Edge tree functional traits and their association with edaphic factors in seasonally dry forests in northern Thailand
iForest - Biogeosciences and Forestry, Volume 15, Issue 4, Pages 273-280 (2022)
doi: https://doi.org/10.3832/ifor3870-015
Published: Jul 26, 2022 - Copyright © 2022 SISEF
Research Articles
Abstract
The relationships between plant traits and soil properties in forest edges can provide insights into tree species recovery in edge habitats. In this study, we investigated the relationships between plant functional traits and soil conditions related to tree species recovery at the edges of two seasonally dry forests, a mixed deciduous forest (MDF) and a deciduous dipterocarp forest (DDF) in northern Thailand. We analyzed differences in functional trait diversity and community-level trait values between forests and performed RLQ analysis to assess the associations among species abundance, plant traits, and soil variables. We found that the MDF site had greater functional diversity and was dominated by plants with high specific leaf area (SLA) and leaf dry-matter content (LDMC) at the community level, whereas the DDF site had lower diversity and was dominated by plants with high wood density (WD) and leaf thickness (LT). The RLQ results indicated that at the MDF site, tree species with greater SLA (e.g., Pterocarpus macrocarpus, Dalbergia cultrata, and Phanera bracteata) were associated with soil clay content and nutrient status (i.e., nitrogen and calcium). Species with greater LDMC and leaf size (e.g., Xylia xylocarpa, Schleichera oleosa, and Chukrasia tabularis) were associated with soil organic matter content. At the DDF site, species with greater WD and LT (e.g., Dipterocarpus obtusifolius, Shorea siamensis, and Buchanania lanzan) were associated with soil sand content and bulk density. These patterns reflect the interplay between soil conditions and plant traits in the edge habitats of seasonally dry forests. Our results indicate that the edge effects on plant communities within seasonally dry forests depend on soil conditions and species-specific plant traits.
Keywords
Forest Edge Effects, Tree Species Recovery, Plant-soil Relationships, Mixed Deciduous Forest, Deciduous Dipterocarp Forest
Introduction
Recent studies have explored the relationships among plant and soil properties, including soil texture, pH, and nutrient availability ([27], [48]). These plant-soil relationships can be used to establish a trait-based framework for investigating plant community assemblages ([28], [39]). Functional trait composition is related to both species diversity and dominance within the plant community, which can be compared in terms of functional diversity indices ([30]) and community-level weighted trait means ([35]), respectively. These indices are essential for understanding the relationships between plant species and soil conditions that influence their assembly into communities ([13]). For example, functional diversity has been used to quantify trait distributions within different plant communities ([32]). Plant community level-weighted trait means have been used to identify the soil factors that limit or promote vegetation recovery through the succession or restoration of plant communities ([42], [2]), and to predict species assemblages along soil nutrient gradients in a natural tropical forest ([21]). Thus, a better understanding of plant trait and soil relationships may enhance our ability to predict vegetation responses to both natural and anthropogenic environmental changes ([31]).
An increased amount of forest edge is characteristic of fragmented forests. These discrete boundaries between forest patches and open areas are typically associated with a transition zone on either side of the edge; this is referred to as an edge effect ([34]). Generally, edge effects are associated with high disturbance regimes ([19], [47]), wherein anthropogenic disturbance and natural disturbance like fire and extreme weather events can interact with vegetation to create a shifting mosaic of diverse vegetation communities at different successional stages ([16], [22]). For example, vegetation structure along edges can be reduced in canopy height with a dense subcanopy layer, thus appearing like secondary forest ([38]). Forest edges are also associated with environmental change in interior habitats when there are abrupt transitions between vegetation communities ([19]). Edaphic factors are influenced by edge effects. Variation in soil nutrient status between edge and interior habitats may have major consequences for forest productivity in fragmented landscapes ([26]). Several studies have shown that soil properties differ between forest edge and interior zones ([49]). In tropical forests, soil organic matter (OM) and soil nutrients (e.g., nitrogen, N; potassium, K; phosphorus, P; calcium, Ca; and magnesium, Mg) are affected by differences in vegetation structure between the forest edge interior, forest edge exterior, and core area ([2]).
Seasonally dry forests are found in tropical and sub-tropical zones under monsoonal climates, where the annual dry season lasts for a minimum of 4-5 months, and are dominated by deciduous canopy trees. Such forests are widespread in southern and southeastern Asia ([11]). Mixed deciduous forest (MDF) and deciduous dipterocarp forest (DDF) are two types of seasonally dry forests found in Thailand and are distributed at similar elevations ([6]). They experience regular disturbances by forest fires and human activities ([29]). Typically, MDFs occur in riparian areas with deep organic soil and gentle slopes (< 700 m a.s.l. - [29], [6]). The dominant tree species include Tectona grandis, Xylia xylocarpa, Pterocarpus macrocarpus, Afzelia xylocarpa, and Dalbergia oliveri. DDFs are found in dry areas with acidic, shallow, sandy, or lateritic soils on gentle slopes at < 900 m a.s.l. in elevation. The dominant species are deciduous dipterocarps including Shorea obtusa, Shorea siamensis, Dipterocarpus obtusifolius, and Dipterocarpus tuberculatus ([6]). Previous work has suggested that soil properties such as OM and nutrient content are higher in mature MDF compared to DDF ([36]), but few studies have compared these properties along edges between the two forest types.
In Thailand, the total proportion of forested land has declined from 53% to 32% over the past 60 years, resulting in forest fragmentation and thus a large amount of forest edges. This decline is partly due to anthropogenic conversion of primary forest for agriculture ([46]). This issue is particularly pronounced in northern Thailand, where forest conversion is contributing to the decline and fragmentation of seasonally dry forest types, i.e., DDF and MDF. For example, in the Mae Khum Mee sub-watershed area, most of the DDF and MDF has been converted to corn fields, with an average forest loss of 173 ha year-1 ([25]). This has led to an increase in forest edges in this area, especially in these two forest types, which have a scattered distribution ([3]). Therefore, understanding plant-soil relationships can provide insight into effect management strategies to promote tree species recovery along edges in seasonally dry forest types. We examined the relationships between soil conditions and plant functional traits in two MDF and DDF stands in Thailand. Specifically, we examined differences in functional diversity and dominance, and their associations with species abundance, plant traits, and soil variables in MDF and DDF. The results of this study will contribute to future predictions of tree species recovery in terms of their responses to environmental disturbances at forest edges.
Materials and methods
Study area
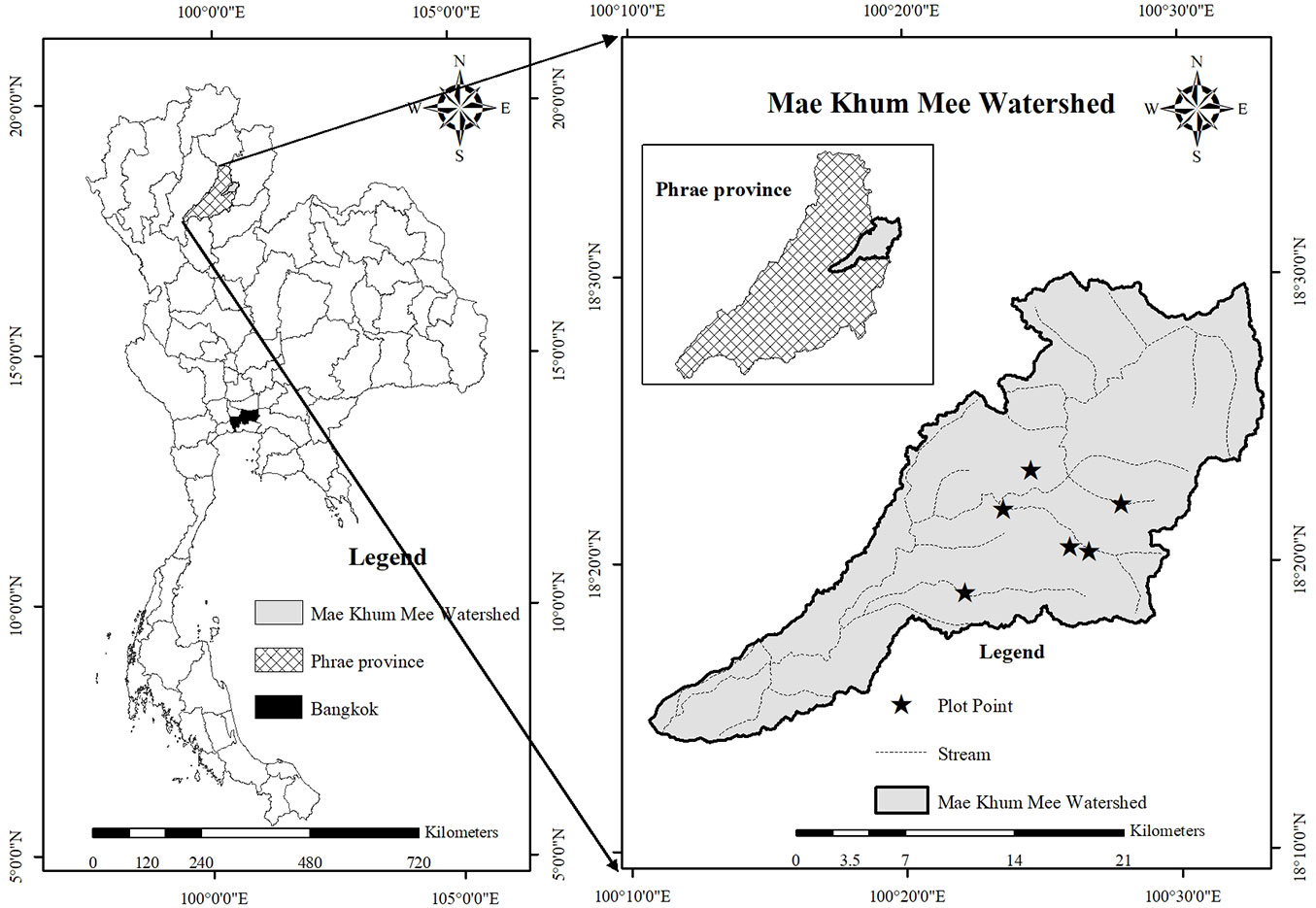
This study was conducted in forest edge areas (i.e., transition zones from the forest edge to the forest interior) in two seasonally dry forest remnants in the Mae Khum Mee sub-watershed area (18° 05′ - 18° 30′ N, 100° 10′ - 100° 35′ E), within an area of 452.4 km2 and at an elevation of 320-540 m above mean sea level, in Phrae Province, northern Thailand (Fig. 1). The region has two main seasons: a wet season (May-October) and a dry season (November-April). The mean rainfall amounts of the wet and dry seasons are 1460 mm and 406 mm, respectively; their mean temperatures are 33.60 °C and 35.35 °C, respectively ([1]). MDF and DDF are the main natural vegetation types, covering 19.03% (80.94 km2) and 0.91% (4.10 km2) of the region, respectively ([25]). Forest fragmentation caused by highland agriculture such as maize cultivation has led to the creation of scattered remnant forest patches throughout the watershed ([9]).
Fig. 1 - Locations of the Mae Khum Mee sub-watershed and study area in Phrae Province, northern Thailand.
Sampling plot selection and mature tree data
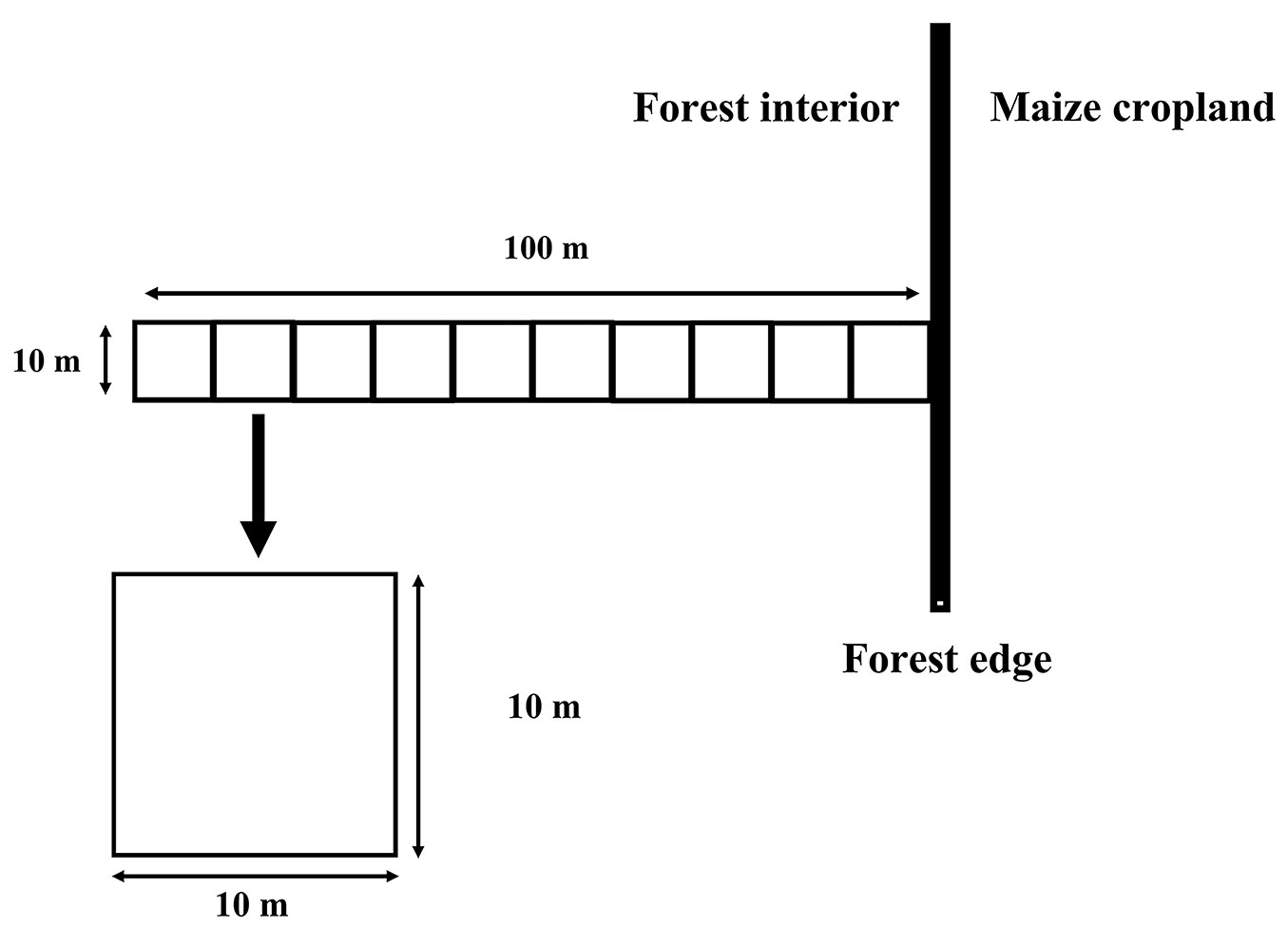
We collected data from January 2018 to December 2018. The DDF and MDF edge sites were selected in similar topographic and geographic settings (elevation: 400 m a.s.l.; slope: 45%; distance to nearest village: approximately 5 km), adjacent to maize fields that had been abandoned at least 3 years previously (as confirmed by interviews with local residents). Within each DDF and MDF site, we established three transect (10 × 100 m - Fig. 2); each of these six transect clearly incorporated an edge zone, such that the species composition reflected edge effects induced by regular disturbance ([26]). The edge boundary was defined as the line separating the interior (i.e., dense mature trees) and exterior (i.e., sparse shrubs, juvenile trees, or bare land) portions of the forest ([15]). We designed each transect to extend 100 m from the forest edge to the interior, perpendicular to the edge line; the transect began near the first mature tree along the edge line. Each transect was subdivided into 10 adjacent plots (10 × 10 m - Fig. 2) to yield 30 plots per forest type i.e., 60 plots (0.6 ha) in total.
Fig. 2 - Transect (10 × 100 m) subdivided into 10 sub-plots (10 × 10 m) were established in the forest interior-edge transition area, extending perpendicular to the edge boundary, in deciduous dipterocarp forest (DDF) and mixed deciduous forest (MDF) stands.
The diameter at breast height (DBH) of all mature trees (height ≥ 1.3 m and DBH ≥ 4.5 cm) was measured in each 10 × 10 m plot. All trees were identified at the species level by comparing collected specimens with standard specimens in the herbarium of the Department of National Parks, Wildlife and Plant Conservation, Thailand. The nomenclature used in this study follows the system used by Pooma & Suddee ([40]).
Soil measurements
In each 10 × 10 m plot, we measured soil variables including soil bulk density (SDb, g cm-3), soil texture (% sand, silt, and clay), pH, OM (%), and soil nutrients (N, %; P, mg kg-1; K, mg kg-1; Ca, mg kg-1; Mg, mg kg-1). For these measurements, we obtained 100-cm3 soil samples from the topsoil layer (0-15 cm) in October 2018 using a soil core sampler. We collected sub-samples from the center and each of the four corners of each plot (five points per plot). Two sets of soil samples were collected from each plot. The first set was used for SDb analysis, in which the SDb was estimated for each soil sample as a proportion of the mass of oven-dried soil to the total volume. The second set of soil samples was used to analyze soil texture, pH, OM, and available N, P, K, Ca, and Mg at the soil laboratory of the Faculty of Forestry, Kasetsart University in Bangkok, Thailand. All samples were collected on the same day, at 10 days after the last rainfall, near the onset of the dry season, i.e., when rain was infrequent. All soil variables were used for our analysis of mature trees in the 10 × 10 m plots.
Functional trait measurements
We selected functional traits that were representative of various plant ecological strategies related to competitive ability, resource exploitation, growth and resistance, and disturbance response ([43]). We examined five functional traits in mature trees: specific leaf area (SLA, cm2 g-1), leaf dry matter content (LDMC, mg g-1), leaf area (LA, cm2), leaf thickness (LT, mm), and wood density (WD, g cm-3). We used sun leaf samples from mature trees in each DDF and MDF forest plot to calculate the representative mean values of leaf traits (SLA, LDMC, LA, and LT) for each species in October 2018. We randomly sampled 2-10 leaves from three individuals for each species that was found in all 10 × 10 m plots. To determine LA, we scanned fresh leaves using the software ImageJ (⇒ http://rsbweb.nih.gov/ij/) and measured LA from those images. Leaf masses were recorded before and after drying at 60 °C for 48 h to a constant weight. SLA was calculated as the ratio of fresh LA to oven-dried mass. LDMC was calculated as the ratio of the oven-dried mass (mg) of a leaf to its fresh mass (g). LT was estimated as the mean leaf blade thickness from five leaf samples, measured using a thickness gauge (China YH-1, Zhejiang, China). Cores for WD determination were collected at breast height (1.3 m) from each tree selected for leaf collection; these cores were collected using 5-mm increment borers. WD was calculated from wood samples (diameter, 0.5 cm; length, 5 cm) as the ratio of the oven-dried mass to fresh volume.
Data analyses
We used Student’s t-test to compare soil variables between DDF and MDF edges. For general analysis of each DDF and MDF site, we calculated the stem density (stem ha-1), stem basal area (m2 ha-1), relative stem density (%), and relative basal area (%). From these results, we derived the importance value index as the sum of relative stem densities and relative basal areas for each forest site to identify the dominant species at each site. The importance value index is an indicator of the relative importance of vegetation species within a site ([10]).
Plant trait data in each 10 × 10 m plot were normalized and used to calculate functional diversity and dominance. We used Rao’s quadratic entropy (RaoQ) as a functional diversity index to characterize the diversity of species traits among the sampled plots. Rao’s quadratic entropy represents community trait divergence based on the sum of dissimilarities among species in trait space, and is weighted by the species’ relative abundances ([45]). It was calculated as follows (eqn. 1, eqn. 2):
where dij is the Euclidean dissimilarity between the traits of each pair of species i and j, Xti is the trait value of i-th species, and T is the number of traits.
To characterize trait dominance in each plot, we analyzed the community-level weighted means (CWMs) of each trait. CWMs were then used to estimate the dominant trait value of the most probable attribute displayed by a species drawn at random from the community ([45]), calculated as follows (eqn. 3):
where pi and tri are the relative abundance and trait value of species i, and n is the total number of species per plot.
RaoQ indices and CWMs were calculated using the “FD” package ([24]) in R software v. 3.4.1 ([44]). Student’s t-test was used to characterize and quantify differences in the mean values of functional trait diversity indices and CWMs of SLA, LDMC, LA, LT, and WD between DDF and MDF edge sites.
To clarify the associations among species abundance, plant traits, and soil variables, we applied RLQ analysis and the fourth-corner method based on the ordination method ([14]) using tree species relative abundance data. RLQ is an extension of co-inertia analysis that simultaneously searches for linear combinations of variables in the species × trait matrix (Q) and site × environmental matrix (R) that maximize the covariance and weighting of the site × species matrix (L). In this study, matrix R consisted of soil variables (SDb, pH, sand, silt, clay, OM, N, P, K, Ca, and Mg contents) measured in each 10 × 10 m plot. We then applied the fourth-corner method to test the correlations between RLQ sample scores (axis R1/axis R2, corresponding to environmental gradients) and species traits (matrix Q) and between RLQ species scores (axis Q1/axis Q2, corresponding to trait variables) and environmental variables (matrix R). For both RLQ and fourth-corner analyses, we used the R package “ade4” ([14]).
Results
Several soil properties differed between the DDF and MDF edge sites (Tab. 1). The DDF edge site had significantly greater soil bulk density and sand and K contents, compared with the MDF edge site (Tab. 1). In contrast, the MDF edge site had significantly greater pH and silt, clay, OM, N, and Ca contents (Tab. 1). P and Mg contents did not differ significantly between the DDF and MDF forest edge sites.
Tab. 1 - Mean ± standard deviation (SD) of soil variables including soil bulk density (SDb), pH, and sand, silt, clay, organic matter (OM), nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg) contents. Data for deciduous dipterocarp forest (DDF) and mixed deciduous forest (MDF) sites were compared using Student’s t-test; t-scores (t) is shown.
| Soil variable | DDF | MDF | df | t | p-value |
|---|---|---|---|---|---|
| SDb (g cm-3) | 1.09 ± 0.68 | 0.82 ± 0.11 | 58 | 2.102 | 0.040 |
| Sand (%) | 57.32 ± 5.04 | 37.67 ± 8.98 | 58 | 10.445 | <0.001 |
| Silt (%) | 18.86 ± 3.42 | 22.21 ± 3.87 | 58 | -3.548 | 0.001 |
| Clay (%) | 23.81 ± 3.54 | 40.11 ± 6.13 | 58 | -12.601 | <0.001 |
| pH | 6.17 ± 0.50 | 6.94 ± 0.59 | 58 | -5.392 | <0.001 |
| OM (%) | 4.50 ± 0.92 | 5.16 ± 1.27 | 58 | -2.313 | 0.024 |
| N (%) | 0.21 ± 0.05 | 0.33 ± 0.11 | 58 | -5.378 | <0.001 |
| P (mg kg-1) | 28.60 ± 15.81 | 15.30 ± 16.53 | 58 | 1.495 | 0.140 |
| K (mg kg-1) | 89.45 ± 31.01 | 62.69 ± 19.33 | 58 | 4.011 | <0.001 |
| Ca (mg kg-1) | 1535.18 ± 807.64 | 3079.60 ± 679.43 | 58 | -4.539 | <0.001 |
| Mg (mg kg-1) | 490.20 ± 337.27 | 417.23 ± 249.88 | 58 | 0.952 | 0.345 |
Plant functional trait data were collected for 49 dominant species, including 24 species present at both DDF and MDF edge sites, 11 species present only at the DDF edge site, and 14 species present only at the MDF edge site (Tab. 2). Based on the importance value index, the dominant species in the DDF site were Pterocarpus macrocarpus, Shorea siamensis, Shorea obtusa, Tectona grandis, and Grewia eriocarpa. By contrast, Pterocarpus macrocarpus, Millettia brandisiana, Schleichera oleosa, Phanera bracteata, and Albizia odoratissima were dominant in the MDF site (Tab. 2).
Tab. 2 - Stem density (stem ha-1), basal area (m2 ha-1), and importance value index (IVI, %) of dominant species, based on plant functional trait data, in a deciduous dipterocarp forest (DDF) and mixed deciduous forest (MDF).
| Species | Code | Density | Basal area | IVI | |||
|---|---|---|---|---|---|---|---|
| DDF | MDF | DDF | MDF | DDF | MDF | ||
| Pterocarpus macrocarpus | PTMAC | 253 | 153 | 0.07 | 0.07 | 25.37 | 21.95 |
| Shorea siamensis | SHSIA | 403 | 7 | 0.05 | 0.08 | 33.42 | 4.72 |
| Millettia brandisiana | MIBRA | 3 | 150 | 0.02 | 0.03 | 1.72 | 20.69 |
| Tectona grandis | TEGRA | 97 | 70 | 0.06 | 0.06 | 15.90 | 13.66 |
| Schleichera oleosa | SCOLE | 17 | 100 | 0.08 | 0.02 | 7.20 | 14.74 |
| Phanera bracteata | PHBRA | 17 | 103 | 0.03 | 0.02 | 4.45 | 14.58 |
| Albizia odoratissima | ALODO | 7 | 30 | 0.08 | 0.13 | 5.82 | 11.91 |
| Chukrasia tabularis | CHTAB | 13 | 47 | 0.06 | 0.07 | 6.27 | 11.24 |
| Xylia xylocarpa | XYXYL | 7 | 53 | 0.05 | 0.05 | 4.58 | 11.23 |
| Canarium subulatum | CASUB | 63 | 13 | 0.04 | 0.23 | 9.89 | 10.64 |
| Senna garrettiana | SEGAR | 10 | 3 | 0.04 | 0.25 | 4.04 | 9.66 |
| Haldina cordifolia | HACOR | 3 | 40 | 0.02 | 0.07 | 2.12 | 8.52 |
| Dalbergia assamica | DAASS | 7 | 13 | 0.05 | 0.11 | 4.15 | 6.23 |
| Dalbergia cultrata | DACUL | 40 | 23 | 0.04 | 0.04 | 8.41 | 6.18 |
| Morinda coreia | MOCOR | 37 | 17 | 0.04 | 0.01 | 8.50 | 4.62 |
| Bombax anceps | BOANC | 3 | 10 | 0.01 | 0.03 | 1.17 | 4.05 |
| Vitex peduncularis | VIPED | 13 | 10 | 0.05 | 0.03 | 5.86 | 3.96 |
| Gardenia obtusifolia | GAOBT | 13 | 13 | 0.02 | 0.02 | 4.12 | 3.95 |
| Mitragyna rotundifolia | MIROT | 20 | 3 | 0.02 | 0.08 | 4.91 | 3.92 |
| Grewia eriocarpa | GRERI | 30 | 7 | 0.10 | 0.04 | 10.80 | 3.52 |
| Lannea coromandelica | LACOR | 27 | 7 | 0.02 | 0.04 | 5.90 | 2.77 |
| Strychnos nux-vomica | STNUX | 33 | 7 | 0.02 | 0.02 | 7.21 | 2.74 |
| Cratoxylum formosum | CRFOR | 7 | 17 | 0.05 | 0.01 | 3.72 | 2.34 |
| Cassia fistula | CAFIS | 7 | 7 | 0.05 | 0.02 | 4.74 | 2.12 |
| Shorea obtusa | SHOBT | 250 | - | 0.02 | - | 22.83 | - |
| Dipterocarpus tuberculatus | DITUB | 33 | - | 0.05 | - | 9.43 | - |
| Dipterocarpus obtusifolius | DIOBT | 43 | - | 0.04 | - | 8.05 | - |
| Dalbergia oliveri | DAOLI | 13 | - | 0.09 | - | 7.77 | - |
| Tristaniopsis burmanica | TRBUR | 53 | - | 0.03 | - | 7.34 | - |
| Gluta usitata | GLUSI | 30 | - | 0.04 | - | 6.49 | - |
| Aporosa villosa | APVIL | 33 | - | 0.02 | - | 6.34 | - |
| Antidesma ghaesembilla | ANGHA | 17 | - | 0.05 | - | 5.03 | - |
| Bridelia retusa | BRRET | 13 | - | 0.03 | - | 4.56 | - |
| Terminalia alata | TEALA | 20 | - | 0.01 | - | 4.54 | - |
| Buchanania lanzan | BULAN | 10 | - | 0.02 | - | 3.49 | - |
| Albizia lebbeck | ALLEB | - | 13 | - | 0.27 | - | 11.24 |
| Lasiobema pulla | LAPUL | - | 23 | - | 0.05 | - | 6.56 |
| Alangium salviifolium | ALSAL | - | 23 | - | 0.06 | - | 6.09 |
| Harrisonia perforata | HAPER | - | 43 | - | 0.01 | - | 6.09 |
| Erythrina subumbrans | ERSUB | - | 10 | - | 0.08 | - | 5.76 |
| Hymenodictyon orixense | HYORI | - | 20 | - | 0.05 | - | 5.59 |
| Vitex canescens | VICAN | - | 13 | - | 0.07 | - | 5.55 |
| Holarrhena pubescens | HOPUB | - | 17 | - | 0.04 | - | 5.46 |
| Aegle marmelos | AEMAR | - | 17 | - | 0.02 | - | 4.80 |
| Walsura trichostemon | WATRI | - | 13 | - | 0.02 | - | 3.95 |
| Colona flagrocarpa | COFLA | - | 10 | - | 0.05 | - | 3.34 |
| Miliusa velutina | MIVEL | - | 10 | - | 0.02 | - | 3.06 |
| Dalbergia volubilis | DAVOL | - | 10 | - | 0.02 | - | 2.22 |
| Terminalia nigrovenulosa | TENIG | - | 10 | - | 0.01 | - | 1.97 |
Trait RaoQ values showed significant differences between the MDF and DDF edge sites (Tab. 3). At the community level, CWM values of SLA and LDMC were significantly greater at the MDF edge than at the DDF edge (Tab. 3). In contrast, CWM values of LT and WD were significantly greater at the DDF edge than at the MDF edge (Tab. 3).
Tab. 3 - Rao’s quadratic entropy (RaoQ) and community-level weighted means (CWMs) of leaf area (LA), specific leaf area (SLA), leaf thickness (LT), leaf dry matter content (LDMC), and wood density (WD) of tree species measured in DDF and MDF edge sites. Means were compared using Student’s t-test. Values are means ± standard deviation; t-scores (t) is shown.
| Functional index |
DDF | MDF | df | t | p-value |
|---|---|---|---|---|---|
| RaoQ | 3.35 ± 1.18 | 3.92 ± 1.29 | 58 | -2.214 | 0.039 |
| CWM-LA | 237.20 ± 94.55 | 300.68 ± 152.67 | 58 | -1.936 | 0.058 |
| CWM-SLA | 109.23 ± 16.91 | 134.49 ± 23.46 | 58 | -4.784 | <0.001 |
| CWM-LT | 0.27 ± 0.03 | 0.22 ± 0.03 | 58 | 6.234 | <0.001 |
| CWM-LDMC | 458.35 ± 27.16 | 481.02 ± 49.34 | 58 | -2.204 | 0.031 |
| CWM-WD | 0.58 ± 0.12 | 0.40 ± 0.17 | 58 | 6.656 | <0.001 |
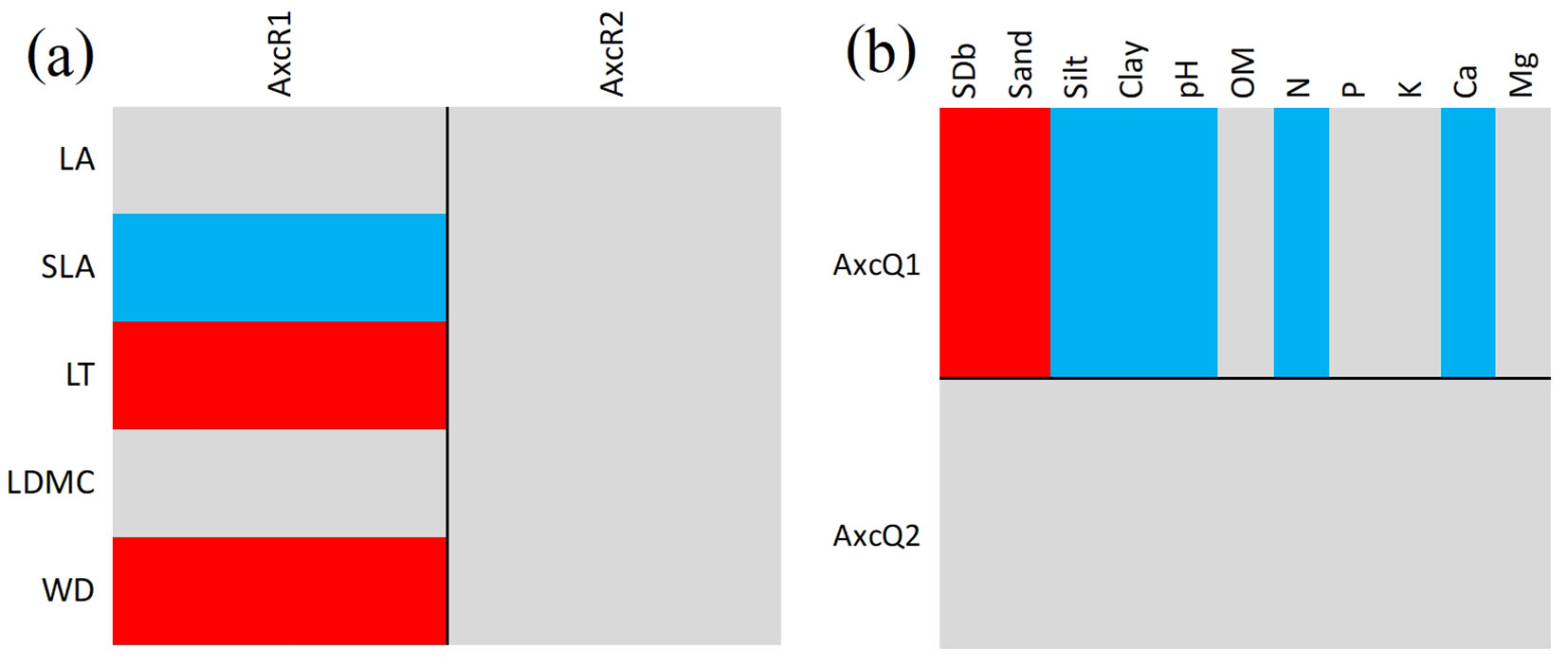
Fourth-corner analysis indicated that soil variables in the DDF and MDF edge sites were significantly correlated with overall tree traits when assessed using species relative abundance (p < 0.001 for model 2, p < 0.01 for model 4; fourth-corner test). Fourth-corner analysis showed similar trends along the DDF-to-MDF forest axis (Fig. 3a, Fig. 3b). The first axis of RLQ sample scores was significantly positively correlated with WD and LT (p < 0.05 and p < 0.001, respectively) and significantly negatively correlated with SLA (p < 0.01 - Fig. 3a). The first axis of RLQ species scores was significantly positively correlated with SDb and sand content (both p < 0.01) and significantly negatively correlated with silt and clay contents (both p < 0.001), as well as pH (p < 0.01) and N and Ca contents (p < 0.01 and p < 0.05, respectively - Fig. 3b). These results suggested that greater soil bulk density and sand content were associated with tree species that exhibited dense wood and thick leaves. In contrast, sites with high pH and silt, clay, N, and Ca contents were associated with tree species with high SLA values.
Fig. 3 - Fourth-corner and RLQ analysis results for (a) functional traits and the first two RLQ axes for soil variables (axis R1/axis R2) and (b) soil variables and the first two RLQ axes for functional traits (axis Q1 and axis Q2) at adjusted p = 0.05. Significant positive and negative associations are indicated by red and blue cells, respectively. Codes for soil variables and traits are explained in Tab. 1and Tab. 2, respectively.
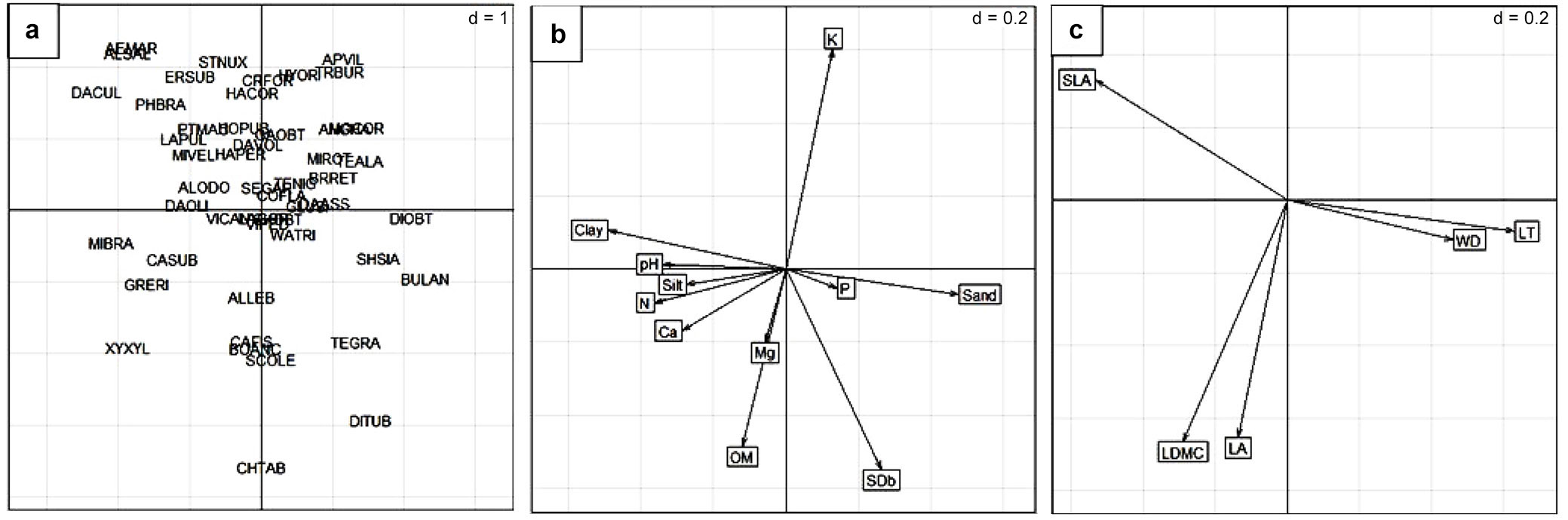
RLQ analysis results were explained almost completely by the first two axes, with eigenvalues of 1.14 and 0.03, respectively, which captured 92% of the covariance in species abundances (L matrix), trait values (Q matrix), and soil variables (R matrix). These results indicated a separation between forest edge sites, but revealed the presence of some dominant species at both DDF and MDF edges (Fig. 4a). Between DDF and MDF sites, differences in species composition were not statistically significant, whereas differences in trait values and soil properties were statistically significant (Fig. 4a-c). The MDF edge was characterized by highly abundant species with greater SLA values, such as Pterocarpus macrocarpus (PTMAC), Dalbergia cultrata (DACUL), Phanera bracteata (PHBRA), Lasiobema pulla (LAPUL), and Miliusa velutina (MIVEL); these species were associated with soils containing greater proportions of silt and clay, as well as greater pH and N and Ca contents. Other highly abundant species that had greater LDMC and larger leaves, including Xylia xylocarpa (XYXYL), Schleichera oleosa (SCOLE), Chukrasia tabularis (CHTAB), Bombax anceps (BOANC), and Cassia fistula (CAFIS), were associated with greater soil OM. The DDF edge was characterized by highly abundant species with dense wood and thicker leaves, such as Dipterocarpus obtusifolius (DIOBT), Shorea siamensis (SHSIA), Buchanania lanzan (BULAN), Tectona grandis (TEGRA), and Dipterocarpus tuberculatus (DITUB), which were associated with greater sand content and soil bulk density (Fig. 4a-c).
Fig. 4 - Results of the RLQ analysis for the first two axes, showing the relationships among (a) dominant species, (b) soil variables, and (c) plant traits. Values of d (upper rightmost) indicate grid size. Species codes are provided in Tab. 2. Codes for soil variables and traits are provided in Tab. 1 and Tab. 3, respectively.
Discussion
Functional trait composition of seasonally dry forest edges
Tree community functional diversity and dominance are the main drivers of ecological processes ([33]). Functional diversity influences ecosystem dynamics and stability, as well as nutrient availability ([18]). In the present study, RaoQ was greater in the MDF site than in the DDF site, which suggested that functional diversity is influenced by both species abundance-based diversity and greater differences among species at the MDF edge than at the DDF edge. RaoQ is the sum of similarities between features among species present in the community; thus, the introduction of a new species into the community increases the species abundance-based diversity, but may reduce the average dissimilarity among species ([5]). High-disturbance regimes, caused by forest fire and adjacent maize agriculture in our study site, can remove species with traits that are poorly adapted to altered conditions, while allowing the recovery of better-adapted species ([33]). Therefore, disturbance may explain the recovery of species that are better adapted to changes in the edge environment, such as those with high SLA (e.g., Pterocarpus macrocarpus, Dalbergia cultrata, and Phanera bracteate), and high LDMC and large leaves (e.g., Xylia xylocarpa, Schleichera oleosa, and Chukrasia tabularis), along the MDF edge, as well as species with dense wood and thicker leaves, such as Dipterocarpus obtusifolius, Shorea siamensis, and Buchanania lanzan, along the DDF edge.
CWMs of trait values (i.e., species trait values weighted by their relative abundance) are commonly used to characterize functional dominance ([45]), which can be helpful for identifying patterns related to community function ([35]). Dominant traits can represent ecosystem resistance and resilience to environmental changes or disturbances; CWMs can also indicate environmentally mediated fitness differences among species with different functional strategies ([35]). In the present study, traits specifically related to the leaf economic spectrum (e.g., SLA) were useful for contrasting the DDF and MDF forest edge communities, reinforcing the importance of these traits in comparative plant ecology ([50]). Leaf and stem traits are involved in the leaf and stem economic spectrum, which is a balance between energy conservation and acquisition ([50], [8]) that is strongly driven by a set of coordinated functional traits ([12]). Our CWM results suggested that the MDF edge community is dominated by acquisitive traits (i.e., high SLA and low WD and LT) rather than conservative traits (i.e., high LDMC). In contrast, the DDF edge community is dominated by conservative traits (i.e., low SLA and high WD and LT) rather than acquisitive traits (i.e., low LDMC), and its traits tended to be associated with productive and highly disturbed environments ([41]).
Edaphic factors affecting functional traits of seasonally dry forests
Forest edge disturbance, combined with fragmentation, affects the prevalence of functional strategies among species in tree communities, demonstrating that chronic disturbance affects trait-environment relationships ([20]). Our results indicated changes in the prevalence of species with specific functional traits in response to soil conditions, which is consistent with previous findings that variation in soil fertility is a key factor affecting tree assemblages in deciduous tropical forests ([7], [4]). The differences between MDF and DDF edge sites were adequately explained by RLQ and fourth-corner analysis results, which suggested that plant trait-soil relationships are sufficiently explained by plant species composition at these sites; notably, plant species composition is driven by the dominant tree species. Thus, the traits of dominant plant species have influenced soil conditions in these sites, and these feedbacks appear to be beneficial in seasonally dry forest edge environments.
The DDF edge site is dominated by spe-cies with denser wood and thicker leaves, which were positively correlated with sand content and soil bulk density (i.e., low soil water) and negatively correlated with soil nutrient contents. These relationships indicated a preference for slow-growing species with greater resilience due to the poor soil at this site (i.e., soil with low water and nutrient contents). Accordingly, previous studies suggested that high stem density and thicker leaves were related to drought tolerance, because they were correlated with high resistance to hydraulic failure under low-water conditions ([39]). The prevalence of more conservative traits (i.e., thicker leaves and denser wood) in less fertile soils may be a result of selection for increased nutrient-use efficiency due to poor soil fertility ([39]). At the MDF edge site, soil nutrient (i.e., N and Ca) and clay and silt contents (i.e., high soil water) were positively correlated with the presence of dominant species with high SLA; these were present in combination with species that had high LDMC and larger leaves, which were positively correlated with OM. These findings indicated that the tree community in the MDF edge area was a complex mixture of faster-growing species (with high SLA and larger leaves) and slower-growing species (with high LDMC). In the MDF site, the functional characteristics that distinguish these strategies are generally related to variation in leaf longevity and nutrient-use efficiency, which constitute a balance between LDMC and SLA. The MDF is also characterized by larger leaves, which are related to soil nutrient stresses and high disturbance levels ([43]). Tree communities with high SLA are frequently found in habitats with good nutrient supply that are characterized by rapid nutrient use ([17]) and are linked to higher growth rates and greater photosynthetic competition ([23]). Thus, the high SLA observed at the MDF site suggests that greater drawdown of internal nutrients is favored to support faster growth and greater water use rates for a specific photosynthetic rate. We observed the reverse conditions at the DDF site, which is characterized by species with high LDMC, associated with OM availability. OM maintains soil structure, especially in fine-textured soil with high cation exchange capacity ([37]). Increased OM during the wet season leads to increased plant growth in DDFs ([29]). This suggests that the leaf traits, such as LA, LDMC, and SLA, of the dominant species of a seasonally dry forest edge may be constrained by soil nutrients.
Conclusion
We found that MDF and DDF forest edges are undergoing a combined recovery process involving characteristic plant strategies and traits. The DDF edge is dominated by species with denser wood and thicker leaves, which were positively associated with sand content and soil bulk density and negatively correlated with soil nutrient contents. These traits show a preference for resilient species in the poorer soil of this site (i.e., low nutrient contents). In contrast, at the MDF edge, soil nutrients (i.e., N and Ca) and clay and silt contents were positively associated with high SLA in the dominant species, whereas dominant species with high LDMC and larger leaves were positively correlated with OM. These traits indicate a preference for species complexity in the fertile soil of this site. These results show that the impacts of edge effects in seasonally dry forests depend on soil conditions and plant traits. As such, prioritizing stable soil conditions and monitoring the recovery process in DDF and MDF edges may improve vegetation community recovery in seasonally dry forest edges.
Acknowledgments
This research was made possible by the assistance of students from the Department of Agroforestry, Phrae Campus, Maejo University, Thailand. We thank the academic and research staff of Phrae Campus for allowing us to conduct this study on their grounds. This study was supported by funds provided by BEDO, the Biodiversity-based Economy Development Office, Bangkok, Thailand, contract no. BEDO.-NRCT.21/2017.
References
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Rungrawee Taweesuk
Torlarp Kamyo 0000-0002-9134-9763
Department of Agroforestry, Maejo University, Phrae Campus, Phrae 54140 (Thailand)
Corresponding author
Paper Info
Citation
Asanok L, Taweesuk R, Kamyo T (2022). Edge tree functional traits and their association with edaphic factors in seasonally dry forests in northern Thailand. iForest 15: 273-280. - doi: 10.3832/ifor3870-015
Academic Editor
Michele Carbognani
Paper history
Received: May 12, 2021
Accepted: May 26, 2022
First online: Jul 26, 2022
Publication Date: Aug 31, 2022
Publication Time: 2.03 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2022
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 30167
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 25476
Abstract Page Views: 2506
PDF Downloads: 1734
Citation/Reference Downloads: 2
XML Downloads: 449
Web Metrics
Days since publication: 1318
Overall contacts: 30167
Avg. contacts per week: 160.22
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2022): 2
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Environmental niche and distribution of six deciduous tree species in the Spanish Atlantic region
vol. 8, pp. 214-221 (online: 28 August 2014)
Research Articles
Short-term effects in canopy gap area on the recovery of compacted soil caused by forest harvesting in old-growth Oriental beech (Fagus orientalis Lipsky) stands
vol. 14, pp. 370-377 (online: 10 August 2021)
Research Articles
Concordance between vascular plant and macrofungal community composition in broadleaf deciduous forests in central Italy
vol. 8, pp. 279-286 (online: 22 August 2014)
Research Articles
Relationships between overstory and understory structure and diversity in semi-natural mixed floodplain forests at Bosco Fontana (Italy)
vol. 9, pp. 919-926 (online: 21 August 2016)
Research Articles
Strong relationships between soil and vegetation in reference ecosystems of a riparian Atlantic rainforest in the upper Doce River watershed, southeastern Brazil
vol. 16, pp. 226-233 (online: 17 August 2023)
Research Articles
Day and night respiration of three tree species in a temperate forest of northeastern China
vol. 8, pp. 25-32 (online: 26 May 2014)
Research Articles
Fine root morphological traits and production in coniferous- and deciduous-tree forests with drained and naturally wet nutrient-rich organic soils in hemiboreal Latvia
vol. 16, pp. 165-173 (online: 08 June 2023)
Short Communications
The importance of forest type when incorporating forest edge deposition in the evaluation of critical load exceedance
vol. 2, pp. 43-45 (online: 21 January 2009)
Research Articles
A comparison between stomatal ozone uptake and AOT40 of deciduous trees in Japan
vol. 4, pp. 128-135 (online: 01 June 2011)
Research Articles
Retranslocation of foliar nutrients of deciduous tree seedlings in different soil condition under free-air O3 enrichment
vol. 9, pp. 835-841 (online: 17 June 2016)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword