Verticillium wilt of Ailanthus altissima in Italy caused by V. dahliae: new outbreaks from Tuscany
iForest - Biogeosciences and Forestry, Volume 13, Issue 3, Pages 238-245 (2020)
doi: https://doi.org/10.3832/ifor3238-013
Published: Jun 19, 2020 - Copyright © 2020 SISEF
Research Articles
Abstract
Verticillium spp., including V. nonalfalfae and V. dahliae, are known vascular wilt pathogens of the invasive Ailanthus altissima (tree-of-heaven) in the United States and in Europe. Herein we provide evidence of the presence of a previously unreported wilt disease of A. altissima in Tuscany (Central Italy). Several isolates were collected from two locations and identified as V. dahliae, based on microscopical features of conidiophores, conidia and microsclerotia. Genomic DNA was extracted from the mycelium, the ITS region was amplified and the sequence was deposited in GenBank as VdGL16 (accession no. MK474459). BLASTn analysis showed 100% similarity with V. dahliae. To confirm pathogenicity of VdGL16, inoculations of Ailanthus seedlings were performed with the root dipping technique whereas mature trees were stem-inoculated. All inoculated seedlings exhibited wilt symptoms after 20 days, while mature Ailanthus trees showed wilting and dieback after six months. The pathogen was easily re-isolated from seedlings and re-identified as V. dahliae, thus satisfying Koch’s postulates. Results from intraspecific resistance screening of nine seed sources from across Italy revealed that Ailanthus provenances from all the six sampled regions were susceptible to V. dahliae. Stem inoculated adult plants exhibited abundant production of epicormic sprouts along the stem within six months, and most of these sprouts wilted following initial dieback of the main stem; furthermore, sprouting from the crown was intense. Petioles and rachises tissues of leaves fallen from infected trees were a good source for re-isolation of the pathogen; we proved that such petioles and rachises can effectively transfer the fungus to healthy Ailanthus seedlings via root infections. Host-specificity of the V. dahliae isolate VdGL16 was also determined on 40 non-target species/varieties/cultivars. The isolate caused disease in herbaceous species belonging to five botanical families: Asteraceae, Lamiaceae, Leguminoseae, Linaceae and Solanaceae. Given the difficulties in countering Ailanthus invasion with mechanical and chemical methods, the biological control using Verticillium may provide an efficient, low cost and sustainable control of this invasive species.
Keywords
Tree-of-heaven, Verticillium dahliae, ITS Region, Accession Number MK474459, Koch’s Postulates, Biocontrol
Introduction
Rapid growth rate ([21]), prolonged and prolific seed production ([56]), allelopathy, clonal proliferation and resistance to herbivory combined with tolerance to environmentally stressful conditions ([26]) make Ailanthus altissima (Mill.) Swingle (also known as tree-of-heaven, Simaroubaceae) a highly invasive species. Ailanthus is an exceptional invader, able to quickly occupy transportation corridors and fallow lands, as well as of natural environments, displacing native vegetation important for biodiversity and damaging infrastructures and archaeological sites ([10], [17], [5], [38]). Native from Eastern Asia, Ailanthus was first introduced into Europe around 1750 ([52]). This species became naturalized on nearly all continents, and now represents a widespread problem in areas where it occurs ([26]). Due to its unpalatability, it rapidly replaces the indigenous flora, jeopardizing the conservation of native biocenoses, and forcing difficult (and usually useless) eradication campaigns ([17], [10]). The growth characteristics of tree-of-heaven make it particularly difficult to control. Cutting the trunk rapidly stimulates multiple root sprouts and young runners even at long distance from the parent tree, so to form clonal stands after disturbance. The above-mentioned actions usually have been accompanied by the periodical use of systemic (and non-selective) chemical herbicides, such as glyphosate, that can be transported to the root system and compromise (but usually only partly) future vegetative renewal ([8]). The use of herbicides is expensive and laborious, requiring repetitive applications, often ineffective against the resprouting ability of Ailanthus ([3]), not to mention the negative impact on non-target vegetation ([27]). Moreover, current approval of glyphosate will expire in 2022, and the use of such herbicides in Europe will face tougher restrictions going forward ([53]). For instance, the Italian PAN (Action plan for the sustainable use of plant protection products - [36]) is leading to serious limitations in the use of chemical pesticides on roads and in urban areas (Action A.5.6); more specifically, Point A.5.6.1 (“Use of herbicide products”) states that “weed-killer treatments are banned and have to be replaced with alternative methods in population centers”. Control of Ailanthus is a major concern because of the lack of long-term conventional methods to limit its invasion. There are ecological, sanitary, economic, and cultural reasons urging the adoption of effective measures of eradication different from chemical herbicides. This is leading to consider the biological control as a possible strategy to counteract the otherwise unrestrainable spread of Ailanthus ([48]).
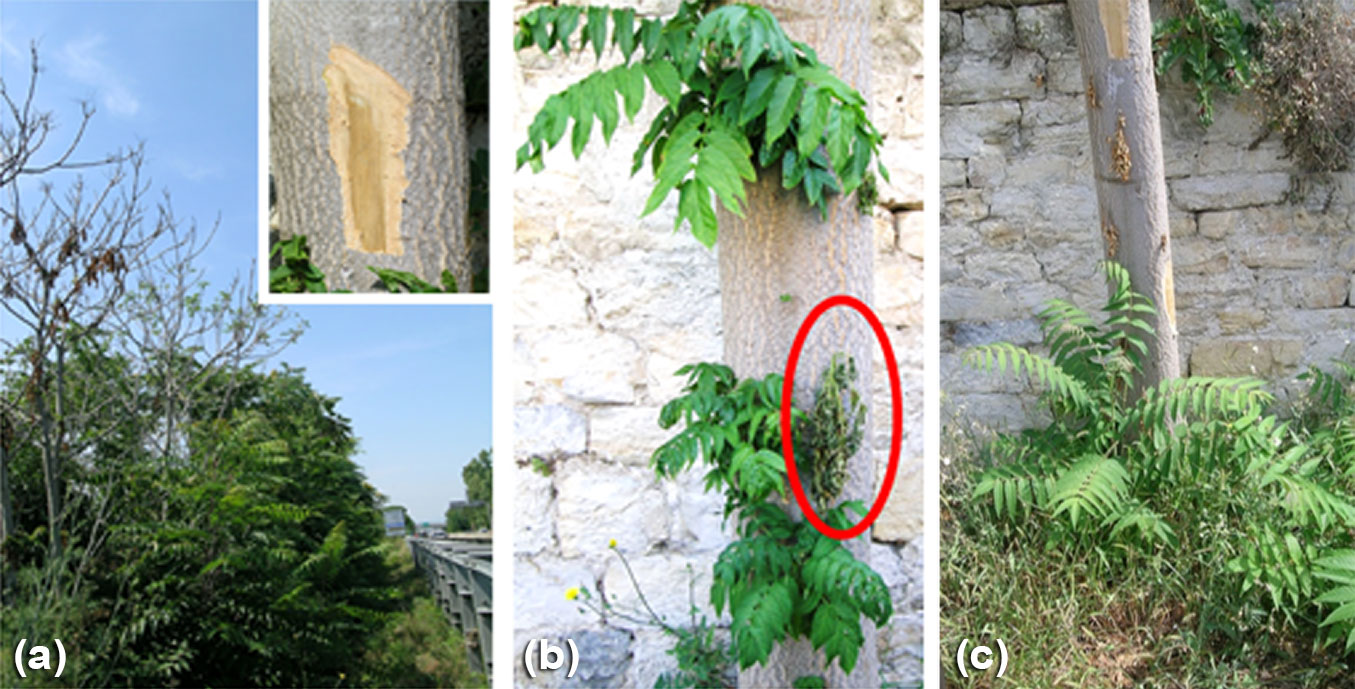
Over the past five years, our group is conducting cursory field observations in Ailanthus populations in several Italian regions looking for candidate mycoherbicide(s) ([29]). During summer 2016, dying Ailanthus suckers were observed in Leghorn (Tuscany, Central Italy, 3 m a.s.l.) that exhibited a typical wilt syndrome, with heavy defoliation and brownish vascular discoloration. Foliar symptoms ranged from slight or sectored yellowing to browning, necrosis and eventual leaf abscission. In spring 2019 a second outbreak was observed about 3.5 km far from the previous one, involving adult plants (Fig. 1a).
Fig. 1 - (a) Symptoms of Verticillium wilt in a natural stand of Ailanthus altissima, including, defoliation, dieback and mortality; (b) abundant production of epicormic sprouts along the stem of an adult Ailanthus tree, following stem inoculation with Verticillium dahliae isolate VdGL16; please note wilting of the encircled sprout; (c) vigorous sprouting from the base of a dying mature Ailanthus tree.
The objectives of the present study were to: (i) identify the pathogen involved in the aforementioned cases; (ii) compare the susceptibility of Ailanthus seedlings grown from seeds collected from various locations across Italy to the isolate; and (iii) evaluate the risk exposure by the pathogen for selected non-target species through artificial root inoculations.
Materials and methods
Pathogen isolation and morphological characterization
Stem samples were collected from symptomatic individuals and petioles and rachises were gathered from the ground around wilting plants in the field. Bark was removed from stem samples. Stem, petiole and rachis samples were cut into 1-cm pieces, surface sterilized with sodium hypochlorite (NaOCl) 0.5% in water for 5 min, and carefully rinsed in distilled sterile water. Small pieces of discolored tissues were excised with a lancet and placed in Petri dishes onto potato dextrose agar (PDA - Sigma-Aldrich, Milan, Italy) amended with streptomycin sulphate (0.1 g l-1 - Gold Biotechnology, Saint Louis, MO, USA). Dishes were incubated at 23 °C under 12 h light/12 h dark, for 15 days. Morphological diagnosis was carried out by observing mycelium and reproductive structures under a stereo microscope (Leica S9®, Leica Microsystems, Buccinasco, Italy) and under a transmitted light/fluorescence contrast microscope (Leica DM4000® B led). Photomicrographs were taken with a Canon PowerShot S50® camera.
Molecular identification
Total genomic DNA was extracted from fresh mycelial plugs, originating from mycelia grown on and harvested from PDA, using the cetyltrimethylammonium bromide (CTAB) protocol, according to the method of Doyle & Doyle ([7]). Fungal tissue (0.1 g) was mixed with 0.5 ml of extraction buffer [1 M CTAB (pH 5); 1 M Tris-HCl (pH 8); 0.5 M ethylenediaminetetraacetic acid (EDTA; pH 8); 5 M NaCl and polyvinylpyrrolidone (PVP 40; 1 g)] and incubated for 30 min at 65 °C. After adding 0.5 ml of chloroform:isoamylic alcohol (24:1 v/v), the mixture was centrifuged at 15.000 g for 15 min at 4 °C, and an equal volume of cold isopropyl alcohol was added to the obtained upper phase in order to favour DNA precipitation. The pellet obtained after centrifugation (15.000 g for 20 min at 4 °C) was washed twice with 70% ethanol (v/v) and dissolved in DNase-free water. DNA extracted was valued with electrophoresis in 1% (w/v) agarose gel and stained with Gel Red® Nucleic Acid Stain (Biotium, Fremont, CA, USA). Amplification of Internal Transcribed Spacer (ITS) region was performed in a Rotor-Gene Q® (QIAGEN, Hilden, Germany) using a standard polymerase reaction (PCR) protocol with the primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′ - Eurofins Genomics, Ebersberg, Germany), according to White et al. ([58]). The PCR reaction mixture (20 μl) included 5 μl DNA template, 10X DreamTaq® Buffer (Thermo Fisher Scientific, Rodano, Italy) with 25 mM MgCl2, 0.2 mM dNTPs (Euroclone, Milan, Italy), 0.5 mM of each primer, 0.025 U μl-1 DreamTaq® polymerase (Thermo Fisher Scientific, Rodano, Italy) and sterilized MilliQ water. PCR setup for amplification was: 5 min at 94 °C, 35 cycles of 45 s at 94 °C (denaturation), 45 s at 55 °C (annealing) and 45 s at 75 °C (elongation); 7 min at 72 °C (final extension). PCR products were detected by electrophoresis in a 1% (w/v) agarose gel, stained with Gel Red® Nucleic Acid Stain, then purified with Wizard SV Gel and PCR Clean-UP® system (Promega, Madison, WI, USA) and sequenced according to the Sanger method (MWG Biotech, Eberberg, Germany). Identification was carried out with BLASTn software (NCBI, Bethesda, MD, USA).
Artificial inoculations
Liquid cultures in Czapek medium (250 ml in 500 ml Erlenmeyer flasks) of the pathogen isolated as described above were incubated on an orbital shaker (711 CT®, Asal, Milan, Italy - 150 rpm) under room conditions for three days. Conidia produced from these cultures were obtained by filtering through layers of sterile cheesecloth and counted with a Bürker chamber. Finally, inoculum concentrations were adjusted to approximately 0.8-1 × 107 conidia ml-1. These conidial suspensions were used for root and stem inoculations. Moreover, petioles and rachises were collected from the soil beneath wilting trees, stored for 3-4 weeks at room temperature, cut into 1-2 cm pieces and mixed thoroughly with a standard potting medium (peat: perlite - 1:1 in vol.). Ten six-month-old Ailanthus seedlings were transplanted into pots filled with this medium and grown in a greenhouse (Tab. 1).
Tab. 1 - Symptoms, defoliation and success of re-isolations of Verticillium dahliae on six months-old Ailanthus seedlings inoculated with petiole and rachis tissues obtained from infected trees.
| Seedling | Symptoms | Defoliation | Re-isolations |
|---|---|---|---|
| 1 | Yellowing | None | No |
| 2 | Wilting | Partly | Yes |
| 3 | No symptoms | None | No |
| 4 | Wilting | Partly | Yes |
| 5 | Yellowing | None | No |
| 6 | Yellowing | Partly | No |
| 7 | Death | Partly | Yes |
| 8 | Death | Totally | Yes |
| 9 | No symptoms | None | No |
| 10 | Death | Totally | Yes |
Susceptibility of Ailanthus seedlings from various seed sources
Ailanthus mature seeds were collected during 2016 to 2018 from a total of nine locations in six Italian regions (Tab. S1 in Supplementary material). Upon arrival, seeds were air dried for 1-2 weeks, placed in paper bags, and stored at room temperature. Up to 50 seeds from each seed source were placed in terracotta bowls containing the standard potting medium described above. Containers were maintained in a greenhouse and regularly watered. Following germination, 10 to 15 seedlings from each seed source were singly transplanted into 500 ml-plastic pots containing the same substrate added with a commercial slow release fertilizer and grown for 4 to 6 months. Inoculation of seedlings was conducted using a root-dip method ([43]). Plants (eight individuals per source) were carefully uprooted from the original substrate, and their roots were thoroughly washed in tap water without intentional wounding, and then submerged in the inoculum suspension for 20 min. Plants were individually transplanted into 20 × 20 cm plastic pots, and 2 ml of additional inoculum suspension pipetted onto the base of each stem. Control plants were “inoculated” dipping them in sterile Czapek solution. Following inoculation, plants were watered as needed and disease severity was evaluated weekly (for around three months) using an ordinal 0-4 rating system, according to the percentage of affected leaves and twigs (0 = no symptoms; 1 = 1-33%; 2 = 34-66%; 3 = 67-99%; 4 = dead plant - [42]). The infection index (or McKinney’s index), which incorporates both the incidence and severity of the disease, was expressed as the weighted means of the disease as a percentage of the maximum possible level ([1]). Symptomatic tissues were plated onto PDA amended with streptomycin sulphate for detection of Verticillium.
Susceptibility of mature Ailanthus trees
Five mature Ailanthus trees in a private garden were stem-inoculated at breast height. Trunk was horizontally punched with an electric drill with a sterilized drill bit, so to produce a 6 mm-hole that completely pierced the stem. Afterwards, ten ml of conidial suspension (see above) were injected with a syringe inside the hole. A rectangular Parafilm M® laboratory film sheet was used for impeding the outflow of the conidial suspension through the other side of the hole sealing it up by wrapping the stem. Other three plants were managed in the same way and “inoculated” with sterile Czapek solution. Ailanthus mature trees were monitored for around one year from inoculation.
Interspecific host range testing
To determine if fungal strain VdGL16 might be pathogenic on other species than Ailanthus, artificial inoculations were performed between May 2018 and August 2019 (i.e., at the same time or close to the inoculations of Ailanthus seedlings) in the greenhouse on potted seedlings/saplings of 40 species/varieties/cultivars (at least eight individuals of each species/variety/ cultivar were tested for around three months from inoculation - Tab. 2). Herbaceous plants were container-grown from seeds and the seedlings of woody species were obtained from a local tree nursery. The same procedures as described before were followed to grow plants, inoculate roots and to evaluate plant responses. Inoculations of species used for the host-range testing were performed under the same greenhouse conditions as inoculations of Ailanthus seedlings.
Tab. 2 - Host range pathogenicity of Verticillium dahliae isolate VdGL16 following artificial inoculations by root dipping. The fourth column represents the McKinney’s index (MI), based on an ordinal 0-4 rating system.
| Family | Species / var. / cv | Susceptibility | MI (%) | Re-isolation |
|---|---|---|---|---|
| Asteraceae | Cichorium endivia var. Latifolium | No | - | No |
| Asteraceae | Cichorium intybus var. Pan di zucchero | No | - | No |
| Asteraceae | Diplotaxis tenuifolia | No | - | No |
| Asteraceae | Helianthus annuus | No | - | No |
| Asteraceae | Lactuca sativa cv Sant’Anna | No | - | Yes |
| Asteraceae | Leuchantemum vulgare | Yes | 85 | Yes |
| Asteraceae | Tagetes patula | No | - | Yes |
| Brassicaceae | Raphanus sativus | No | - | Yes |
| Brassicaceae | Sinapis alba | No | - | No |
| Caprifoliaceae | Viburnum lantana | No | - | No |
| Cucurbitaceae | Citrullus lanatus cv Sugar baby | No | - | No |
| Cucurbitaceae | Cucurbita pepo var. Romanesco | No | - | No |
| Fagaceae | Quercus cerris | No | - | No |
| Fagaceae | Quercus ilex | No | - | No |
| Lamiaceae | Ocimum basilicum var. Citriodorum | No | - | No |
| Lamiaceae | Ocimum basilicum var. Napoletano | No | - | No |
| Lamiaceae | Ocimum basilicum var. Red rubin | No | - | Yes |
| Lamiaceae | Ocimum basilicum var. Tigullio | No | - | No |
| Lamiaceae | Ocimum basilicum var. Verde italiano | Yes | 59 | Yes |
| Lamiaceae | Lavandula sativa | No | - | No |
| Leguminoseae | Cicer arietinum | Yes | 94 | Yes |
| Leguminoseae | Hedysarum coronarum | Yes | 94 | Yes |
| Leguminoseae | Medicago sativa cv Itaca | No | - | - |
| Leguminoseae | Phaseolus vulgaris var. Nano dolico dall’occhio | No | - | No |
| Leguminoseae | Trifolium repens | No | - | No |
| Leguminoseae | Trifolium subterraneum | Yes | 91 | Yes |
| Leguminoseae | Vicia faba var. major cv Aguadulce | No | - | No |
| Leguminoseae | Vicia faba var. minor | Yes | 91 | Yes |
| Linaceae | Linum usitatissimum | Yes | 97 | Yes |
| Lythraceae | Punica granatum cv Parfianca | No | - | No |
| Magnoliaceae | Liriodendron tulipifera | No | - | No |
| Oleaceae | Olea europaea cv Leccino | No | - | No |
| Sapindaceae | Acer rubrum | No | - | No |
| Solanaceae | Capsicum annuum cv Quadrato d’Asti | No | - | No |
| Solanaceae | Solanum lycopersicum cv Canestrino | No | - | No |
| Solanaceae | Solanum lycopersicum cv Roma | No | - | No |
| Solanaceae | Solanum melongena cv Violetta di Rimini | Yes | 91 | Yes |
| Solanaceae | Solanum melongena cv Black beauty | Yes | 91 | Yes |
| Solanaceae | Solanum melongena cv Viola lunga | Yes | 84 | Yes |
| Vitaceae | Vitis vinifera cv Sangiovese | No | - | No |
| Simaroubaceae | Ailanthus altissima | Yes | 98 | Yes |
Results
Pathogen identification
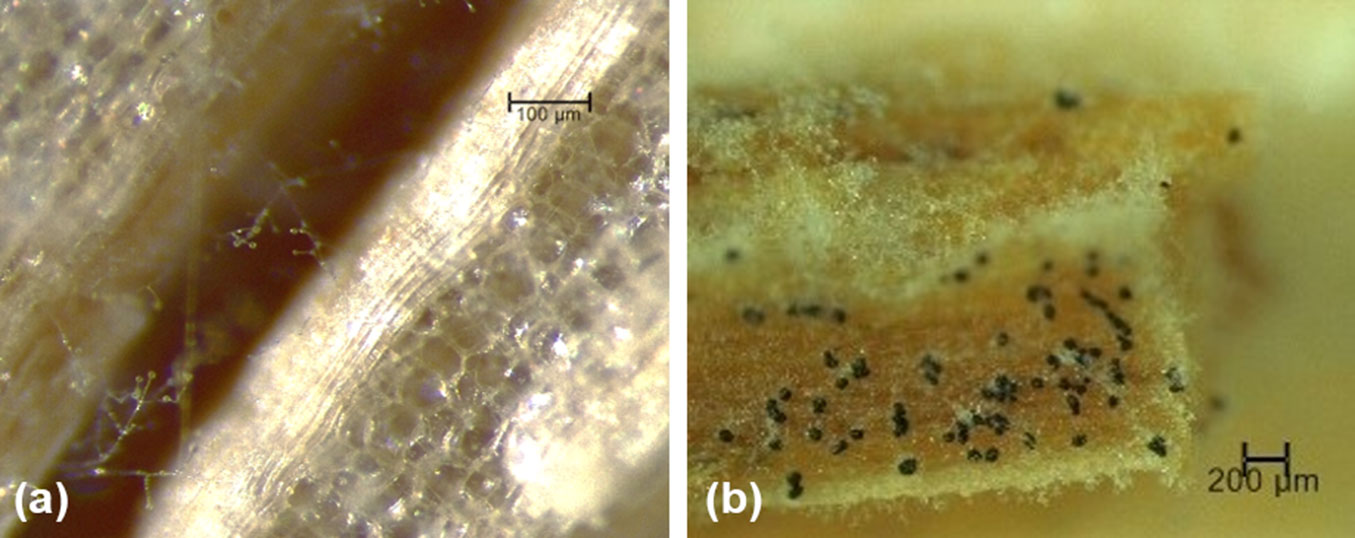
Fungal isolates from symptomatic plants were identified morphologically as a putative pathotype of the genus Verticillium, based on microscopical observation of: (i) hyaline and non-septate hyphae; (ii) verticillate conidiophores; (iii) cylindrical or ellipsoid 1-celled conidia (mean ± SD: 3.8 ± 1.1 μm × 1.8 ± 0.6 μm, n = 50); and (iv) presence of melanized microsclerotia (20 to 100 μm) in woody tissues (Fig. 2) and on PDA dishes ([41], [18]). BLASTn search at VertShield database, an online resource that supports Verticillium research and species identification, confirmed the putative Verticillium isolate (from now on identified as VdGL16) as V. dahliae, matching 100% similarity with other V. dahliae GenBank strains (e.g., MKO939 77, MH392569 and MG910491). ITS sequence has been deposited in GenBank with the accession number MK474459 (February 2019). A standard PCR using specific primers (designed with Primer 3 software) Vert-1F (Vert1F: 5′-GTTGGTGAACCAGCGGAGGG-3′) and Vert-1R (Vert1R: 5′-AGGGTTGAAACGACGCTCGGA-3′) was carried out in order to check the match. PCR setup for amplification was: 2 min at 94 °C, 32 cycles of 19 sec at 94 °C (denaturation), 20 sec at 55 °C (annealing) and 60 sec at 75 °C (elongation); 6 min at 72 °C (final extension).
Fig. 2 - Conidiophores (a) and microsclerotia (b) of Verticillium dahliae developed on woody tissue of symptomatic Ailanthus tree.
Microsclerotia and/or conidiophores and conidia of V. dahliae were microscopically detected in 16 out of 26 (61.5%) leaf petioles and rachises collected from the soil beneath an infected tree.
Artificial inoculations of Ailanthus seedlings from different seed sources
Our results indicate that V. dahliae strain VdGL16 is pathogenic to Ailanthus with 9 of 9 seed sources from six regions showing susceptibility. All inoculated Ailanthus plants grown from seeds exhibited vascular discoloration, wilt symptoms and defoliation within 3-4 weeks while control individuals remained asymptomatic. The pathogen was consistently re-isolated from symptomatic seedlings, and morphological characteristics of the resulting colonies were identical to VdGL16.
Artificial inoculations of Ailanthus mature trees and seedlings inoculated with petioles
Within six months from stem inoculation with VdGL16, all five Ailanthus mature trees inoculated in a private garden exhibited abundant production of epicormic sprouts along the stem, and some of these sprouts wilted following initial dieback of the main stem (Fig. 1b). Vigorous sprouting from the base of the trunk of an inoculated mature tree was observed (Fig. 1c).
Artificial inoculations based on petioles and rachises as inoculum for six-month-old Ailanthus seedlings showed that after 3-4 weeks 8 out of 10 inoculated individuals showed typical symptoms such as wilt, defoliation and dieback (rate of mortality: 30%). V. dahliae was isolated successfully from 50% of these plants (Tab. 1).
Host range analyses
In addition to Ailanthus provenances, 40 woody and herbaceous species / varieties / cultivars were tested for susceptibility to artificial inoculations with Verticillium dahliae VdGL16. Results are summarized in Tab. 2. Ten (25%) of these sources exhibited vascular discoloration, wilt and dieback and V. dahliae was easily reisolated from them (Fig. S1 in Supplementary material). They belong to five botanical families: Asteraceae, Lamiaceae, Leguminoseae, Linaceae and Solanaceae. All of the susceptible plants were herbaceous, whereas none of the woody species tested was responsive to VdGL16. The behaviour of Ocimum basilicum (sweet basil) deserves attention: five commercial varieties were tested, and one of them (Verde italiano) was susceptible (McKinney Index = 59%); another three (Citriodorum, Napolitano and Tigullio) exhibited no outward symptoms and fungal re-isolation was not successful whereas a tolerant host response was observed in the variety Red rubin, where Verticillium was recovered from apparently healthy inoculated individuals. In contrast, no cultivarietal differential response was observed in Solanum melongena (eggplants: three cultivars tested, all of them highly susceptible) and in Solanum lycopersicum (tomato: two cultivars assayed, both non responsive). From the two Trifolium species tested, T. repens proved to be resistant (no symptoms, no re-isolation), whereas T. subterraneum was highly susceptible (McKinney Index 91%).
Discussion and conclusive remarks
Verticillium wilt of tree-of-heaven has appeared sporadically in the past in the phytopathological literature. The first report of a disease causing Ailanthus decline and death in Europe was at the end of the XIX century, in Paris, but no pathogen was then recognized ([30] - V. dahliae was firstly described in 1913, cit. in [18]). The same outbreak was investigated in detail three decades later by Arnaud & Barthlet ([2]), who ascribed the case to V. dahliae with an exhaustive treatise including chapters on histopathology, epidemiology and physiological plant pathology. In this context, the description of the presence of mycelium in the foliar petioles and rachises of infected plants is particularly noteworthy and an unprecedented aspect at the time. The very first report from Italy of Ailanthus decline is due to G. Goidànich, who in 1935 described the presence of two mature trees affected by Verticillium “near the railway station of Loano, in Liguria” ([11]). In the meantime, the literature reports the first cases from Eastern United States, such as Pennsylvania, Virginia and New York ([46], [12], cit. in [22]), showing Ailanthus as one of the earliest known perennial hosts of Verticillium wilt (due to V. albo-atrum sensu lato - [9]) in the United States. During the 1990s, Verticillium wilt of Ailanthus was observed in Greece (caused by V. dahliae - [50]) and in Austria (causal species not determined - [6]). After that, the issue has been neglected until 2005, when the research team led by D. Davis started to study widespread mortality of Ailanthus in the Eastern United States ([47]), whose causal agent was identified as the newly described species V. nonalfalfae previously classified as V. albo-atrum and morphologically indistinguishable from this ([18]). Ailanthus wilt caused by V. nonalfalfae was also reported in Ohio and Virginia ([44], [51]). Results of a survey in eastern Austria ([33], [35]) indicated a widespread occurrence of V. dahliae and a rare occurrence of V. nonalfalfae on declining Ailanthus natural stands. Recently, Longa et al. ([28]) reported a lethal outbreak of Ailanthus in Northern Italy (Eastern Italian Alps) and identified V. dahliae as the causal agent. A similar report was given from Izsépi et al. ([20]) from Hungary and the impact of V. dahliae on A. altissima was recently observed and assessed in Virginia, USA ([4]).
Here, we provide the first evidence of Verticillium wilt on A. altissima in Central Italy (Tuscany). Several isolates were collected from two locations and identified as V. dahliae, based on microscopial features of conidiophores, conidia and microsclerotia, as well as by molecular analysis (VdGL16 is the isolate deposited in GenBank). The detection of V. dahliae on wilting Ailanthus in Italy supports the hypothesis of Inderbitzin & Subbarao ([19]) that the natural spread of V. nonalfalfae is likely confined to areas with temperate climate. However Maschek & Halmschlager ([34]) have clearly demonstrated the co-existence of V. dahliae and V. nonalfalfae in close vicinity. Furthermore, differences in detection frequency between the widely distributed V. dahliae and the rarely occurring V. nonalfalfae might explain the fact that V. nonalfalfae has not been detected yet on Ailanthus in Italy.
In our studies, Koch’s postulates were fulfilled using VdGL16, and both Ailanthus seedlings (from nine seed sources collected in six Italian regions) as well as mature trees inoculated with our isolates showed wilt symptoms and defoliation, with mature trees also showing formation of epicormics sprouts along the stem that also wilted (this does not seem to be a general rule, and this has sometimes been related to a high dosage of conidial inoculum - [41]). These symptoms were already described for both V. dahliae ([41]) and V. nonalfalfae ([22]).
Therefore, the potential of these Verticillium species as biocontrol agents to counteract the highly invasive Ailanthus might deserve attention, given the need of effective, affordable non-chemical biocontrol agents. To be clear: biological plant protection products also need to be registered according to EU legislation, and the application of these products/biological agents in the field requires authorisation of each study plot by the national plant protection authority as long as the product has not been officially approved as plant protection product. This should be positively evaluated in terms of “augmentative biological control” ([16]), with emphasis on endemic host-adapted pathogens such as Verticillium (e.g., the selected and thoroughly tested strains of V. nonalfalfae or the V. dahliae VdGL16 described here), provided that its pest-risk assessment is regarded as positive. The huge potential of selected strains of V. nonalfalfae as biocontrol agents against invasive A. altissima was already demonstrated in the United States ([22], [23], [39], [40], [47]) and in Austria ([32], [33]). Moreover, a commercial product based on a fairly specific strain of V. nonalfalfae has been placed on the market in Austria in 2019 ([13]).
According to Inderbitzin et al. ([18]) V. nonalfalfae is genetically related to V. dahliae but differs morphologically by the formation of resting mycelium (characterized by a shorter life-span) instead of the formation of microsclerotia (which can persist up to 14 years in the soil) that are found in V. dahliae. Furthermore, V. nonalfalfae has a greater aggressiveness and effectiveness compared to V. dahliae ([14], [49], [47]), and due to the short life-span of resting mycelium and a rapid host mortality there may be less opportunities to infect other susceptible hosts ([34], [35]). Up to now, V. nonalfalfae has been found on a few hosts such as cotton, hop, petunia, potato, soil, spinach, tomato and wild celery, although more work would be needed to expand knowledge on its host range and distribution ([19]). Moreover, although intraspecific root grafts and clonal growth within Ailanthus stands have been easily demonstrated ([40]), natural spread of V. nonalfalfae seems to be more difficult than in V. dahliae.
On the contrary, V. dahliae has the greatest economic impact and is among the most widespread plant diseases worldwide ([24]). Although no exact statistics exist on the number of species that are susceptible to V. dahliae, it was estimated that at least 400 plant species, ranging from annuals to woody perennials, are affected ([25]). Large spread of V. dahliae is due to the fact that its microsclerotia can survive in the soil up to 14 years during the non-parasitic phase ([57], cit. in [25]), either as dispersed propagules or embedded within plant debris, mainly in the upper layer of the soil from where they can be easily spread by wind, rain or irrigation water, human and animal activities, and agricultural tools and machines ([41]). Due to its wide host range and long lasting persistence of microsclerotia in soil plant debris, comprehensive risk analyses have to be carried out (preferably in enclosed environments such as a greenhouse) in order to assess the potential of V. dahliae strain VdGL16 for the biological control of Ailanthus in the warmer Mediterranean basin.
Differences in pathogenicity and symptom development due to V. dahliae infections observed in different hosts might be attributed to: (i) differences in virulence as a pathogen attribute; (ii) different levels of tolerance in the plant/host; and/or (iii) a consequence of specific plant/pathotype interactions in the soil ([31]). Nevertheless, isolates of V. dahliae are considered host-adapted (rather than host-specific) since they were commonly pathogenic on different hosts but are more virulent to the host from which they are isolated ([31]). This was confirmed by the present study since the inoculated Ailanthus seedlings showed the highest disease severity (i.e., McKinney index), compared to the few non-target species that proved to be susceptible to VdGL16 strain in our host-range analyses. Among the 40 non-target species/varieties/ cultivars on which the virulence of the V. dahliae isolate VdGL16 was tested, only 25% were susceptible, all being herbaceous species belonging to five botanical families (Asteraceae, Lamiaceae, Leguminosae, Linaceae and Solanaceae), whereas no tree species was affected yet, though more tree species have to be investigated. Another interesting outcome of the present study was the fact that some of the tested hosts (i.e., Lactuca sativa cv Sant’Anna, Tagetes patula, Raphanus sativus, Ocimum basilicum var. Red rubin) were successfully colonized by VdGL16 but were lacking disease symptoms. Asymptomatic infections of V. dahliae have been already reported in the past, mainly in cereal crops and weeds ([31]) but also in other plant species (e.g., olive, red and sugar maple, and tulip-poplar trees - [23], [24]). This suggests that V. dahliae could colonize some plants without inducing visible symptoms, only becoming a reservoir of inoculum that could initiate epidemics of Verticillium wilt disease ([24]).
Petiole tissues of leaves fallen from infected trees were a good source for re-isolation of the pathogen in our case. In addition, we proved that some petioles and rachises can effectively transfer the fungus to healthy Ailanthus seedlings. Such a transfer of V. dahliae from diseased plants to the rizosphere of healthy plants by means of leaf petioles and rachises that contain microsclerotia has been shown for several tree species, such as Acer spp. ([59], [15]), Liriodendron tulipifera ([37]), Olea europaea ([54], [42]) and Fraxinus excelsior ([45]). As mentioned above, microsclerotia may survive for years in the soil and become available as inoculum for new infections ([24]). So, the role of windblown leaves originating from naturally or artificially infected Ailanthus plants in the medium-distance dispersal of V. dahliae deserves closer attention, because spread of the fungus might not only be limited to adjacent Ailanthus trees but might also occur to non-target species (as suggested by the fact that VdGL16 induced wilt disease in other ten tested species, in addition to Ailanthus).
To conclude, this study not only reports a Verticillium wilt disease of A. altissima in the warm Mediterranean basin, but also proposes to deserve attention to V. dahliae as a potential biological agent to counteract the highly invasive Ailanthus. Although V. dahliae is highly virulent, widely distributed and not host specific (conversely to V. nonalfalfae), it has been the only pathogen isolated from dying A. altissima in the Mediterannean basin so far. At the moment, only some herbaceous species of horticultural and forage concern have been proved to be susceptible to our strain, but more investigations need to be carried out, especially on tree crops of economic importance in Italy and already resulted susceptible to Verticillium, such as olive ([42], [24]) and kiwifruit, on which infection caused by Verticillium dahliae was recently observed in Turkey ([55]). The response of non-target species must be evaluated in a forceful pest-risk analysis for regulatory issues associated with the use of the pathogen in the open field.
Acknowledgements
This research was supported by funding from the University of Pisa, PhD innovative projects. We thank Dr. Mariagrazia Tonelli for assistance in the microscopical investigations and Dr. Ferruccio Filippi and Ms Simona Ciangherotti for helping in seed collection, running the greenhouse facilities and growing of test plants.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Alessandra Marchica 0000-0002-4634-2968
Rodolfo Bernardi 0000-0001-7513-7712
Antonella Calzone 0000-0001-8182-5351
Lorenzo Cotrozzi 0000-0002-4401-3896
Cristina Nali 0000-0002-8228-0258
Elisa Pellegrini 0000-0001-9237-5341
Giacomo Lorenzini 0000-0002-6371-5105
Department of Agriculture, Food and Environment of the University of Pisa, v. del Borghetto 80 - 56124 Pisa (Italy)
Corresponding author
Paper Info
Citation
Pisuttu C, Marchica A, Bernardi R, Calzone A, Cotrozzi L, Nali C, Pellegrini E, Lorenzini G (2020). Verticillium wilt of Ailanthus altissima in Italy caused by V. dahliae: new outbreaks from Tuscany. iForest 13: 238-245. - doi: 10.3832/ifor3238-013
Academic Editor
Alberto Santini
Paper history
Received: Sep 13, 2019
Accepted: Apr 18, 2020
First online: Jun 19, 2020
Publication Date: Jun 30, 2020
Publication Time: 2.07 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 42321
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 34607
Abstract Page Views: 4020
PDF Downloads: 2948
Citation/Reference Downloads: 3
XML Downloads: 743
Web Metrics
Days since publication: 2061
Overall contacts: 42321
Avg. contacts per week: 143.74
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 16
Average cites per year: 2.67
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Natural spread of Verticillium wilt as effective constraint on Ailanthus altissima invasion
vol. 18, pp. 391-398 (online: 22 December 2025)
Review Papers
Ulmus laevis in the Iberian Peninsula: a review of its ecology and conservation
vol. 8, pp. 135-142 (online: 07 August 2014)
Research Articles
Concordance between vascular plant and macrofungal community composition in broadleaf deciduous forests in central Italy
vol. 8, pp. 279-286 (online: 22 August 2014)
Research Articles
Temporal development of collar necroses and butt rot in association with ash dieback
vol. 10, pp. 529-536 (online: 05 May 2017)
Short Communications
Local spread of an exotic invader: using remote sensing and spatial analysis to document proliferation of the invasive Asian chestnut gall wasp
vol. 5, pp. 255-261 (online: 24 October 2012)
Research Articles
Effect of environmental gradients on leaf morphological traits in the Fandoghlo forest region (NW Iran)
vol. 13, pp. 523-530 (online: 13 November 2020)
Research Articles
Variation in resistance to the rust fungus Melampsora larici-populina Kleb. in Populus nigra L. in the Czech Republic
vol. 9, pp. 146-153 (online: 26 October 2015)
Review Papers
Dutch elm disease and elm bark beetles: a century of association
vol. 8, pp. 126-134 (online: 07 August 2014)
Research Articles
Phytopathogenic fungi in forest nurseries of Middle Siberia
vol. 13, pp. 507-512 (online: 05 November 2020)
Research Articles
Ectomycorrhizal fungal community in mature white poplar plantation
vol. 14, pp. 540-547 (online: 26 November 2021)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword