Culturable fungi associated with wood decay of Picea abies in subalpine forest soils: a field-mesocosm case study
iForest - Biogeosciences and Forestry, Volume 11, Issue 6, Pages 781-785 (2018)
doi: https://doi.org/10.3832/ifor2846-011
Published: Nov 28, 2018 - Copyright © 2018 SISEF
Short Communications
Abstract
Fungi are the principal wood decomposers in forest ecosystems and their activity provides wood necromass to other living organisms. However, the wood decay mechanisms and the associated microbial community are largely unknown, especially in Alpine areas. In this study, the culturable fraction of fungal communities associated with the decomposition of Norway spruce (Picea abies [L.] Karst) deadwood in subalpine forest soils were determined using microbiological methods coupled with molecular identification. Fungal communities were evaluated using in-field mesocosms after one year of exposition of P. abies wood blocks along an altitudinal gradient ranging from 1200 up to 2000 m a.s.l. comprising eight subalpine sites, four of them located at north- and other four at south-facing slopes. Although many saprotrophic species were isolated from the wood blocks, several white-rot species as the pathogenic fungi Armillaria cepistipes and Heterobasidion annosum, along with soft-rot fungi such as Lecytophora sp. were identified. Our results further indicated that the wood-inhabiting fungal community was mainly influenced by topographic features and by the chemical properties of the wood blocks, providing first insights into the effect of different slope exposure on the deadwood mycobiome in the subalpine forest ecosystem.
Keywords
Wood-inhabiting Fungi, Basidiomycota, Subalpine Forest, Wood Decomposition, Norway Spruce, Slope Exposure
Introduction
In forest ecosystems, a wide variety of wood-decaying fungi are saproxylic species that depend on deadwood during some stages of their life cycle ([32]). The most important wood-decaying species belong to Basidiomycota and have generally been classified into two main groups, white-rot and brown-rot fungi, based on the cell wall component degraded ([30], [24]). Another important form of wood decay known as soft-rot includes several species belonging to mainly Ascomycota, which typically attack wood with a higher moisture content and preservative-treated wood ([29]). The rate of wood decomposition is influenced by the chemical properties and the cell structures of the wood, the nature and the abundance of the wood-decomposing organisms, as well as by different ecological and climatic factors ([28], [12]). In fact, the influence of slope exposure on wood decay dynamics has recently been demonstrated in subalpine environments ([7], [22], [23], [10]) and in subtropical and tropical forests ([9], [26]). However, it is so far unknown how exposure and, in general, climate affect the wood-inhabiting microbiota. In this context, we performed a study on the culturable wood-inhabiting fungi (WIF) in-field mesocosm experiment with the purpose of evaluating the fungal community colonizing Norway spruce (Picea abies [L.] Karst) deadwood after one year of exposition to natural conditions in subalpine forests.
Material and methods
The investigation area is located in Val di Rabbi in the south Alpine belt in northern Italy between a rather warm Insubrien and a cold Alpine climate. Eight sites along an altitudinal gradient ranging from 1200 up to 2000 m a.s.l. were investigated (Tab. S1 and Fig. S1 in the Supplementary material); four sites were positioned at north-facing slopes (N1-4) and other four at south-facing (S6-9) slopes ([6], [2]). At each study site a field experiment using soil mesocosms was set up as described in Fravolini et al. ([7]). Mesocosms (PVC tubes having diameter = 10.2 cm and height = 25 cm) were installed into the natural soil in August 2012, that is one year prior to the addition of the wood blocks of P. abies, at a distance of > 1 m from large trees and > 0.5 m from the adjacent mesocosms, leaving at the surface a border of about 1 cm. Wood blocks from the same P. abies tree and with a uniform size (2 × 5 × 5 cm) were placed on the soil surface in each of the mesocosm tubes. Three replicate mesocosms were installed in each of the eight study sites (Fig. S2 in the Supplementary material). P.abies wood samples and soil samples (0-5 cm depth) were collected from each mesocosm in June 2014, placed in polyethylene bags and transported on ice to the laboratory for their physico-chemical characterization (Tab. S2 in the Supplementary material). Wood and soil physico-chemical properties were assessed as described by Fravolini et al. ([7]) and Bardelli et al. ([2]), respectively.
For the determination of the cultivable fungal decomposers all the wood blocks were surface sterilized by flaming and five small samples were cut out and placed on Petri dishes containing Hagem agar (HA) medium ([34]) with addition of chloramphenicol (0.035 g L-1, Sigma, MO, USA) and streptomycin (0.018 g L-1, Sigma) in three replicates. All the inoculated plates were incubated at room temperature and continuously observed for 2 weeks. Soil samples were analysed by the Dilution Plate Technique: 10 g of soil were diluted with sterile water 1:10 (w/v) and mechanically shaken for 20 min. The suspensions were further diluted 1:10 and 1 mL aliquots of the suspension 10-4 were homogeneously distributed and incubated at room temperature and continuously observed for 2 weeks. Fungal colonies were afterwards sub-cultured and the species in pure culture were identified based on classical morphological features using light microscopy. The fungal morphological identification was further confirmed by sequencing of the ITS2 region ([20]) using the ITS3 (5′-GCATCGATGAAGAACGCAGC-3′) - ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) primer pair ([35]) as described by Maresi et al. ([19]). The ITS sequences were compared to those from the NCBI database (⇒ http://www.ncbi.nlm.nih.gov/) to ascertain closest sequence matches. To visualize the WIF community compositions, we used three dimensional non-metric multidimensional scaling (3D-NMDS) analysis based on the Bray-Curtis dissimilarity index calculated in R ([27]). Abiotic factors (including site, altitude and exposure) and chemical properties of the soil and deadwood were fitted to the NMDS ordination plots using the “envfit” function in the “vegan” package of R, and goodness-of-fit statistics (R2) were calculated with P values based on 999 permutations ([21]).
Results and discussion
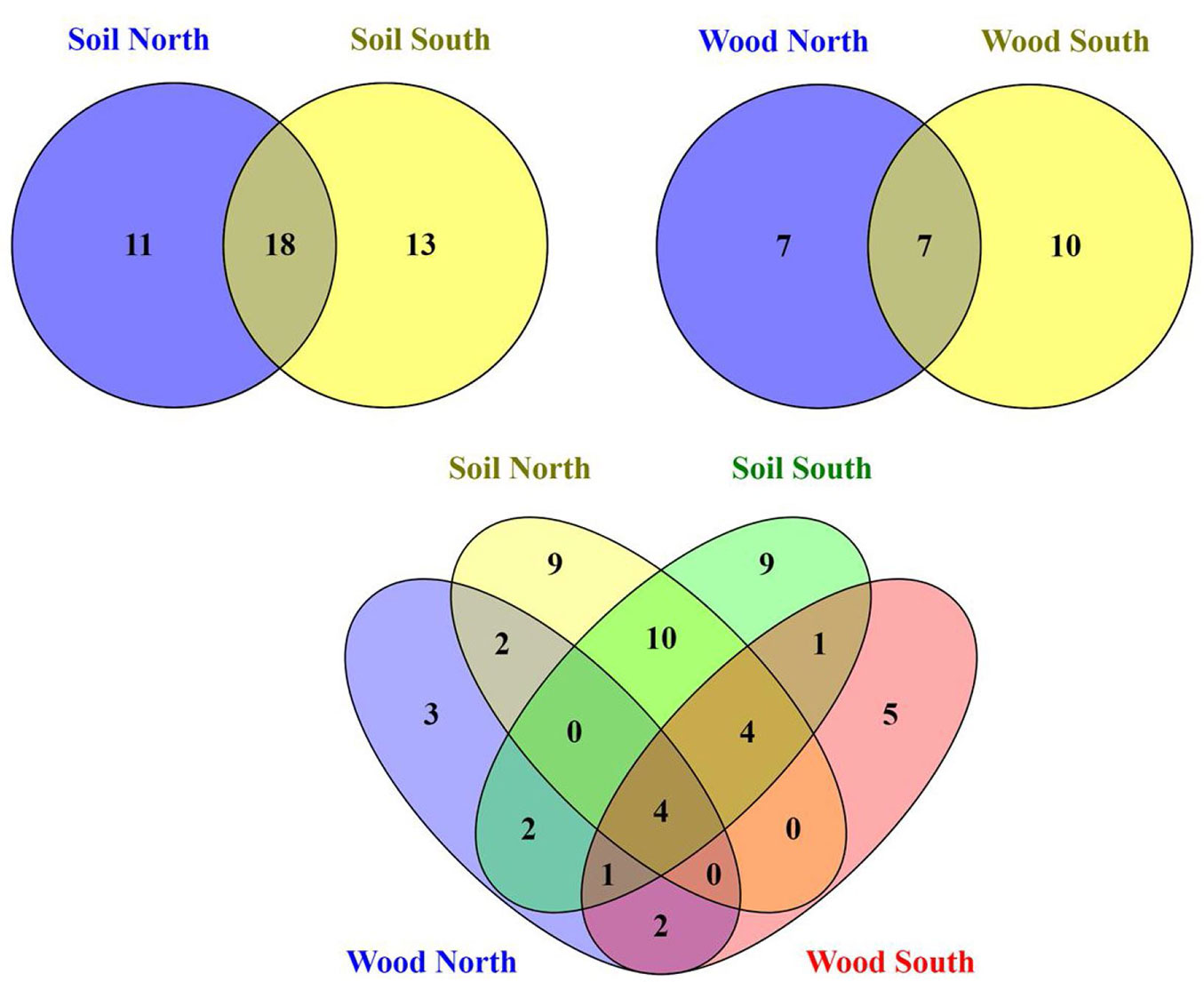
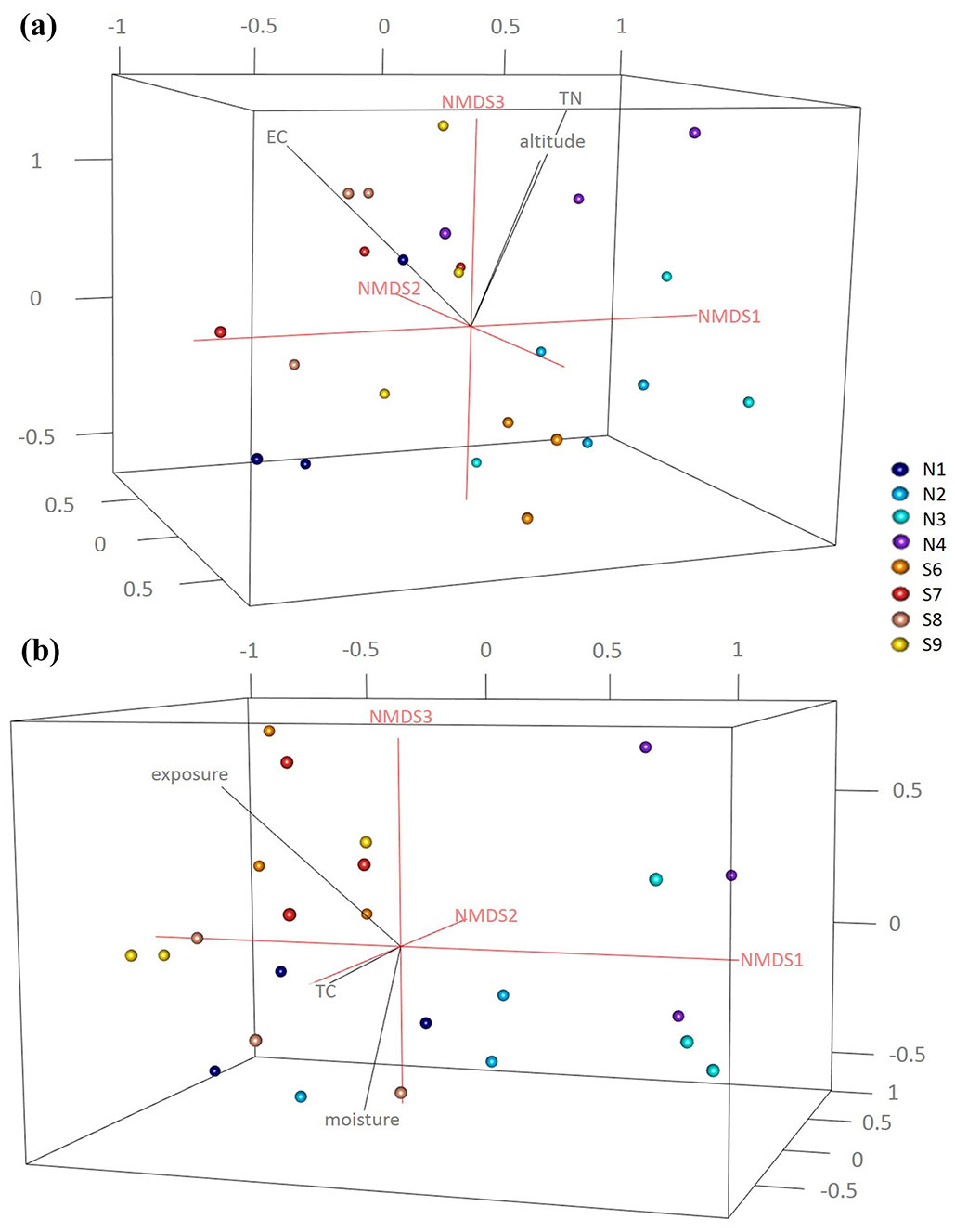
In total 215 fungal isolates were cultured and 52 fungal taxa were morphologically identified and their identification was complemented by molecular analysis based on ITS sequences. The isolates on BLAST analyses showed 97-100% identity to the available sequences in NCBI (Tab. S3 and Fig. S3 in Supplementary material). Overall, Ascomycota was the dominant phylum (32/52 taxa), followed by Zygomycota (11/52), Basidiomycota (8/52) and sterile mycelia. In particular, 42 taxa were isolated from the soil samples in direct contact with the wood blocks; 18 taxa were common to both slopes, while 11 taxa were exclusively isolated from north- and 13 taxa from south- facing sites (Fig. 1). The soil cultivable fungal population was mainly characterized by Ascomycota (26 taxa) and Zygomycota (10 taxa) with a lower proportion of Basidiomycota (5 taxa). Differences in community composition among the different sites were also observed (Fig. 1, Fig. 2a, Fig. 2b), and total nitrogen (TN), electrical conductivity (EC) and altitude correlated significantly with the soil fungal community composition (Tab. 1). From the wood blocks, a total of 24 fungal species were identified, of which 13 belong to Ascomycota, 6 to Zygomycota and 4 to Basidiomycota, while for one isolate no match in the NCBI database was found (Tab. S3 in the Supplementary material). These results are in line with the study by Rajala et al. ([28]) and Kazartsev et al. ([14]), who reported that Ascomycota was the most dominant fungal phylum during the early stages of P. abies wood decay in boreal forests. Although Ascomycota are generally weak lignin decomposers, as the enzyme systems of most ascomycetes do not contain the typical lignin-modifying enzymes (with the exception of laccases), they may regulate the wood decomposition rate by interacting and competing with Basidiomycota in the early stage of decomposition ([18], [13]). Among the WIF isolated, 7 were common to both slopes, 7 were isolated only at the north-facing site and other 10 exclusively at the south-facing site (Fig. 1). These differences in community composition among the north- and south-facing sites (R = 0.31, p <0.05) were also reflected in the NMDS ordination plot (Fig. 2a, Fig. 2b) as the WIF clustered mainly as a function of slope exposure. Indeed, the WIF community composition correlated significantly with exposure, wood moisture and total carbon (TC), while lignin and cellulose contents did not contribute significantly in shaping the structure of WIF community (Tab. 1). Permutational Multivariate Analysis of Variance (PERMANOVA) confirmed that the soil and wood communities were significantly affected by altitude and exposure (Tab. 2). In line with our results, slope, elevation and wood physicochemical properties such as water content have been reported as determinant drivers for WIF richness and community composition in forest ecosystems ([28], [25], [26], [13], [10]). Parallel studies conducted on the same P. abies wood blocks (in-field mesocosm experiment) investigated in this work reported that the deadwood mass decay dynamics was related to wood pH and moisture, soil texture, temperature and topographic features including exposure and altitude ([7], [10]), corroborating the considerable effect of abiotic and climatic factors on WIF community and consequently on P. abies deadwood degradation. Furthermore, about 60% of the WIF isolated from the wood blocks were also found in the top 5 cm soil layer, suggesting that soil biotic attributes are also important drivers of initial deadwood decay in sub-alpine Norway spruce forests. However, 10 taxa were isolated only from the wood samples, and among them, we identified several decay fungi commonly associated with wood decomposition such as Armillaria cepistipes (at sites S7 and S8), Bjerkandera adusta (at site S6) and Athelia decipiens and Heterobasidion annosum (at site N3). These fungi were also identified in a recent study on the WIF population associated with P. abies deadwood using culture-independent molecular techniques on forest plots of the German Biodiversity Exploratories in south-western Germany ([13]). Furthermore, these species represent necrotrophic and saprotrophic fungi including some of the most detrimental pathogens in conifer forests, which are capable of degrading wood, infecting the roots and stems and causing root white-rot ([15], [11], [24]). Lecytophora sp. and Humicola sp. were isolated from the wood blocks at site N1 and from soil at site S6, respectively, and species affiliated with these genera have already been associated with soft-rot decay of treated wood ([5]). Interestingly, we found that three WIF taxa affiliated with the Trichoderma genus (T. viridescens, T. atroviride, T. citrinoviride) were also isolated in a recent study on wood decay fungi across European forest ecosystems ([3]). Although they are frequently isolated in decaying wood and plant material ([17]), Trichoderma spp. are weak decomposers of non-decayed wood, but the delignification performed by white-rot fungi improves the accessibility of the woody material ([8]). The basidiomycete Coprinellus radians was isolated from the wood blocks at sites S7 and S8 and from the soil samples at N2 and S8 and it has been hypothesized that Coprinoid species might have abilities of white-rot fungi on pre-decayed wood ([1]). Moreover, several saprotrophic ascomycetes species found in this study like Aspergillus sp., Penicillium sp., Cladosporium sp. and Epicoccum sp., along with members of the phylum Zygomycetes may influence the decay process in large woody debris, but in general they play a subordinate role as direct agents of wood decay ([33]) because they primarily utilize compounds derived from the action of wood degraders ([16]).
Fig. 1 - Venn diagrams showing specific and common fungal taxa between the north- and south-facing sites isolated from the wood- and soil samples in direct contact with the wood blocks.
Fig. 2 - 3D-Nonmetric multidimensional scaling (NMDS) ordination of a) soil (stress = 0.11) and b) wood (stress = 0.14) fungal community structure at the north- and south-facing sites using the plot3d and ordigl functions in R. The NMDS ordination plots were fitted with the significant soil physico-chemical and topographic factors using the envfit command in “vegan”.
Tab. 1 - Goodness-of-fit statistics (R2) for factors fitted to the three dimensional non-metric multidimensional scaling (3D-NMDS) ordination of the soil and wood fungal community composition. The significance was based on 999 permutations. (nd): not detected; (EC): electrical conductivity; (TC): total carbon; (TN): total nitrogen; (Ptot): total phosphorous; (Pav): available phosphorous; (*): P < 0.05; (**): P < 0.01; (***): P < 0.001.
| Variable | Soil | Wood | ||
|---|---|---|---|---|
| R2 | P | R2 | P | |
| Exposure | 0.209 | 0.168 | 0.358 | 0.025* |
| Altitude | 0.305 | 0.045* | 0.184 | 0.378 |
| Moisture | 0.168 | 0.284 | 0.303 | 0.048* |
| Volatile solids | 0.199 | 0.218 | 0.095 | 0.585 |
| pH | 0.136 | 0.389 | 0.018 | 0.943 |
| EC | 0.410 | 0.013* | 0.0210 | 0.924 |
| TC | 0.205 | 0.206 | 0.461 | 0.006** |
| TN | 0.427 | 0.009** | nd | nd |
| NH4+ | 0.248 | 0.116 | - | - |
| NO3- | 0.279 | 0.077 | - | - |
| Ptot | 0.089 | 0.617 | - | - |
| Pav | 0.086 | 0.629 | - | - |
| Cellulose | - | - | 0.182 | 0.227 |
| Lignin | - | - | 0.095 | 0.588 |
Tab. 2 - PERMANOVA table showing differences in community composition (based on a Bray-Curtis dissimilarity matrix) among altitude and exposure, for the soil and wood fungal communities. (*): P < 0.05; (**): P < 0.01; (***): P < 0.001.
| Variable | Soil fungi | Wood fungi | ||
|---|---|---|---|---|
| R2 | P | R2 | P | |
| altitude | 0.18 | 0.001*** | 0.07 | 0.05* |
| exposure | 0.06 | 0.05* | 0.2 | 0.001*** |
| altitude : exposure | 0.11 | 0.01** | 0.12 | 0.01* |
In summary, the study of cultured fungi associated with the early stage of P. abies deadwood decay in subalpine forest soils resulted in a large number of species mainly belonging to the phyla Ascomycota and Zygomycota, which are generally saprotrophs with low impact as direct wood decay agents. Most of the isolates could be cold-adapted as they belong to species that are reported in periglacial soil at about 2500 m a.s.l. ([31]). In fact, methods based on culturing are known to favor rapidly-growing fungi (like saprotrophs), and on the contrary, obliged plant pathogens or mutualistic biotrophic fungi such as ecto-mycorrhizal species are notoriously difficult or impossible to isolate ([4]). However, several white-rot species affiliated with Basidiomycota - that have been previously associated with the decay process of deadwood in forest ecosystems - were isolated and identified with the cultivation method used in the present study. Some of these species were only isolated from wood blocks at particular sites, indicating that, besides deadwood chemical properties, altitude and exposure are important drivers for WIF community composition in subalpine forest ecosystems. A further step in the identification of the fungal taxa associated with deadwood decay processes would consist in a taxonomical characterization of the fungal community of the studied wood blocks using high-throughput next generation sequencing technologies.
Acknowledgements
M. Gómez-Brandón and J. Ascher-Jenull have been funded by the Fonds zur Förderung der wissenschaftlichen Forschung (FWF), Austria (Project I989-B16) and partially by the Ente Cassa Risparmio di Firenze (Florence, Italy). M. Gómez-Brandón also acknowledges support by the Programa Ramón y Cajal (RYC-2016-21231; Ministerio de Economía y Competitividad, Spain). T. Bardelli has been funded by a PhD grant from the University of Florence (Italy) and by the Doctoral Scholarship Programme for Junior Researchers (2017/3/BIO-1) of the University of Innsbruck (Austria).
References
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Department of Sustainable Agroecosystems and Bioresources, Research and Innovation Centre, Fondazione Edmund Mach, v. E. 1, 38010, San Michele all’Adige (Italy)
Plant Ecology and Nature Conservation Group, Wageningen University, P.O. Box 47, 6700AA Wageningen (The Netherlands)
Institute of Microbiology, University of Innsbruck, Technikerstraβe 25d, 6020 Innsbruck (Austria)
Departamento de Ecología y Biología Animal, Universidad de Vigo, Vigo 36310 (Spain)
Tommaso Bardelli
Heribert Insam
Institute of Microbiology, University of Innsbruck, Technikerstraβe 25d, 6020 Innsbruck (Austria)
Tommaso Bardelli
Giacomo Pietramellara
Department of Agrifood and Environmental Science, University of Florence, p.le delle Cascine 18, 50144 Florence (Italy)
Department of Geography, University of Zürich, Winterthurerstrasse 190, 8057 Zürich (Switzerland)
Museo delle Scienze (MUSE), c.so del Lavoro e della Scienza 3, 38122 Trento (Italy)
Corresponding author
Paper Info
Citation
Oliveira Longa Claudia M, Francioli D, Gómez-Brandón M, Ascher-Jenull J, Bardelli T, Pietramellara G, Egli M, Sartori G, Insam H (2018). Culturable fungi associated with wood decay of Picea abies in subalpine forest soils: a field-mesocosm case study. iForest 11: 781-785. - doi: 10.3832/ifor2846-011
Academic Editor
Alberto Santini
Paper history
Received: May 09, 2018
Accepted: Oct 01, 2018
First online: Nov 28, 2018
Publication Date: Dec 31, 2018
Publication Time: 1.93 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 46565
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 38809
Abstract Page Views: 3922
PDF Downloads: 2879
Citation/Reference Downloads: 9
XML Downloads: 946
Web Metrics
Days since publication: 2625
Overall contacts: 46565
Avg. contacts per week: 124.17
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 5
Average cites per year: 0.63
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Decomposition of Norway spruce and European larch coarse woody debris (CWD) in relation to different elevation and exposure in an Alpine setting
vol. 9, pp. 154-164 (online: 28 August 2015)
Research Articles
Wood-soil interactions in soil bioengineering slope stabilization works
vol. 2, pp. 187-191 (online: 15 October 2009)
Research Articles
Effect of plant species on P cycle-related microorganisms associated with litter decomposition and P soil availability: implications for agroforestry management
vol. 9, pp. 294-302 (online: 05 October 2015)
Research Articles
Effects of arbuscular mycorrhizal fungi on microbial activity and nutrient release are sensitive to acid deposition during litter decomposition in a subtropical Cinnamomum camphora forest
vol. 16, pp. 314-324 (online: 13 November 2023)
Research Articles
Analysis of dust exposure during chainsaw forest operations
vol. 10, pp. 341-347 (online: 23 February 2017)
Research Articles
Mechanical and physical properties of Cunninghamia lanceolata wood decayed by brown rot
vol. 12, pp. 317-322 (online: 06 June 2019)
Research Articles
Contrasted growth response of hybrid larch (Larix × marschlinsii), jack pine (Pinus banksiana) and white spruce (Picea glauca) to wood ash application in northwestern Quebec, Canada
vol. 14, pp. 155-165 (online: 06 April 2021)
Research Articles
The effect of soil conditions on submountain site suitability for Norway spruce (Picea abies Karst.) in Central Europe
vol. 16, pp. 210-217 (online: 31 July 2023)
Research Articles
Soil CO2 efflux in uneven-aged and even-aged Norway spruce stands in southern Finland
vol. 11, pp. 705-712 (online: 06 November 2018)
Research Articles
Potential relationships of selected abiotic variables, chemical elements and stand characteristics with soil organic carbon in spruce and beech stands
vol. 14, pp. 320-328 (online: 09 July 2021)
iForest Database Search
Google Scholar Search
Citing Articles
Search By Author
- M Oliveira Longa Claudia
- D Francioli
- M Gómez-Brandón
- J Ascher-Jenull
- T Bardelli
- G Pietramellara
- M Egli
- G Sartori
- H Insam
Search By Keywords