 7.47% (B), 6.49% (pH4.8+B) and 7.29% (pH4.0+B). At the later decomposition stage, the monthly decomposition amount was 4.08% (CK), 3.71% (pH4.8), 2.55% (pH4.0), 2.49% (B), 3.07% (pH4.8+B) and 2.04% (pH4.0+B).
Fig. 1 - The changes in mass remaining of C. camphora leaf litter under acid deposition and AMF suppression. The center line represents the median, box limits represent the upper and lower quartiles, whiskers represent 1.5 times interquartile range (n = 5). Values followed by the same capital letter among acid deposition intensities under the same benomyl treatment, and values followed by the same lowercase letter between different benomyl treatments under the same acidity level, do not significantly differ (P > 0.05) after Tukey’s HSD test.
Tab. 1 - Effects (indicated by F values from repeated-measures ANOVA) of sampling time (repeated factor, T), acid deposition (A) and AMF suppression (benomyl addition, B) and their interactions on mass remaining, enzymatic activity and soil nutrients. (Mr): Mass remaining; (Inv): invertase; (Ure): urease; (Pho): phosphatase; (Pro): protease; (Cat): catalase; (SOM): soil organic matter; (AN): alkali-hydrolyzable nitrogen; (AP): available phosphorus; (Wcs): soil water content; (*): p<0.05; (**): p<0.01; (***): p<0.001, (ns): not significant.
| Litter Decomposition |
Factor | Mr | Inv | Ure | Pho | Pro | Cat | SOM | AN | AP | Wcs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| The early 6-months | A | 1.78 ns | 13.23** | 39.88*** | 0.09 ns | 2.33 ns | 10.02** | 8.82** | 12.92** | 86.62*** | 1.14 ns |
| B | 0.001 ns | 217.30*** | 0.14 ns | 3.83 ns | 0.27 ns | 0.04 ns | 77.36*** | 355.20*** | 6.70 ns | 1.86 ns | |
| T | 157.84*** | 182.90*** | 277.81*** | 489.97*** | 192.98*** | 52.53*** | 7.83** | 540.07** | 125.91*** | 85.11*** | |
| A×B | 1.90 ns | 30.67*** | 8.42* | 2.10 ns | 12.63** | 30.33*** | 10.75** | 9.43*** | 14.86** | 2.58 ns | |
| A×T | 1.18 ns | 47.79*** | 4.69** | 0.64 ns | 2.68 ns | 6.69*** | 7.13*** | 9.13*** | 21.68*** | 0.81 ns | |
| B×T | 0.81 ns | 62.99*** | 13.34*** | 0.93 ns | 8.67*** | 2.33 ns | 3.98* | 6.59** | 2.98 ns | 0.64 ns | |
| A×B×T | 0.80 ns | 23.47*** | 5.84** | 0.26 ns | 8.23*** | 9.03*** | 8.35*** | 31.10*** | 4.43** | 1.47 ns | |
| The later 6-months | A | 20.02*** | 4.16 ns | 117.20*** | 0.16 ns | 14.86** | 1.49 ns | 10.24** | 274.50*** | 16.06** | 18.91*** |
| B | 19.47* | 0.05 ns | 145.50*** | 2.74 ns | 2.57 ns | 1.05 ns | 1.58 ns | 144.90*** | 5.31ns | 48.41** | |
| T | 15.34*** | 120.82*** | 150.95*** | 64.85*** | 8.80*** | 78.62*** | 18.31*** | 255.84*** | 130.32*** | 123.46*** | |
| A×B | 4.24 ns | 10.90** | 180.80*** | 0.45 ns | 18.48*** | 40.31*** | 18.02*** | 276.60*** | 0.95 ns | 7.89* | |
| A×T | 0.49 ns | 35.18*** | 11.61*** | 0.61 ns | 5.13** | 12.75*** | 4.26** | 17.22*** | 0.94 ns | 7.08*** | |
| B×T | 0.49 ns | 51.48*** | 9.00*** | 0.13 ns | 0.73 ns | 92.06*** | 6.40** | 10.40*** | 3.34 ns | 8.03** | |
| A×B×T | 0.74 ns | 22.14*** | 24.79*** | 0.87 ns | 10.47*** | 40.17*** | 6.06** | 11.80*** | 1.94 ns | 11.05*** |
After one-year in situ decomposition, the final litter mass remaining were 32.31% (CK), 40.84% (pH4.8), 43.39% (pH4.0), 40.20% (B), 42.61% (pH4.8+B) and 43.99% (pH4.0+B) (Fig. 1). Both acid deposition and AMF suppression inhibited the decomposition of C. camphora leaf litter. The remaining mass of C. camphora leaf litter increased with the decrease of acidity, and further increased after AMF was suppressed. At the sixth month, the difference in remaining mass between acid treatments and control was the smallest. Thereafter, the difference was gradually and constantly amplifying. The remaining mass under acid deposition was significantly lower than the control group, while the presence of AMF significantly reduced this difference (P < 0.05). On the other hand, acid deposition significantly reduced the colonization rate of AMF on litter, and AMF colonization was dramatically decreased by benomyl (P < 0.05 - see Fig. S1 in Supplementary material). Under acid deposition, the addition of benomyl further inhibited AMF colonization.
Negative exponential decay model of litter decomposition
Based on the fitting curve of negative exponential decay model, the decomposition rates under acid deposition and AMF suppression were lower than CK (Tab. 2, Fig. S2 in Supplementary material). According to the exponential decay model, it would take 6.99 and 30.47 months for 50% and 95% litter decomposition, respectively, under natural conditions. Acid deposition prolonged the time by 25.13% (pH4.8) and 23.88% (pH4.0) for 50%, and 26.78% (pH4.8) and 26.49% (pH4.0) for 95% litter decomposition, respectively. After AMF was suppressed in the natural state, the time for 50% and 95% litter decomposition was prolonged by 17.84% and 20.26%, respectively. In the presence of both acid deposition and AMF suppression, the time was 26.81% (pH4.8+B) and 28.27% (pH4.0+B) longer for 50%, and 29.78% (pH4.8+B) and 34.31% (pH4.0+B) longer for 95% litter decomposition than control, respectively. Under acid deposition, AMF suppression prolonged the time by 1.34% (pH4.8+B) and 3.54% (pH4.0+B) for 50%, and by 2.37% (pH4.8+B) and 6.18% (pH4.0+B) for 95% litter decomposition, respectively. These results suggested that AMF promote C. camphora leaf litter decomposition, and that AMF relieve the negative effect of acid deposition on litter decomposition.
Tab. 2 - Fitting results of exponential decay model for C. camphora leaf litter decomposition. The decomposition coefficients (k, month-1) are reported as means (n = 5) and 95% confidence intervals; t0.5 and t0.95 indicate the time (month) required for decomposing 50% and 95% leaf litter, respectively. CK is the control, +B indicates the benomyl addition treatment.
| Treatment | k | R2 | t0.5 (month) |
t0.95 (month) |
|---|---|---|---|---|
| CK | 0.098 ± 0.005 | 0.9896 | 6.99 | 30.47 |
| pH4.8 | 0.077 ± 0.003 | 0.9931 | 8.76 | 38.67 |
| pH4.0 | 0.077 ± 0.007 | 0.9681 | 8.67 | 38.58 |
| B | 0.081 ± 0.006 | 0.9772 | 8.25 | 36.68 |
| pH4.8+B | 0.075 ± 0.005 | 0.9806 | 8.88 | 39.58 |
| pH4.0+B | 0.072 ± 0.006 | 0.9681 | 8.98 | 40.96 |
Effects of acid deposition and AMF on detritusphere soil microbial activity
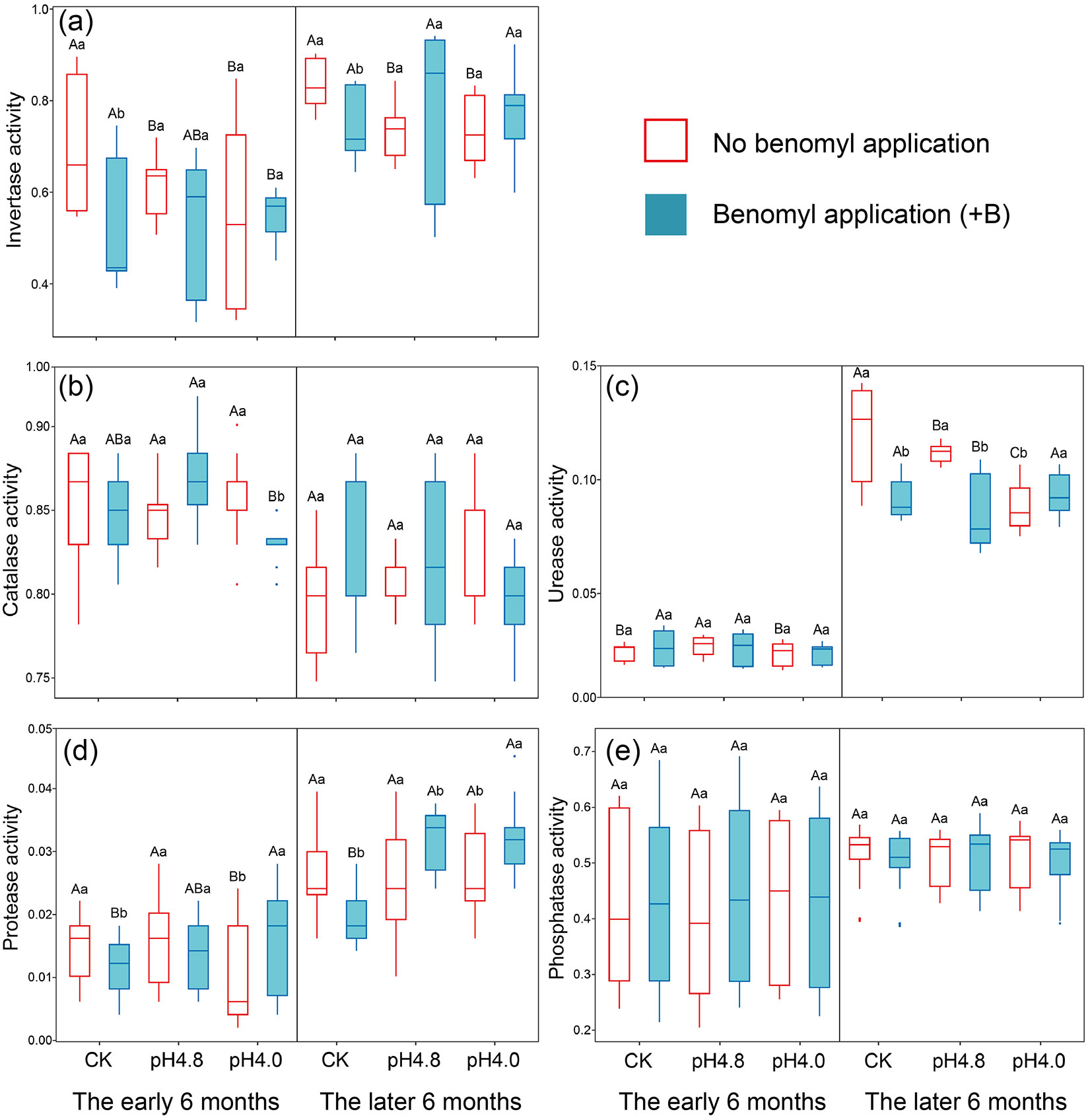
Repeated measures ANOVA showed that acid deposition, AMF suppression and their interactions had significant impacts on invertase and catalase activities at early decomposition stage, while these effects seem time-dependent at the later decomposition stage (P < 0.01 - Tab. 1). During the whole experimental period, acid deposition and AMF suppression significantly reduced invertase activity (P < 0.05 - Fig. 2a, Fig. S3 in Supplementary material). At the later 6-month decomposition, AMF suppression weakened the negative effect of acid deposition on invertase activity. On the other hand, AMF suppression reduced catalase activity, and catalase activity decreased significantly after inhibiting AMF under the condition of acid deposition (P < 0.05). At later decomposition stage, acid deposition increased catalase activity without AMF suppression.
Fig. 2 - The enzymatic activity dynamic in detritusphere soil of the early (a) and later (b) 6-month decomposition under acid deposition and AMF suppression. The center line represents the median, box limits represent the upper and lower quartiles, whiskers represent 1.5 times interquartile range (n = 15). Values followed by the same capital letter among acid deposition intensities under the same benomyl treatment, and values followed by the same lowercase letter between different benomyl treatments under the same acidity level, do not significantly differ (P > 0.05) after Tukey’s HSD test. The units of the enzyme activity were mg g-1 d-1.
Almost all effects of AMF suppression, acid deposition, sampling time and their interactions were significant on the activities of nitrogen-cycling enzymes (protease and urease, P < 0.05 - Tab. 1). Nitrogen-cycling enzymatic activities of detritusphere soil in the later 6-month decomposition stage showed obviously higher than the first 6-month (Fig. 2b, Fig. 2c, see also Fig. S3 in Supplementary material). Acid deposition significantly inhibited urease activity at the later decomposition stage, and AMF suppression significantly reduced urease activity except for under high acid-deposition (P < 0.05). AMF suppression significantly reduced protease activity during the whole experimental period, and at the early decomposition stage, high-intensity acid deposition significantly inhibited protease activity (P < 0.05). Besides sampling time, the effect of which was significant (P < 0.001), acid deposition, AMF suppression or their interaction all had no significant effect on phosphatase activity during the one-year decomposition period (P > 0.05 - Tab. 1, Fig. 2d).
Effects of acid deposition and AMF on the dynamics of detritusphere soil nutrients
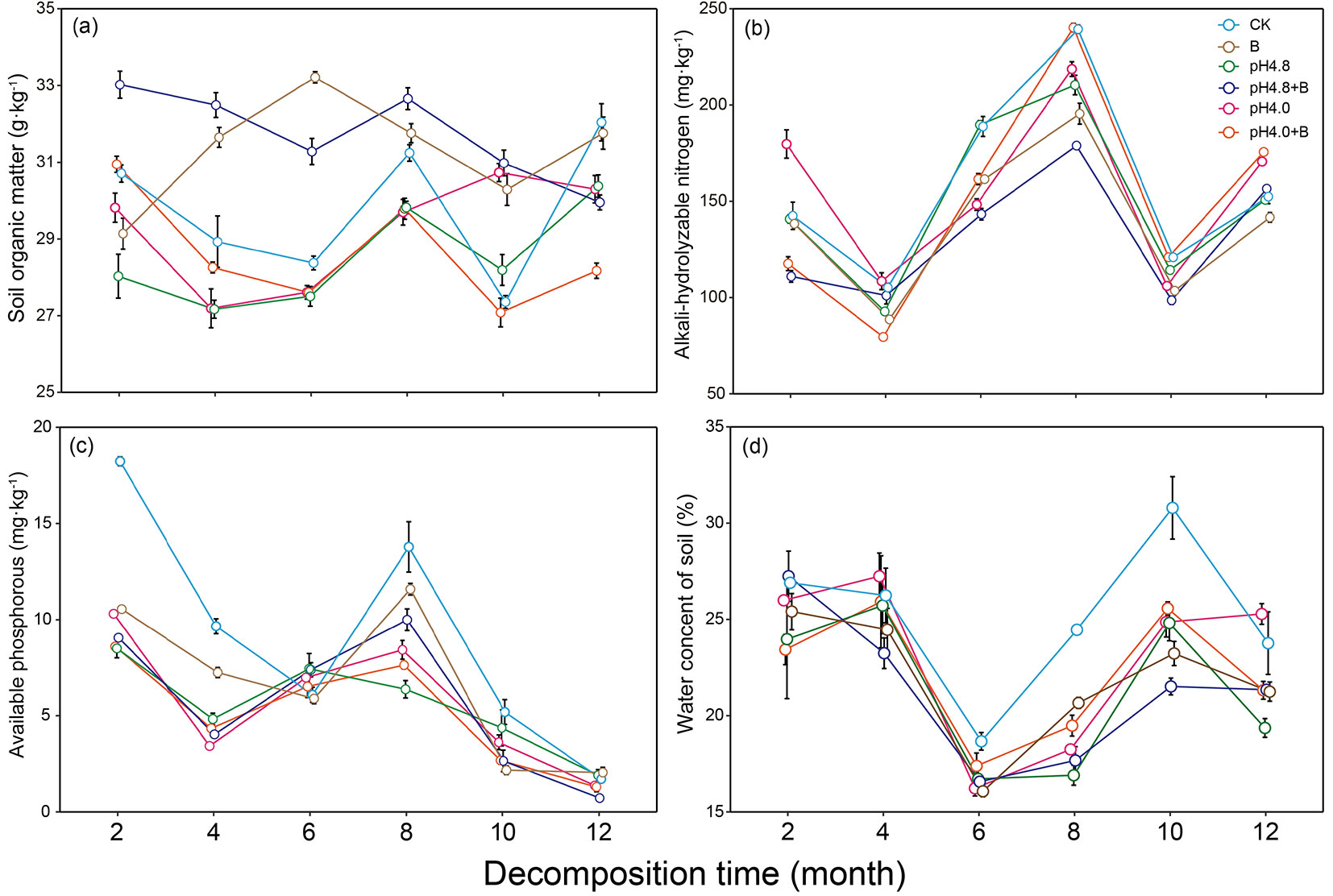
During the one-year decomposition, except for SOM content, the contents of AP, AN and water in detritusphere soil fluctuated with sampling month, and had a similar monthly variation pattern (Fig. 3). Changing of water content lagged behind that of AP and AN, suggesting they were highly sensitive to water content (Fig. S4 in Supplementary material). Soil water content was the lowest after 6-month (in September) and the highest after 10-month (in January) decomposition. Soil AN and AP contents were the lowest after 4-month and the highest after 8-month decomposition. SOM content showed no monthly fluctuation for B and pH4.8+B treatments, or weak fluctuation for other treatments.
Fig. 3 - The dynamics of soil organic matter (a), alkali-hydrolyzable nitrogen (b), available phosphorus (c) and water (d) contents in detritusphere soil under acid deposition and AMF suppression during one-year litter decomposition. Values are mean ± standard error (n = 5).
Repeated measures ANOVA showed that all effects of acid deposition, AMF suppression and their interactions on SOM and AN were significant (P < 0.05), except for AMF suppression on SOM at later decomposition stage (Tab. 1). AMF suppression significantly increased SOM content and decreased AN content under no or low acid deposition (P < 0.05 - Fig. 3a, Fig. 3b, Fig. S5). Under high acid level, AMF suppression decreased SOM content during the whole decomposition period and AN content at the early decomposition, while recovered AN content at late decomposition especially at 12th month. Acid deposition regardless of level significantly reduced SOM content. Low acid deposition had no effect on AN content, while high acid deposition intensity significantly increased AN content at 2nd and 12th month, and decreased AN content significantly at other months (P < 0.05 - Fig. 3b, Fig. S5 in Supplementary material).
Acid deposition and its interaction with AMF suppression or sampling time significantly affected AP content at early decomposition stage, while at late decomposition stage, only acid deposition and its interaction with AMF suppression showed significant effects on AP (P < 0.05 - Tab. 1). Both acid deposition and AMF suppression significantly reduced soil AP content, CK had obviously higher AP content than other treatments during the whole decomposition period (P < 0.05 - Fig. 3c, Fig. S5 in Supplementary material). After 4-month decomposition, soil water content was significant reduced by acid deposition and AMF suppression (P < 0.05 - Fig. 3d, Fig. S5 in Supplementary material), and at later decomposition stage, soil water content was significantly affected by acid deposition, AMF suppression and their interactions (P < 0.05 - Tab. 1).
Response of AMF effects on microbial activity and nutrient contents to acid deposition
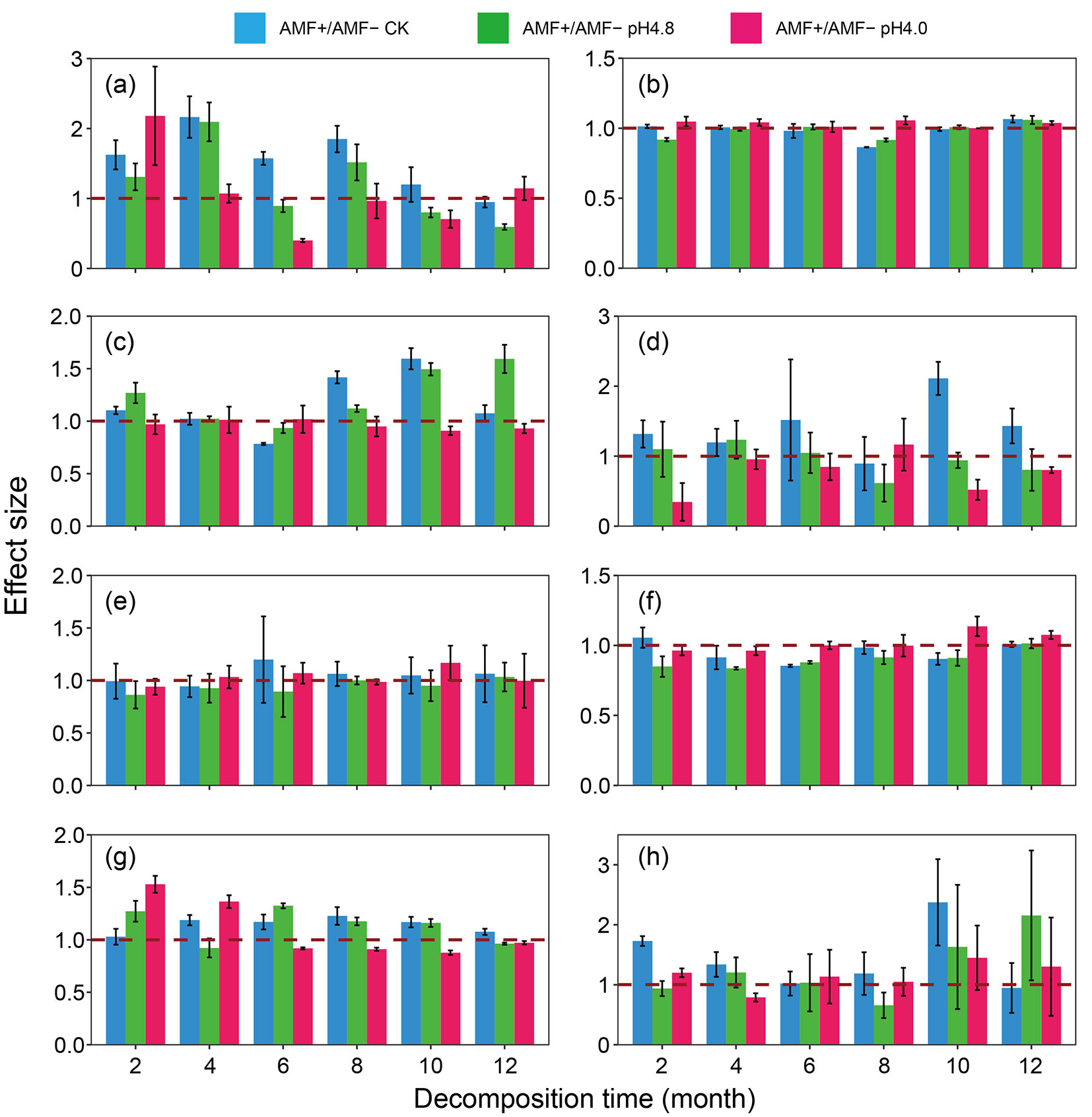
Without acid deposition, AMF positively affect the activities of invertase, urease, phosphatase and protease but had no or negative effect on catalase activity (Fig. 4a-e). The positive AMF effect on invertase activity decreased while on urease activity increased gradually with decomposition time. Besides, AMF effect on protease activity was obviously positive while on catalase activity showed no or negative effect. Acid deposition dramatically weakened, especially under high-intensity, the AMF effects on invertase and protease activities throughout the decomposition period, as well as on urease at the late decomposition stage. In contrast, high-intensity acid deposition slightly promoted the AMF effect on phosphatase and catalase, which were not sensitive to AMF effect.
Fig. 4 - The response of AMF effects on enzyme activity and nutrient contents of detritusphere soil to acid deposition. Diagrams a, b, c, d and e indicate the activities of invertase, catalase, urease, protease and phosphatase, respectively; diagrams f, g and h indicate the contents of soil organic matter, alkali-hydrolyzable nitrogen and available phosphorus, respectively. Values are mean (n = 5) and 95% confidence intervals, intervals that do not cross the 1 dashed line indicate significant effects.
Without acid deposition, AMF negatively affect SOM content while positively affect contents of AP and AN (Fig. 4f-h). High-intensity acid deposition weakened the negative AMF effect on SOM and positive AMF effect on AP. Compared with high-intensity acid deposition, the negative AMF effect on SOM content was stronger without and under low-intensity acid deposition (Fig. 4f). On the whole, the positive effect of AMF on AP content gradually weakened with the increase of acid deposition, and the difference is more obvious with the progress of decomposition (Fig. 4g), indicating the highly sensitivity of AMF to acid deposition. On the other hand, at the early stage of the experiment, acid deposition enhanced the positive effect of AMF on AN, and this effect increased with the increase of acid deposition intensity (Fig. 4h). With decomposition time, acid deposition gradually weakened the positive AMF effect on soil AN content.
The relationship of mass remaining, microbial activity and soil nutrients
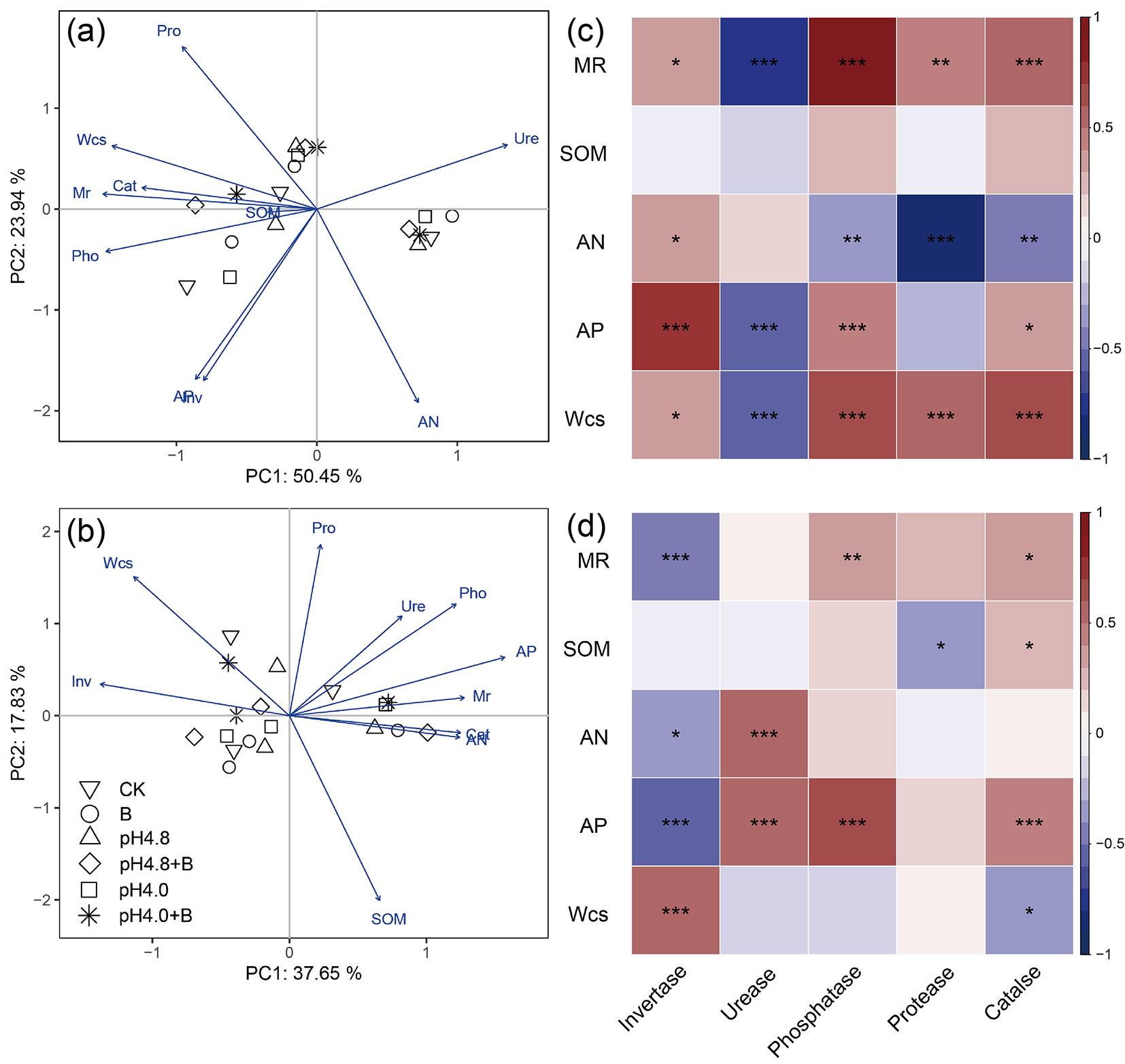
PCA result showed that PC1 explained 43.05% and 34.96%, and PC2 explained 21.48% and 15.98% of the total variance of the early and late litter decomposition stages, respectively (Fig. 5a, Fig. 5b). At the early decomposition stage, the remaining mass of litter was closely related to phosphatase and catalase activities and soil water content. At the later decomposition stage, litter remaining mass is more closely related to AP and AN content.
Fig. 5 - Principal component analysis (PCA) ordination and Spearman’s correlation heatmap of mass remaining, microbial activity and soil property at the early (a, c) and later stage (b, d) of litter decomposition. The traits were centered and standardized prior to ordination. The abbreviations are the same as shown in Tab. 1. (*): p<0.05; (*): p<0.01; (*): p<0.001.
AN was significantly correlated with activities of invertase (positive), and phosphatase, protease and catalase (negative) at early decomposition stage, while with invertase (negative) and urease (positive) activities at late decomposition stage (P < 0.05 - Fig. 5c, Fig. 5d). AP was significantly correlated with activities of urease (negative), invertase, phosphatase and catalase (positive) at the early decomposition stage, and with activities of invertase (negative), urease, phosphatase and catalase (positive) at late decomposition stage (P < 0.05). Most enzyme activities showed significant correlation with soil water content at the early decomposition stage, while only invertase and catalase activities showed significant correlation with soil water content at the late decomposition stage (P < 0.05). SOM content was significantly correlated with protease (negative) and catalase (positive) at late decomposition stage (P < 0.05 - Fig. 5d).
Discussion
Effects of acid deposition and AMF on litter decomposition
Benomyl can effectively control the growth and activity of AMF, while has little impact on other microorganisms, and has been widely used in field experiments ([41]). Application of benomyl also effectively suppressed AMF growth on the leaf litter of C. camphora in our study. On global scale, climate factors are recognized as the basic factors affecting litter decomposition ([43]). While on local scale, litter decomposition rate is mainly determined by litter quality and decomposition microenvironment, and microbial community is the main driving force ([44]). Consistently with our first hypothesis, both acid deposition and AMF suppression decelerated the decomposition of C. camphora leaf litter, but this occurred only at late decomposition stages. Litter quality was the predominant controlling factor and labile carbon was the main components to be decomposed in early-stage litter decomposition ([10]). The high decomposition rate of the high-quality fresh C. camphora leaf litter at this stage might mask the weak effects of acid deposition and AMF suppression. Besides, initial leaching process plays a crucial role at early-stage decomposition. Thus, soil water content might be the main abiotic factor affecting litter decomposition. Indeed, PCA showed that soil water content was closely related to the remaining mass of litter. At the early decomposition stage, soil water content decreased significantly and was the lowest at the 6th month (September). The low water content might weak the effect of acid deposition and AMF suppression on litter decomposition by restricting microbial activity, as most enzyme activities were significantly and positively correlated with soil water content at this stage. In addition, high soil water content due to high precipitation in the first 4-month decomposition might dilute or wash away the active ingredient of sulfuric acid and benomyl in the forests before they exerted effects on litter decomposition.
At later decomposition stages, the decomposition rate of C. camphora leaf litter slowed down rapidly under acid deposition and AMF suppression, especially in the pH4.0 treatment. The continuous application of sulfuric acid and benomyl during the experiment period might have resulted in the accumulation of these materials, which were unfavorable for activity of microbial decomposers and AMF. Besides, the remaining recalcitrant components (e.g., lignin) to be decomposed at this stage have high demand for microbial decomposers which are easily affected by soil property. In this study, the greater the acidity of acid deposition, the higher the remaining mass of C. camphora litter, indicating that intensification of acid deposition would further decelerate litter decomposition. Acid deposition can directly affect litter decomposition through soil acidification, which affects microbial community structure and weakens the extracellular enzyme activity of microbial decomposers ([22]). AMF activity was also significant inhibited by acid deposition in our study. It is generally believed that the mycelia of AMF can transport low molecular weight sugars (hyphal exudates) into the decomposition system, which generates priming effect and enhances microbial activity, thus promoting litter decomposition ([12], [15], [25], [26]). We found that suppression of AMF significantly and negatively affected litter decomposition, prolonging the time for decomposing 50% and 95% C. camphora leaf litter. Under acid deposition, this time was further prolonged, which in turn suggested that the presence of AMF may mitigate the inhibitory effect of acid deposition on litter decomposition. However, which AMF species or which guild of microbial community mediated by AMF play the mentioned buffer role needs further exploration in future work. Besides, which microbial guild benefits from this buffer effects also need further research.
Effects of acid deposition and AMF on detritusphere soil microbial activity
Microorganisms are the main contributors to litter decomposition, and the activities of enzymes secreted by microbial decomposers related to C, N and P cycling can reflect the changes of decomposition microenvironment ([27]). Consistent with a previous research ([45]), the activities of invertase, urease and protease in our study were significantly reduced after AMF suppression throughout the decomposition period, and the catalase activity was significantly reduced at later decomposition stage. However, AMF suppression had no significant effect on phosphatase activity, which is inconsistent with most previous studies ([47]). This study was carried out in an artificial C. camphora forest. The environmental factors in the field are complex, and the floor of the C. camphora forest are rich in lateral roots. Rhizodeposition might compensate to some extent for the negative effect of AMF suppression on phosphatase.
By reducing soil pH, acid deposition can directly affect enzyme activity through influencing enzyme catabolic dynamics. Besides, acid deposition can affect enzyme secretion by affecting the composition and activity of microbial decomposer community, and then indirectly affect enzyme activity ([37]). Consistently with previous researches ([23], [37]) and our second hypothesis, despite AMF promoting microbial activity, acid deposition significantly decreased microbial activity. The activity of invertase throughout the decomposition period and of protease at the early decomposition stage were significantly inhibited by acid deposition. It is generally believed that carbohydrate and protein substances in plant litter were the first to be decomposed ([16]). Acid hydrolysis of sucrose could reduce the substrate of invertase ([5]), which might inhibit the synthesis and secretion of extracellular enzymes by soil microorganisms. Acid deposition with high intensity made the decomposition environment more acidic, thus reducing protease activity ([40]). Besides, the activities of phosphatase and urease involved in organic P and N mineralization are not significantly affected by acid deposition at the early-stage decomposition, which is inconsistent with previous researches ([19]). This might be due to the fact that the experimental plot is located in the acid rain area of western Hunan ([37]), where soil microorganisms are more resistant to acid stress, and the complex habitat of forest soil may also buffer to some degree the impact of acid deposition.
AMF can improve the activities of most enzymes except for polyphenol oxidase, but this effect strongly depends on the abiotic environment ([31]). Under acid deposition, especially under high-intensity, the positive AMF effects on invertase and protease activities throughout the decomposition period, and on urease at the late decomposition stage, were dramatically weakened. This indicated that AMF effects on these enzyme activities are highly sensitive to acid deposition. The positive AMF effect on invertase activity decreased gradually with decomposition time, suggesting the priming effect of AMF mainly exert on easily decomposed labile C. On the other hand, suppression of AMF enhanced the inhibition effects of acid deposition on the activities of invertase, urease and catalase, indicating that AMF could relieve the negative effect of acid deposition. AMF can relieve microbial activity by providing low molecular weight sugars, and improve the resistance of microbial communities to adverse environments ([13]). At the same time, the recruitment of functional microorganisms such as alkaline-phosphatase producing bacteria and N-fixing bacteria by AMF mycelia, or the release and transfer of nutrients in litter, can also mitigate the negative impact of acid deposition on microbial community ([48]).
Enzyme secreting pattern, in which different enzymes are secreted under different conditions, reflects the resource allocation of the decomposer community, the relative nutrient availability ([11]), and the energetic trade-offs associated with enzyme production ([35]). We observed that N-cycling enzymatic activities (protease and urease) were obviously higher in later than in early decomposition stage. This suggested the high N demand of soil microorganisms at later decomposition, as the decomposition of recalcitrant litter substances requires multiple enzymes to work together, which requires adequate N ([50]). High alkali-hydrolyzable N content in the detritusphere soil verified the high activities of N-release enzymes. Meanwhile, this also implies the high loss of N to the soil, which would adverse to AMF for absorbing litter N. AMF is reported to acquire substantial N from decomposing litter, and exported up to one-third litter N to host plant ([17]). In order to absorb litter N, AMF preferentially colonized plant litter instead of an additional host plant ([12]). On the other hand, the later decomposition stage started from November of that year to March of the next year, which was the time of the second peak litterfall of C. camphora. Fresh C. camphora leaf litter falling on to the forest floor might stimulate the N-cycling enzymatic activity.
Effects of acid deposition and AMF on detritusphere soil nutrient dynamics
Nutrient release from litter decomposition is a key process for soil nutrient cycling, and is expected to be controlled by soil nutrient availability and litter quality as well as environmental conditions ([34]). During the one-year decomposition period, we observed a strong fluctuation of available P, alkali-hydrolyzable N and water contents, and a weak fluctuation of SOM content in detritusphere soil with sampling month. After 8-month decomposition (in November), contents of available P and alkali-hydrolyzable N were recovered to the highest. This might be due to the fact that autumn was the second peak litterfall season of C. camphora, which resulted in large amount input of fresh leaf litter. Aboveground litter input is reported to significantly increase soil nutrients ([7]). Besides, priming effect by this fresh litter input might stimulated the nutrient release of sublayer decomposing litter ([21]). Despite the seasonal effect on litter nutrient release was overwhelming, we still observed the effects of acid deposition and AMF suppression on C. camphora litter nutrient release, thus the seasonal effects could not affect our conclusions.
Nutrient release of C. camphora litter revealed a surprisingly complex pattern; different nutrients showed different release patterns under different conditions, which was inconsistent with our third hypothesis. Under no or low acid deposition, AMF suppression significantly increased SOM content and decreased alkali-hydrolyzable N content. This indicated that the presence of AMF not only stimulate the decomposition of C. camphora leaf litter but also of detritusphere SOM. N-cycling enzymatic activity was not stimulated under AMF suppression, thus the decreased litter N release might result in the decreased alkali-hydrolyzable N content ([15]). However, under high acid level, AMF suppression on the contrary decreased SOM content during the whole decomposition period and alkali-hydrolyzable N content at the early decomposition stage. High acid deposition might shorten the AMF lifespan and accelerate their life cycle, as we observed AMF hyphal colonization was seriously inhibited. Subsequently, the death AMF extraradical mycelium become part of the SOM and alkali-hydrolyzable N ([8]). However, whether high acid level accelerate the death of AMF needs further investigations. At late decomposition stages, that AMF suppression recovered alkali-hydrolyzable N content to the level of control under high acid deposition might be due to the fast decomposition during the second peak litterfall of C. camphora. On the other hand, AMF suppression also showed to significantly decrease available P content, consistently to previous researches ([25]). This may be due to the decreased activity of P-decomposing microorganisms after inhibiting AMF, thereby reducing the mineralization efficiency of organic P ([48]).
Acid rain can promote soil organic carbon accumulation by decreasing microbial activity and litter decomposition rate ([23]). However, in our study, acid deposition regardless of level significantly reduced SOM content during the first 8-month decomposition period. The effect of acid rain on SOM storage is reported to depend on the amount of acid deposition and forest type. In acidic red soil of south China, leaching by acid deposition generally results in the decrease of SOM content ([18]). The opposite ordination between water and SOM content in PCA in our study also suggested this strong effect of leaching on SOM content (Fig. 5b). Besides, the decomposition of SOM to smaller molecular weight compounds, such as fulvic acid, also could lead to the decrease of SOM content ([18]). Thus, the decreased decomposition of litter into SOM and increase of leaching and transformation of SOM under acid deposition resulted in the decrease of SOM in our study.
Acid rain could lead to soil acidification and decreases the availability of NO3--N, mineral N, P ([23]). Consistently with these researches, acid deposition with high intensity significantly decreased soil available P content, and alkali-hydrolyzable N content except for at 2nd and 12th month (in March). March is the litterfall month of C. camphora, fast decomposition of fresh leaf litter might prime the litter N release, resulting in the increase of alkali-hydrolyzable N content. We found that AMF effects on available P and alkali-hydrolyzable N contents were highly sensitive to acid deposition, implying that prolonged acid deposition would dramatically affect litter nutrient release through soil microorganisms.
Conclusions
Acid deposition and AMF suppression negatively affected the decomposition of C. camphora leaf litter at late decomposition stage. The remaining mass of litter and microbial activity were highly sensitive to water content, which was the main factor restricting litter decomposition at early decomposition stage. Besides, we found that nitrogen-cycling enzymatic activity of detritusphere soil was significantly higher in later than in early decomposition stages, and acid deposition and AMF suppression significantly decreased microbial activity. However, nutrient release of C. camphora litter revealed a complex pattern. AMF effects on activities of invertase and N-release enzymes, and on soil alkali-hydrolyzable N and available P contents were highly sensitive to acid deposition. Thus, global climatic changes (e.g., CO2 enrichment and nitrogen deposition) leading to soil acidification would dramatically affect the AMF-mediated ecological processes. Given the complexity of the microbial network relationship in AMF functions under acid deposition in subtropical forest ecosystems, further research is needed to understand the relationship between AMF and other soil microorganisms under acid deposition and the impact of these relationships on ecological process.
Author contribution
Xiangshi Kong and Xingbing He formulated the overarching research goals and aims, and designed the study. Can Wu conduct the field work, analyzed the results, and wrote the first version of the manuscript. Yonghui Lin and Zaihua He participated in data analysis. Xiangshi Kong, Xingbing He, Yuehong Gao and Qin Kong reviewed and edited the paper. All authors have read and approved the final version of the paper.
Funding
This study was supported by the National Natural Science Foundation of China (32160356, 32060332, 31670624, 31560205), the Key Program of Scientific Research projects of Hunan Provincial Education Department (21A0334 and 20A400), Postgraduate Scientific Research Innovation Project of Hunan Province (CX20221122), the Natural Science Foundation of Hunan Province (2021JJ30555 and 2020JJ5455), and the Open Foundation of Hunan Key Laboratory of Ecotourism (STLV2108).
Data availability
The authors declare that the data supporting the findings of this study are available within the paper or its Supplementary information files or from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Xiangshi Kong 0000-0003-3541-5663
Xingbing He 0000-0001-6726-1799
Yonghui Lin
Zaihua He 0000-0002-4975-8141
Yuehong Gao
Qin Kong
College of Biology and Environmental Sciences, Jishou University, Jishou, 416000 (China)
Key Laboratory for Ecotourism of Hunan Province, School of Tourism, Jishou University, Jishou, 416000 (China)
Corresponding author
Paper Info
Citation
Wu C, Kong X, He X, Lin Y, He Z, Gao Y, Kong Q (2023). Effects of arbuscular mycorrhizal fungi on microbial activity and nutrient release are sensitive to acid deposition during litter decomposition in a subtropical Cinnamomum camphora forest. iForest 16: 314-324. - doi: 10.3832/ifor4324-016
Academic Editor
Claudia Cocozza
Paper history
Received: Feb 08, 2023
Accepted: Sep 12, 2023
First online: Nov 13, 2023
Publication Date: Dec 31, 2023
Publication Time: 2.07 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2023
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 18369
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 14271
Abstract Page Views: 2224
PDF Downloads: 1487
Citation/Reference Downloads: 8
XML Downloads: 379
Web Metrics
Days since publication: 819
Overall contacts: 18369
Avg. contacts per week: 157.00
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effect of plant species on P cycle-related microorganisms associated with litter decomposition and P soil availability: implications for agroforestry management
vol. 9, pp. 294-302 (online: 05 October 2015)
Research Articles
Arbuscular mycorrhizal fungal symbiosis with Sorbus torminalis does not vary with soil nutrients and enzyme activities across different sites
vol. 8, pp. 308-313 (online: 03 September 2014)
Research Articles
Shifts in the arbuscular mycorrhizal fungal community composition of Betula alnoides along young, middle-aged plantation and adjacent natural forest
vol. 13, pp. 447-455 (online: 07 October 2020)
Review Papers
Arbuscular mycorrhizal fungi as a tool to ameliorate the phytoremediation potential of poplar: biochemical and molecular aspects
vol. 7, pp. 333-341 (online: 17 April 2014)
Research Articles
Soil fauna communities and microbial activities response to litter and soil properties under degraded and restored forests of Hyrcania
vol. 14, pp. 490-498 (online: 11 November 2021)
Review Papers
Soil fungal communities across land use types
vol. 13, pp. 548-558 (online: 23 November 2020)
Research Articles
The effect of soil conditions on submountain site suitability for Norway spruce (Picea abies Karst.) in Central Europe
vol. 16, pp. 210-217 (online: 31 July 2023)
Research Articles
Soil stoichiometry modulates effects of shrub encroachment on soil carbon concentration and stock in a subalpine grassland
vol. 13, pp. 65-72 (online: 07 February 2020)
Research Articles
The manipulation of aboveground litter input affects soil CO2 efflux in a subtropical liquidambar forest in China
vol. 12, pp. 181-186 (online: 10 April 2019)
Research Articles
Potential spread of forest soil-borne fungi through earthworm consumption and casting
vol. 8, pp. 295-301 (online: 26 August 2014)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword