Effect of environmental gradients on leaf morphological traits in the Fandoghlo forest region (NW Iran)
iForest - Biogeosciences and Forestry, Volume 13, Issue 6, Pages 523-530 (2020)
doi: https://doi.org/10.3832/ifor3391-013
Published: Nov 13, 2020 - Copyright © 2020 SISEF
Research Articles
Abstract
The purpose of this study was to analyze the effects of altitude, the position of the trees along a gradient of canopy cover, and the orientation of their crown on leaf traits of 18 deciduous woody species belonging to 10 families in the Fandoglo forest region in Ardabil, North West of Iran. We measured eight leaf traits (leaf width, length, area, thickness, water content, leaf mass per area, specific leaf area, and dry matter concentration) of trees sampled at sites subjected to different light regimes (forest edge, forest understory, and isolated trees). All traits were measured on more than 3600 leaves from 90 trees sampled in two altitudinal ranges (low: 1300-1500 m a.s.l.; high: 1500-1700 m a.s.l.). A two-way ANOVA and t-test for independent samples were applied to test for differences in leaf traits between different altitudes and degree of canopy cover. The results confirmed that species’ leaf traits were more strongly correlated with the altitude and canopy cover rather than the orientation of the crown. No relationship between leaf traits and crown orientation was detected. All leaf traits had significantly higher values at low than at high elevation, indicating that environmental factors such as atmospheric CO2 concentration, temperature, light, irradiance, and wind deeply impact on foliar morphology and function; however, water content and specific leaf area showed an opposite trend. Also, species with different positions along the gradient of canopy cover could have different responses to elevation. Our results indicate that the variation of functional (morphological and physiological) traits in different tree species are affected by altitude and light regime. This might provide a theoretical basis for afforestation and forest management activities in the Fandoghlo forest region.
Keywords
Leaf Morphological Traits, Fandoglo Forest Region, Altitude, Tree Position, Crown Orientation
Introduction
Forest trees adapt to environmental factors like climate, soils, and topography by evolving specific subpopulations adapted to the constraints of their local environments ([15]). Leaves are the organ most directly affected by the environment and display a series of attributes linked to specific functions and responses to biotic and abiotic stress factors. These attributes may be subdivided into: (i) morphological traits; (ii) chemical traits; (iii) physiological traits; and (iv) symptoms ([15], [8]). Leaf morphology is incredibly variable both among and within species ([17]). Leaf morphological traits of most woody plants change with the local environmental conditions as a response to change in abiotic factors like soil moisture ([22]), air temperature ([16]), solar radiation ([6]), and atmospheric CO2 concentration ([43]). Understanding the adaptive modifications of leaf traits to changes in environmental conditions, e.g., along elevation gradients, is vital ([3]). Altitudinal variation involves changes in many environmental factors, such as air temperature, humidity, heat and sunlight radiations, soil depth, and wind speed ([39], [48]). Temperature decreases, while precipitation and radiation increase with increasing altitude ([27], [46]). The orientation of tree crown according to the cardinal points can also have a complex impact on climatic parameters by modifying temperature and light conditions, which also influence leaf morphology and physiology ([5]). For example, south exposed leaves are subject to higher light radiation and harsher drought conditions than leaves exposed northwards. Furthermore, forest canopy features might be one of the most predominant determinants of tree species distributions, especially in mountain environments ([26]). Forest canopy characteristics (in terms of composition and density) affect the patterns of resource availability (i.e., light, water, soil nutrients) on the forest floor, which in turn influence the foliar morphology ([45]).
Numerous studies focused on the acclimation of leaves, through leaf traits analysis, to environmental changes on species and community level. According to Bussotti et al. ([6]), individual leaf surface decreases under drought conditions, while the thickness of leaves and their specific dry weight increases. The analysis of morphological characteristics of leaves of Dodonaea viscosa subsp. angustissima (DC.) J. G. West in South Australia indicated that leaf width is linked to regional maximum temperature (latitude gradient) and leaf area to local minimum temperature (altitude gradient - [13]). Akinlabi et al. ([3]) revealed that plants generally respond to changes in altitudinal gradient, showing a reduction of dimensions at higher altitudes. Researchers also reported that high light and low altitudes are limiting factors for growth in bamboo ([26]). De la Riva et al. ([9]) observed that species with high leaf mass per area (LMA) and leaf density (LD) could be found in habitats with low water availability. Studies carried out in temperate and subtropical forests showed that dry matter content (DMC) was highest in trees, followed by shrubs and herbs ([28]). Higher DMC increases the resistance of gas diffusion in leaves ([11]).
Several studies in forest stands along environmental gradients have been previously carried out in the Hyrcanian forest of Northern Iran. For example, Yousefzadeh et al. ([50]) studied the variation in leaf characters of Parrotia persica (DC.) C.A. Mey. as a consequence of their position in the canopy along an altitudinal gradient. These authors observed a significant increase in few leaf features (width of lamina, base angle, number of pair vein of leaf, top and end of leaf figure) with increasing altitude. The results of the studies carried out on the effects of altitude on leaf morphology of Caucasian alder (Alnus subcordata C. A. Mey) showed a substantial reduction in lamina length with elevation ([2]). A study of the variability in leaf morphology of three chestnuts (Castanea sativa Mill.) natural populations using principal components analysis (PCA) showed that most of the variation (85%) was explained by the first four components; leaf size emerged as the most important variable in the corresponding eigenvectors ([51]). Paridari et al. ([33]) investigated the altitudinal variation of Carpinus betulus L. using leaf morphological traits, showing that altitude, and related temperature and rainfall, represent an essential driving factor of leaf morphological variation. Mohebbi Bijarpas et al. ([30]) focused on changes in leaf morphological characteristics of oriental beech (Fagus orientalis Lipsky) along altitudinal gradients; leaf length, leaf area index, and specific dry weight decreased significantly with increasing altitude, while the opposite was true for petiole length, petiole index, BW, leaf area, SLA, and relative water content. The results of Mohebbi Bijarpas et al. ([31]) also showed that leaf area, SLA, and relative water content of samples collected from two opposite parts of crowns (southern and northern exposures) in oriental beech increased with increasing elevation and decreased from 1200 to 1700 m a.s.l. The effects of climatic variations on some leaf morphological traits of Crataegus meyeri Pojark. were studied by Hamzehee et al. ([14]); statistical analysis of climatic data and morphological traits showed that wind and temperature were the most influential factors affecting various morphological traits.
According to the above studies, leaf morphological traits in forest trees are affected by altitude (or temperature), water availability, light radiations, soil fertility, and topography. Despite the essential role of altitude in controlling the energy and mass exchange characteristics of terrestrial vegetation such as photosynthesis, respiration, transpiration, carbon and nutrient cycle, and rainfall interceptions, not much is known about the spatial variation in leaf morphology traits of Hyrcanian forests at a landscape scale. The most apparent general trend is a decrease in plant height with increasing altitude ([4]). The position of trees along a gradient of forest canopy cover and orientation of their crowns, which have been considered for their possible effects on leaf traits besides altitude, has not been investigated in detail in the forests until now. In this study, we attempt to establish relationships between leaf attributes and environmental conditions at a landscape level. Our specific goal was to investigate the effects of altitude, the position of trees along a gradient of canopy cover, and orientation of the crown on leaf traits in a broadleaved forest of the Fandoghlo region in Ardabil province (NW Iran), with the aim of better clarifying the role of environmental variables in the foliar morphology and physiology of species. These characteristics can be utilized as a monitoring tool to fulfill the following goals: (i) to predict plant performance under changing environmental conditions; (ii) to detect plant responses promptly after performing the treatment; (iii) to discover the main factors that can cause changes in the plant condition and composition; and (iv) to predict short-term growth response and long-term forest development.
Material and methods
Study area
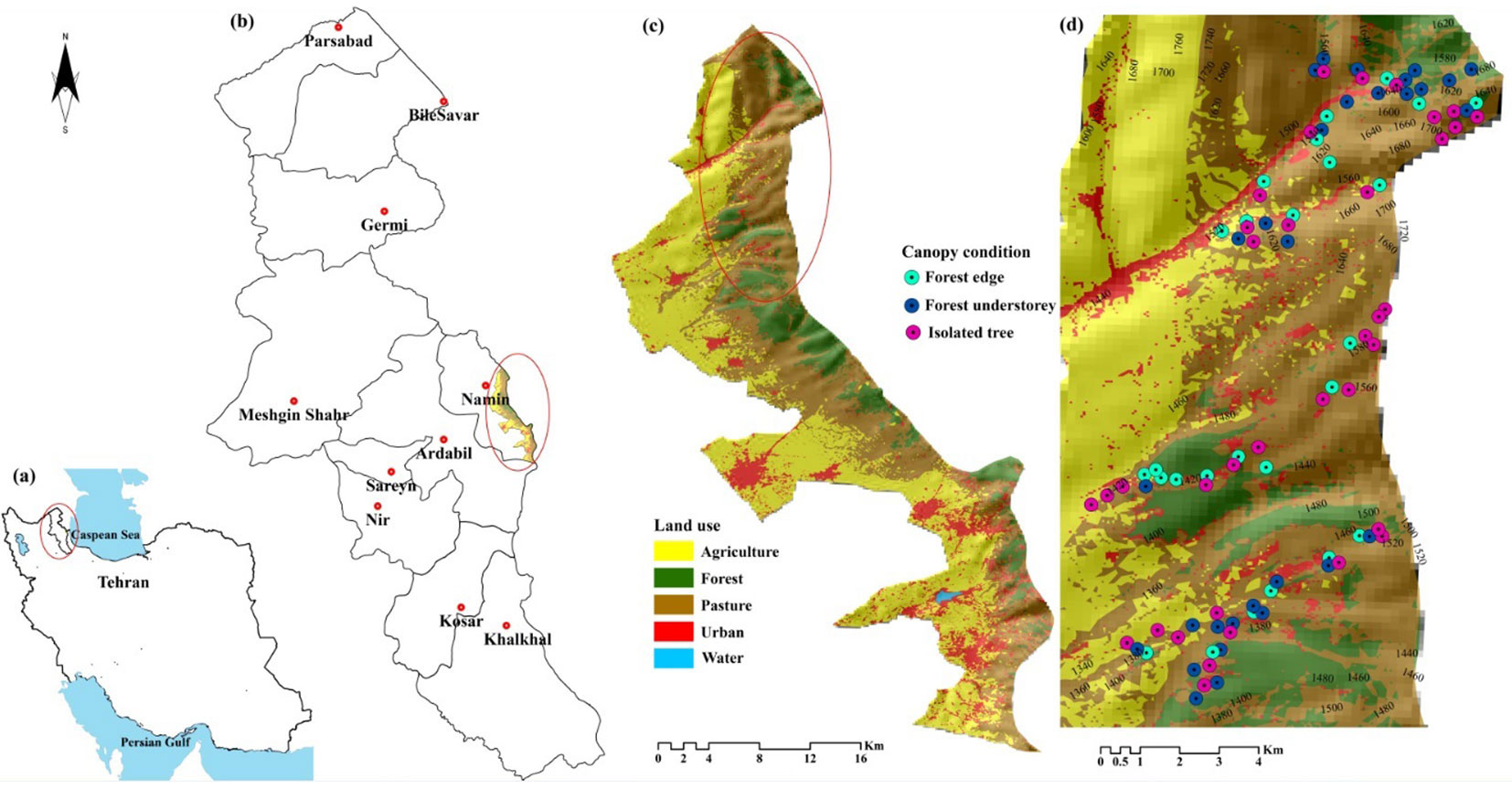
The Fandoghlo forest region is part of Namin municipality, Ardabil, NW Iran, located between latitude 38° 09′ 82″ to 38° 28′ 59″ N and longitude 48° 29′ 27″ to 48° 42′ 36″ E, with elevation ranging from 1320 to 2320 m a.s.l. (Fig. 1, Tab. 1). It borders Azerbaijan on western and northeastern sides and the Guilan province of Iran to the east. The area is influenced by the Caspian Sea on the one hand and by the mountainous climate of Sabalan on the other hand. Most of the rainfall occurs from June to September and snowfall is common in winter (November to March). The average annual rate of precipitation is 430 mm (2008-2018) at the Namin weather station (1480 m a.s.l.), with maximum monthly rainfall in October (50 mm) and no rainfall in August. The mean average temperature is 10 °C. Monthly mean minimum and maximum temperatures are -3.9 °C (January) and 35 °C (August), respectively. Soils are predominantly calcareous brown forest soils, and the organic matter content is moderate. Fandoghlo covers 18.034 ha, of which 41.6% is agricultural lands, 35% pastures, 11.7% forests, and 11.5% urban areas (Fig. 1). Concerning floristic characteristics, the Fandoghlo region can be divided in two parts: forest area and pasture area. Most species in the study area belong to the Euro-Siberian (Hyrcanian) and Irano-Turanean regions ([42]). The deciduous forests is composed by coppice (99% of the area) and high forest (1% - [49]).
Fig. 1 - (a) Location of Ardabi province in NW Iran; (b) the Fandoghlo region in eastern Ardabil province; (c) land use map of the region; (d) the study area in the north part of Fandoghlo region, with indication of the sampled trees and their elevation (in m a.s.l.). Spot colors refer to the different positions of the sampled trees along a gradient of canopy cover (i.e., forest understory, forest edge, and isolated trees).
Tab. 1 - Characteristics of the study area.
| Characteristics | Value |
|---|---|
| Latitude N | 38° 09′ 82″ 38° 28′ 59″ |
| Longitude E | 48° 29′ 27″ 48° 42′ 36″ |
| Area (ha) | 18.034 |
| Elevation (m a.s.l.) | 1320-2320 |
| Mean slope (%) | 43 |
| Soil type | Brown forest |
| Mean annual temp (°C) | 9 |
| Mean annual rainfall (mm) | 430 |
Study design and sampling
Two altitudinal zones were investigated, referring to here as “low” (1300-1500 m a.s.l.) and “high” (1500-1700 m). At both altitudes, leaves were collected from trees in the following three positions along a gradient of canopy cover (Tab. 2): forest edge (FE), forest understory (FU), and isolated trees (IT). The canopy characteristics at each sampling point were recorded and are summarized in Tab. 2. Sampling at the forest edge (FE) included broadleaved trees and shrubs such as Corylus avellana L., Quercus castaneaefolia C.A.Mey., Quercus macranthera Fisch.& C.A.May., Fraxinus excelsior L., Acer campestre L., Mespilus germanica L., and Cornus mas L. Sampling in the forest understory (FU) included the same woody species and others such as Carpinus betulus, Crataegus meyeri, Crataegus microphylla Koch., Malus orientalis Uglitzk, Punica granatum L., Prunus divaricata Ledeb., with canopy cover ranging from 50% to 90% (Tab. 2). Isolated trees (IT) were sampled in open areas, where trees are typically sparse. There were significant differences between the sites where trees were sampled in terms of light intensity and water availability, because of the crown position and crown exposure to light. The age of trees was assessed using increment cores taken at breast height from at least five trees per site. Sampled trees were approximately 80 years old and more than 8 m tall, on average. Trees were randomly chosen within an area of 200 ha at low altitude and 500 ha at high altitude, with an average distance between trees of about 0.2 km (Fig. 1d).
Tab. 2 - General characteristics of the sampling sites of Fandoghlo region at Namin, Ardabil, Iran.
| Sites | Canopy cover (%) | Altitude (m) |
No. Trees |
Slope (°) | Dominant canopy species |
|---|---|---|---|---|---|
| Forest edge (FE) | 65.45 ± 5.20 | 1300-1500 (Low) |
45 | 0-15 | Acer campestre, Carpinus betulus, Corylus avellana, Crataegus meyeri, Crataegus microphylla, Frangula alnus Mill., Malus orientalis, Populus deltoides Bartr. ex Marsh., Prunus divaricata, Quercus macranthera, Quercus castaneaefolia, Salix aegyptiaca L., Viburnum opulus L. |
| Forest understory (FU) | 70.52 ± 12.02 | ||||
| Isolated trees (IT) | - | ||||
| Forest edge (FE) | 45.00 ± 8.50 | 1500-1700 (High) |
45 | 0-23 | Carpinus betulus, Crataegus meyeri, Crataegus microphylla, Cornus mas, Fraxinus excelsior, Malus orientalis, Mespilus germanica, Punica granatum, Prunus divaricata, Quercus castaneaefolia, Quercus macranthera, Rosa canina L. |
| Forest understory (FU) | 55.52 ± 10.15 | ||||
| Isolated trees (IT) | - |
Overall, ten different families of trees were sampled. More than 3600 leaves were randomly collected from four to ten trees for each species, totaling 90 trees. The detailed information on families, species and trees sampled are listed in Tab. 3.
Tab. 3 - List of the 18 tree species sampled in the study area.
| Species Code | Family | Scientific Name | No. Trees |
|---|---|---|---|
| Ac | Sapindaceae | Acer campestre | 4 |
| Vi | Adoxaceae | Viburnum opulus | 4 |
| Co1 | Betulaceae | Corylus avellana | 4 |
| Ca | Carpinus betulus | 4 | |
| Co2 | Cornaceae | Cornus mas | 4 |
| Qu1 | Fagaceae | Quercus castaneaefolia | 6 |
| Qu2 | Quercus macranthera | 4 | |
| Pu | Lythraceae | Punica granatum | 4 |
| Fr1 | Oleaceae | Fraxinus excelsior | 4 |
| Fr2 | Rhamnaceae | Frangula alnus | 4 |
| Cr1 | Rosaceae | Crataegus meyeri | 8 |
| Cr2 | Crataegus microphylla | 10 | |
| Ma | Malus orientalis | 6 | |
| Me | Mespilus germanica | 6 | |
| Pr | Prunus divaricata | 6 | |
| Ro | Rosa canina | 4 | |
| Po | Salicaceae | Populus deltoides | 4 |
| Sa | Salix aegyptiaca | 4 |
Pérez-Harguindeguy et al. ([37]) recommended sampling at the peak of the growing season when leaves are fully expanded. At each site, forty mature and healthy leaves were collected on sunny days during the leaf-expansion season (July 2019). All leaves were taken from the upper outer part of the crown, completely sunny exposed, from four orientations (ten leaves for each north, south, east, and west exposures) at the same height (1.5-2 m above the ground). The detached leaves were carefully pressed between damp paper towels, sealed in marked plastic bags and preserved at 4 °C, and then carried to the laboratory for further analysis.
Morphology measurements
The full list of morphological traits measured on sampled leaves is reported in Tab. S1 (Supplementary material). Leaf size parameters (leaf width: LW; leaf length: LL; leaf area: LA; leaf thickness: LT; fresh weight: FW; saturated weight: SW; dry weight: DW) are critical ecological indicators per se, since many stress factors reduce the productivity and growth of the whole plant and individual organs ([8]). LW (maximum width of the blade), LL (base of petiole to leaf tip), and LT (at the central part of the lamina, half-way between midrib and margin) were all measured using digital vernier calipers with 0.1 mm resolution. The fresh leaves were scanned at 300 dpi resolution, and the leaf area was determined by digital analysis of the images using the software Image-Pro Plus® v. 4.5 (Media Cybernetic Inc., Rockville, MD, USA). After measuring the LA, the leaf lamina was blotted dry with tissue paper to remove any surface water and immediately weighed to determine the saturated weight (SW). Leaf dry weight (DW) was determined after oven-drying at 70 °C for 72 h. Water content (WC) was calculated based on the ratios between FW, SW, and DW, as indicated in Tab. S1 (Supplementary material). Based on leaf size parameters and the measured weights, leaf mass per area (LMA), specific leaf area (SLA), and dry matter concentration (DMC) were calculated. LMA (mg cm-2) is defined as the ratio between leaf mass (expressed as DW) and leaf area. SLA (cm2 mg-1) is the reciprocal of LMA (SLA=1/LMA) and expresses the leaf surface development per mass unit. SLA is positively correlated with moisture and nutrient availability and is mainly determined by leaf density and thickness ([1]). DMC (mg cm-3) is the dry mass per volume of plant organ.
Data analysis
We employed two-way ANOVA and independent-sample t-test for all variables to investigate the effects of altitude (low, high), the position of trees (forest edge, forest understory, and isolated trees), and the orientation in the crown (N, S, E, and W) on leaf morphology. Differences were considered significant at p<0.05 level. It is worth to stress that all the results have been analyzed at the community level over the study area by pooling the different species. A boxplot was also used to compare morphological traits of leaves among sites. XLSTAT® ver. 2019.1 (Addinsoft, Paris, France) was used for all analyses. All values presented are means ± standard error.
Results
Variations of leaf traits at different altitudes
Foliar morphological traits of woody species in the study area at two different altitudes (1300-1500 m and 1500-1700 m a.s.l.) were investigated to establish their response to varying altitudes. Except for LW, LL, and LA, all measured leaf traits showed a significant difference (p <0.05) between the two altitudinal zones considered (Tab. 4). LW ranged from 0.85 to 13.2 cm with an average of 4.7 cm at low altitude, while at high altitude it ranged from 0.93 to 10.81 cm, with an average of 4.39 cm (Fig. 2). The mean LL was 7.81 cm and 7.21 cm at low and high altitudes, respectively. The average LA at low altitude was 27.95 cm2 ranging from 3.55 to 63.25 cm2; at high altitude, average LA was 24.15, ranging from 2.52 to 59.32 cm2. The average LT was lower at high altitude (184.29 µm) than at low altitude (231.08 µm). Regarding water relations, WC was the highest at high altitudes; the average WC was 34.63% and 43.75% at low and high altitudes, respectively. SLA in trees from low altitudes ranged from 0.52 to 13.27 cm2 mg-1, with an average of 3.31 cm2 mg-1, which by far exceeded that of trees from high altitude (average of 5.37 cm2 mg-1). The opposite pattern was found regarding LMA, irrespective of the position of the trees along the gradient of canopy cover (Fig. 2). The average LMA was 6.01 and 4.39 mg cm-2 at low and high altitudes, respectively. DMC at low altitude was ranging from 0.08 to 2.46 mg cm-3, with an average of 0.6 mg cm-3, and was significantly higher than that at high altitude, ranging from 0.06 to 1.82 mg cm-3, with an average of 0.5 mg cm-3.
Tab. 4 - ANOVA summary of the effect of altitude, position of the trees, crown orientation, and their interactions on leaf morphological traits of woody species in the study area. F-values and significance levels are displayed. (df): degrees of freedom; (***): p<0.001; (**): p<0.01; (*): p<0.05 (ns): not significant.
| Effect | df | LW (cm) |
LL (cm) |
LA (cm2) |
LT (µm) |
WC (%) |
LMA (mg cm-2) |
SLA (cm2 mg-1) |
DMC (mg cm-3) |
|---|---|---|---|---|---|---|---|---|---|
| Altitude (low, high) | 1 | 0.01ns | 0.01ns | 0.03ns | 8.46** | 40.45*** | 18.48*** | 33.72*** | 5.52* |
| Position of the trees (FE, FU, IT) | 2 | 3.59* | 17.44*** | 16.57*** | 18.16*** | 4.31* | 6.79** | 13.3*** | 8.61*** |
| Orientation of the crown (N, S, W, E) | 3 | 0.11ns | 0.24ns | 0.08ns | 0.23ns | 1.02ns | 0.31ns | 0.29ns | 0.15ns |
| Altitude × Position of the trees | 2 | 1.86ns | 4.36* | 2.15ns | 10.79*** | 0.83ns | 2.83ns | 4.09* | 2.12ns |
| Altitude × Orientation of the crown | 3 | 0.25ns | 0.66ns | 0.19ns | 0.03ns | 0.26ns | 0.55ns | 0.22ns | 0.54ns |
| Position of the tree × Orientation of the crown | 6 | 0.01ns | 0.01ns | 0.02ns | 0.10ns | 0.17ns | 0.06ns | 0.11ns | 0.09ns |
Fig. 2 - Differences in leaf morphological traits between two altitudes (low, high) in forest edge, forest understory, and isolated trees. (LW): leaf width; (LL): leaf length; (LA): leaf area; (LT): leaf thickness; (WC): water content; (LMA): leaf mass per area; (SLA): specific leaf area; (DMC): dry matter concentration. Data are shown as average ± SE. The same letters indicate no significant difference (p<0.05) between means.
Almost all the traits tended to be lower at high altitude than at low altitude, except WC and SLA, regardless of the position of the sampled tree (FU, FE, IT - Fig. 2). Also, the results showed that LT reduced significantly with increasing altitude (from 234.34 ± 13.17 to 186.39 ± 13.03 µm, p<0.001). In contrast, LMA increased significantly (p<0.001) from 6.01 ± 0.72 mg cm-2 at low altitude to 4.39 ± 0.48 mg cm-2 at high altitude. Both WC and SLA increased significantly (p<0.001) from low to high altitudes.
Variations of leaf traits along the gradient of canopy cover
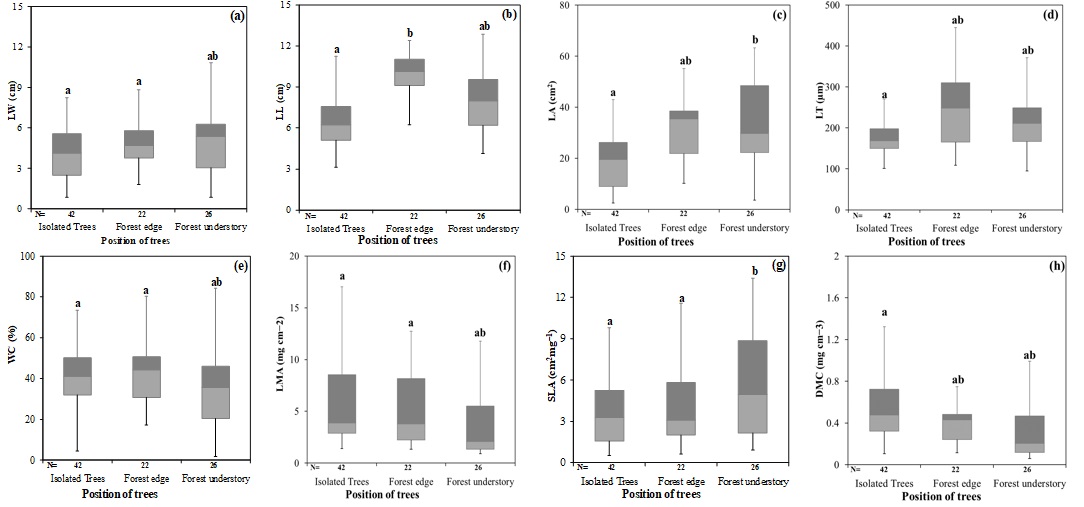
The position of sampled trees along the gradient of canopy cover (FU, FE, IT) had a significant effect on all morphological traits (p<0.05). Average LW was 5.19 cm at forest edges (FE), 4.92 cm at forest understory (FU), and 4.03 cm for isolated trees (IT). Additionally, the mean LL was 9.21, 7.97, and 6.46 cm for the leaves collected at forest edge, forest understory, and from isolated trees, respectively. LA differed significantly from FE and FU (about 32 cm2) to IT (about 20 cm2). We found that leaves of trees from the forest edge were ticker (mean LT = 256.08 µm, range = 109-445 µm) as compared with leaves from forest understory and isolated trees (mean LT of 220.29 and 177.16 µm, respectively). WC was slightly different among the three positions. LMA and SLA differed significantly between FE and FU (p < 0.05), but these traits in the IT were close to those of FE. Mean LMA ranged from 3.64 mg cm-2 at the forest understory to 6.10 mg cm-2 in isolated trees, and 5.19 mg cm-2 at the forest edge. On average, the values of SLA in FE, FU, and IT were 4.15, 5.72, and 3.65 cm2 mg-1, respectively. Moreover, the mean values of DMC were 0.39 and 0.43 mg cm-3 at the forest edge and forest understory, respectively, while in the isolated trees the mean DMC was 0.74 mg cm-3 (Fig. 3). Furthermore, species with different positions had different responses in the different altitudinal zones. Leaf size parameters (LW, LL, LA, and LT) tended to be lower at high altitude than at low altitude for FE and FU, but this trend was the opposite for IT. However, these differences were not significant among the three different positions, except for LL and LT in isolated trees and forest edge, respectively (Tab. 5). SLA was significantly higher in forest understory than at forest edge only at low altitude. At the same time, all other traits were significantly higher (p<0.05) at the forest edge than in forest understory, regardless of low or high altitude (Tab. 5, Fig. 2).
Fig. 3 - Paired boxplots of leaf morphological traits by the position of trees along a gradient of canopy cover (forest edge, forest understory, and isolate trees). Boxes with the same letter indicate means that were not significantly different (p<0.05).
Tab. 5 - Mean values (± SE) of leaf morphological traits for different positions of trees along a gradient of canopy cover (FE, FU, IT) at low (1300-1500 m a.s.l.) or high (1500-1700 m a.s.l.) elevation. t-values and significance of differences after t-test are given. (***): p<0.001; (**): p<0.01; (*): p<0.05; (ns): not significant.
| Leaf traits | Forest edge (FE) | t | Forest understory (FU) | t | Isolated trees (IT) | t | |||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | ||||
| LW (cm) | 5.66 ± 0.71 | 4.26 ± 0.42 | -1.31ns | 4.95 ± 0.36 | 4.86 ± 0.70 | -0.13ns | 3.64 ± 0.42 | 4.24 ± 0.23 | 1.36ns |
| LL (cm) | 9.75 ± 0.53 | 8.14 ± 0.82 | -1.64ns | 8.23 ± 0.41 | 7.62 ± 0.50 | -0.91ns | 5.75 ± 0.25 | 6.84 ± 0.31 | 2.36* |
| LA (cm2) | 34.47 ± 2.51 | 26.67 ± 2.22 | -1.95ns | 33.63 ± 3.51 | 31.11 ± 4.17 | -0.45ns | 16.67 ± 2.53 | 20.89 ± 1.24 | 1.66ns |
| LT (µm) | 299.54 ± 18.41 | 169.17 ± 13.71 | -4.56*** | 225.18 ± 13.66 | 213.45 ± 18.58 | -0.51ns | 178.29 ± 7.45 | 176.56 ± 6.80 | -0.16ns |
| WC (%) | 38.04 ± 7.58 | 49.52 ± 28.25 | 3.33*** | 32.87 ± 5.62 | 39.82 ± 3.47 | 3.51*** | 42.72 ± 7.27 | 38.76 ± 8.10 | 4.54*** |
| LMA (mg cm-2) | 5.77 ± 6.65 | 3.64 ± 0.22 | -4.33*** | 4.71 ± 4.23 | 3.41 ± 3.53 | -1.03ns | 6.70 ± 6.95 | 5.21 ± 5.01 | -2.80*** |
| SLA (cm2 mg-1) | 3.89 ± 0.26 | 3.70 ± 0.64 | 8.45*** | 3.32 ± 0.64 | 6.82 ± 0.92 | 1.37ns | 3.08 ± 0.32 | 5.26 ± 0.34 | 4.32*** |
| DMC (mg cm-3) | 0.42 ± 0.02 | 0.87 ± 0.01 | -4.21*** | 0.61 ± 0.04 | 0.39 ± 0.04 | -0.001ns | 0.74 ± 0.05 | 0.53 ± 0.04 | -2.76** |
Interaction of the position of trees along the gradient of canopy cover and altitude had a significant effect on LL, LT, and SLA (p<0.001). It should be noted that for isolated trees, the level of LL at high altitude was considerably higher than low altitude (p<0.05). However, this parameter showed no significant differences between FE and FU to their altitude. LT at high altitude was significantly lower (p<0.001) than at low altitude in FE, though no significant differences for this parameter were found between the two altitudinal ranges in FU and IT (Tab. 5, Fig. 2). WC showed an overall trend of increase with increasing altitude, especially at the forest edge and in forest understory (Tab. 5, Fig. 2). LMA of all species decreased with increasing altitudes. SLA of all species showed an overall trend of increase with increasing altitudes. Furthermore, the DMC of species in FE and IT showed a decrease along the altitude gradient. There was no noticeable effect of elevation on DMC in the forest understory. The effect of the different positions of the sampled trees on leaf traits is also shown in Fig. 3.
Variations of leaf traits in different orientation of the crown
The main effects of altitude and crown orientation on leaf trait variation are presented in Tab. 4and Tab. 6. We found no significant difference (p>0.05) in the mean values of leaf morphological traits among different orientations of the crown (Tab. 4). Furthermore, there were no significant differences (p>0.05) in the interactions of the orientation of the crown with altitude, as well as the position of the trees (Tab. 4).
Tab. 6 - The main effects of the different orientation of tree crown (± SE) on leaf traits.
| Leaf traits | Orientation of the crown | |||
|---|---|---|---|---|
| North | South | East | West | |
| LW (cm) | 4.39 ± 0.37 | 4.68 ± 0.38 | 4.61 ± 0.38 | 4.48 ± 0.37 |
| LL (cm) | 7.35 ± 0.39 | 7.69 ± 0.40 | 7.63 ± 0.39 | 7.36 ± 0.40 |
| LA (cm2) | 25.80 ± 2.33 | 26.58 ± 2.46 | 26.45 ± 2.40 | 25.18 ± 2.28 |
| LT (µm) | 211.00 ± 11.93 | 200.56 ± 10.95 | 211.10 ± 13.38 | 205.78 ± 11.81 |
| WC (%) | 36.51 ± 8.36 | 38.69 ± 7.45 | 40.81 ± 8.35 | 41.22 ± 10.23 |
| LMA (mg cm-2) | 5.24 ± 0.44 | 4.91 ± 0.65 | 5.46 ± 0.80 | 5.18 ± 0.69 |
| SLA (cm2mg-1) | 4.48 ± 0.49 | 4.49 ± 0.50 | 3.99 ± 0.44 | 4.47 ± 0.52 |
| DMC (mg cm-3) | 0.56 ± 0.04 | 0.57 ± 0.04 | 0.60 ± 0.04 | 0.56 ± 0.03 |
Discussion
Variations of leaf traits at different altitudes
Our results confirmed that altitude had remarkable effects on almost all leaf morphological traits of the woody plant community. Leaf water content (WC) and specific leaf area (SLA) at high altitude (1500-1700 m a.s.l.) had values higher than those at low altitude (1300-1500 m a.s.l.), while all other parameters investigated, such as leaf area (LA), were significantly higher at lower altitudes. Higher values of leaf size parameters at low altitude might result from lower light due to understory competition, suggesting a higher environmental stress at low altitude ([26]).
This result can also provide some indications on the physiological drought conditions at high altitudes due to low air temperature, soil depth, high wind speed, and the short vegetative periods ([19], [26]). According to Paridari et al. ([33]), trees at the higher altitude have smaller leaf lamina than those at lower altitudes. Moreover, it has been reported that tree populations from higher altitudes generally exhibit reduced growth, as well as smaller and thicker leaves ([34]). In this study, the effect of the shortened growing season with increasing altitude is evident in the decreased leaf thickness (LT). However, such effect is controversial in the literature, as in some cases an increased altitude has been claimed to result in thicker leaves (mainly due to intense irradiance - [6]), while in other cases in thinner leaves ([41]), reflecting the drought conditions at high altitudes which hamper leaf growth. We found that leaf mass per area (LMA) decreased (p<0.001) with increasing altitude. High LMA under low-light conditions does not enhance light capture ([12]). This result is consistent with the findings of Paridari et al. ([33]) in the Hyrcanian forest, where LMA was higher at low altitudes. In contrast, the results of some studies confirmed that leaves at high altitudes had higher LMA than at lower altitudes ([38]), suggesting that different leaf properties may be fostered under different environmental conditions. Among leaf traits, LMA, or its inverse value SLA, has been frequently used as an indicator of differential functional strategies in plant species ([47], [10]). The low LMA/high SLA leaves of trees could also respond to low photosynthate concentrations ([35]). In contrast, high values of LMA can be the consequence of a high density of mesophyll tissues, or leaf thickness. This could lead to misinterpretations of the ecological significance of LMA, since DMC and LT have different ecological interpretations ([24]). Both WC and SLA increased (p<0.001) with increasing altitude. Higher SLA at high altitude reflects a high capacity to absorb active photosynthetic radiation ([36], [26]). This result is in contrast with previous studies reporting that SLA decreases with increasing altitude as a likely response to decreasing temperature rather than increasing radiation ([23]) or changes in water availability ([36]). Our findings suggest that leaves generally respond to changes in altitude with a reduction of several morphological traits such as LW, LL, LA, and LT. At the same time, a general increase in water relations in terms of WC and SLA was observed. These results highlight the influence of altitude on plant physiological parameters and consequently on the autoecology of the species.
Variations of leaf traits under different canopy cover
In this study, the variation of leaf morphological traits of species reflected the heterogeneous light environment (in terms of sunlight radiation) which occurs in different locations of the forest environment, such as in the understory, at the forest edge, and in open spaces. Indeed, we observed that the position of the sampled trees along the gradient of canopy cover had a substantial effect on leaf morphological traits of the tree species considered. All morphological traits except SLA were higher at the forest edge than in the forest understory. At higher light environments, SLA tended to decrease ([26], [25]), as observed in the present study. LMA increases with decreasing forest cover, likely due to the increased light availability and water stress ([32]). The results of this study also showed a significant increase in LMA with increasing light at the isolated trees, similar to the findings of previous studies ([40], [44]). LMA and DMC were significantly higher in isolated trees than at the forest edge and in the understory, regardless of low or high altitude (p<0.001). The low LMA and DMC observed in this study in forest edge and forest understory is consistent with the findings of Liu et al. ([28]), as higher moisture and lower evaporation under low light favor a high leaf water content, thus leading to a lower leaf dry mass to fresh mass ratio. Results for the effect of canopy cover and altitude on morphology showed that in forest edge and forest understory, leaf size parameters (LW, LL, LA, and LT) decreased at higher altitude due to the change of moisture and temperature conditions. In contrast, the parameters increased in the case of isolated trees under full light. The high WC might be due to the reduction of LA with increasing altitude ([46]). LMA decreased with increasing altitude for all trees in three different sites. LMA at high altitude was significantly lower than at low altitude at forest edge and in isolated trees (p<0.001); however, this decrease is not significant in the forest understory. DMC decreased in forest edge and isolated trees with increasing altitude, and such effect was not significant in the forest understory. Generally, a high DMC indicates few intercellular spaces and a high mesophyll resistance to the diffusion of gases. These features are detrimental to photosynthetic function due to the low availability of CO2 within the mesophyll. Also, the DMC increases following many environmental stresses, such as oxidative stress ([7]).
Variations of leaf traits at different orientation of the crown
The differences between the data measured were not significant for any crown orientation. This result could not have been explained in this study, and further studies should focus on this topic. However, this finding confirms previous studies showing no significant differences in the main leaf features among different geographical sides of the crown ([50]). Nonetheless, some previous studies have reported that various orientations of the crown differ significantly in respect of some leaf morphological characteristics, due to differences in light environment ([18], [20], [21]).
Conclusions
We carried out in situ observations of leaf morphological traits by sampling 18 different deciduous woody species at their full leaf expansion. Due to differences in light and water conditions, the growth rate of singular leaves may be influenced by their position within the canopy and across the stand. We also considered isolated trees in the open field with a few other trees nearby. Leaf morphological traits varied considerably between the two altitudinal ranges and at different positions of the sampled trees along the light gradient. Overall, most leaf traits, including leaf width, length, area, and thickness, decreased significantly with increasing altitude. Furthermore, water relations, such as water content and specific leaf area ratios markedly increased. There were significant positive relationships between both altitude and position of the trees on the one hand and leaf morphological traits on the other. In contrast, both were negatively related to the orientation of the crown. Altitude and position of the trees were significant for all morphological traits of the species (except for the effect of altitude on leaf width, length, and area).
The results confirmed that, in terms of leaf morphology, all the species analyzed had similar responses to the heterogeneous light environment occurring at different locations characterized by different canopy cover. All leaf morphological traits except SLA tended to be higher at the forest edge and at lower altitudes than in forest understory and at higher altitudes. These results indicate that species shift their morphological characters to adapt to a specific environment and confirm earlier reports on the response of plants to changes in environmental parameters. Information about variability in both leaf morphological and ecophysiological traits, and its roles in growth, development, and productivity of trees and shrubs in a forest ecosystem, is needed for foresters and forest scientists for forest management and planning further research projects.
References
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Supplementary Material
Authors’ Info
Authors’ Affiliation
Faculty of Agriculture and Natural Resources, University of Mohaghegh Ardabili, Ardabil (Iran)
University of Florence, Department of Agriculture, Food, Environment and Forestry (DAGRI), Firenze (Italy)
Corresponding author
Paper Info
Citation
Jahdi R, Arabi M, Bussotti F (2020). Effect of environmental gradients on leaf morphological traits in the Fandoghlo forest region (NW Iran). iForest 13: 523-530. - doi: 10.3832/ifor3391-013
Academic Editor
Claudia Cocozza
Paper history
Received: Mar 02, 2020
Accepted: Sep 14, 2020
First online: Nov 13, 2020
Publication Date: Dec 31, 2020
Publication Time: 2.00 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 39058
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 32659
Abstract Page Views: 3262
PDF Downloads: 2512
Citation/Reference Downloads: 4
XML Downloads: 621
Web Metrics
Days since publication: 1907
Overall contacts: 39058
Avg. contacts per week: 143.37
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 3
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Successional leaf traits of monsoon evergreen broad-leaved forest, Southwest China
vol. 10, pp. 391-396 (online: 16 March 2017)
Research Articles
A comparative study of growth and leaf trait variation in twenty Cornus wilsoniana W. families in southeastern China
vol. 10, pp. 759-765 (online: 02 September 2017)
Research Articles
Effect of family, crown position, number of winter buds, fresh weight and the length of needle on rooting ability of Pinus thunbergii Parl. cuttings
vol. 9, pp. 370-374 (online: 11 January 2016)
Research Articles
Effects of mild drought on the morphology of sun and shade needles in 20-year-old Norway spruce trees
vol. 12, pp. 27-34 (online: 10 January 2019)
Research Articles
Estimation of stand crown cover using a generalized crown diameter model: application for the analysis of Portuguese cork oak stands stocking evolution
vol. 9, pp. 437-444 (online: 02 December 2015)
Research Articles
Adaptability and interspecific variability in growth and leaf traits of eucalypt
vol. 14, pp. 560-568 (online: 09 December 2021)
Research Articles
Seedling emergence capacity and morphological traits are under strong genetic control in the resin tree Pinus oocarpa
vol. 17, pp. 245-251 (online: 16 August 2024)
Research Articles
Edge tree functional traits and their association with edaphic factors in seasonally dry forests in northern Thailand
vol. 15, pp. 273-280 (online: 26 July 2022)
Research Articles
Trade-offs and spatial variation of functional traits of tree species in a subtropical forest in southern Brazil
vol. 9, pp. 855-859 (online: 07 July 2016)
Research Articles
The influence of age and crown position on growth efficiency along a Scots pine chronosequence
vol. 12, pp. 474-479 (online: 14 October 2019)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword