Temporal development of collar necroses and butt rot in association with ash dieback
iForest - Biogeosciences and Forestry, Volume 10, Issue 3, Pages 529-536 (2017)
doi: https://doi.org/10.3832/ifor2407-010
Published: May 05, 2017 - Copyright © 2017 SISEF
Research Articles
Abstract
In recent years collar necroses and butt rot associated with the ash dieback disease occurred with alarming frequency in Fraxinus excelsior. We analysed tree ring structures to identify the year of necrosis initiation on a set of 507 necroses on 155 stem discs from nine severely diseased south-western German stands. The number of first-time infections of trees was highest from 2010 to 2012 and slightly decreased in 2013 and 2014, whereas the total number of newly emerging individual necroses remained high. Logistic modelling of disease progression suggests that collar rot infection has almost reached its maximum incidence and that a fraction of trees will remain healthy at the root collar. On average, Hymenoscyphus fraxineus was isolated more frequently from younger collar necroses, whereas older necroses were more often colonized by Armillaria spp. Advanced stages of rot that may pose a risk to forest workers, visitors and traffic were observed already in two years-old necroses infected by Armillaria spp.
Keywords
Ash Dieback, Collar Necrosis, Disease Progression, Armillaria, Butt Rot, Epidemiology
Introduction
Populations of Common ash (Fraxinus excelsior L.) and Narrow-leaved ash (F. angustifolia Vahl) in Europe are currently devastated by ash dieback, a disease of outstanding severity that strongly impairs the silvicultural utilization of these important broadleaf tree species. The agent of ash dieback is the ascomycete Hymenoscyphus fraxineus (T. Kowalski) Baral et al. (syn. H. pseudoalbidus Queloz et al.; anamorph: Chalara fraxinea T. Kowalski). According to current knowledge, the fungus has been introduced from Far East Asia to Europe about 25 years ago ([11], [5]). In south-western Germany, first traces of ash dieback have been dated back to the year 2006. Since 2009, the disease is omnipresent throughout this area ([24]).
Characteristic crown symptoms of ash dieback are necroses on leaves and dieback of shoots. Already in 2005, diseased root collars of ash trees have been reported in combination with crown symptoms of ash dieback, but were assigned to secondary pathogens ([19], [32], [1]). Only in 2012, it became clear that H. fraxineus can frequently be detected in diseased root collars ([15]).
The path of infection on root collars still remains unknown and it was not yet possible to directly demonstrate that H. fraxineus is the primary agent of these symptoms, but there is strong indirect evidence ([3]). Potential infection courts for H. fraxineus at root collars may be lenticels, as hypothesized by Husson et al. ([15]). After successful infection by H. fraxineus, the cambium is killed and characteristic, tongue-shaped discoloured bark necroses develop at the root collar. The sapwood proximal to the affected area dies and turns to a darker, brownish colour. Bark cracks become visible within one or two years. Xylem proximal to necroses is colonized by secondary agents that can cause butt and root rot. Very frequent pathogens in this regard are species of honey fungus, mainly Armillaria gallica and A. cepistipes, but also A. mellea and A. borealis ([19], [1], [15], [6], [12], [3], [20]). In addition, some wood decay fungi of other genera and of minor importance have been reported ([13], [18]).
Spatial patterns of collar necroses prevalence suggest an influence of site factors and there are indications for a predisposition of humid and wet sites ([15], [6], [27], [20]). Moreover, there is a genetic component in susceptibility to collar necroses ([27]), as previously demonstrated for crown symptoms of ash dieback ([21] and references therein, [8]). Collar necroses occur more frequently on trees with high susceptibility to crown symptoms, but can also be observed on otherwise healthy trees ([15], [6], [27]). Underlying resistance mechanisms of the different symptoms and possible relationships between them are still unknown.
Collar rots can occur in high prevalence, often cause substantial mortality and eventually are the most damaging factor associated with H. fraxineus ([15], [26], [3], [20]). Root and butt rot reduce the stability of the trees and can be a risk for foresty staff, forest visitors and traffic ([25], [17], [26], [33]). Collar necroses also occur on narrow-leaved ash (F. angustifolia Vahl - [12]).
The aim of this study was to investigate the initiation and progression of this important disease in F. excelsior trees and stands, including the time course of the colonization of necroses by wood rotting fungi. The results are expected to provide a basis for decision-making and enhanced disease management in ash stands.
Material and methods
Study sites and sampling
In the context of consulting activities of the Forest Research Institute of Baden-Württemberg, nine pure ash stands with high prevalence of collar necroses have been reported by local forest authorities (Tab. 1). Harvesting operations were carried out shortly before investigation in these stands. Stumps of currently felled trees were investigated with differing intensity as described below. Discolorations of sapwood due to necroses and rots can be easily detected on stumps of recently felled trees (Fig. 1) and allow a more reliable survey of collar lesion prevalence than surveys on standing trees. In total, 155 stem discs with at least one necrosis were selected randomly (Tab. 2) and collected by sawing less than 10 cm above ground. The stem discs were about 4 cm in width.
Tab. 1 - Location and characteristics of study sites. (1): According to forestry site maps (InFoGIS / ForstBW); (2): according to documents of local forest authorities or the mean number of year rings at stumps; (3): the number of stumps affected by collar necroses was counted either directly in the stands or on sampled stem discs.
| Site | Coordinates | Altitude (m a.s.l.) |
Site conditions (1) | Sampling date |
Mean diameter |
Age (2) | No. diseased (3) |
Percentage diseased (3) |
|---|---|---|---|---|---|---|---|---|
| AC | 48°38′ 01″ N 08°01′ 15″ E |
135 | Fresh fluvial loam | 02.2014 | 21.8 | 25 | 229 | 77.6 |
| WE | 48°13′ 33″ N 07°13′ 50″ E |
167 | Moist floodplain | 10.2014 | 36.0 | 45 | 27 | 54.0 |
| SE | 47°49′ 33″ N 09°31′ 06″ E |
560 | Marshy lower slope | 12.2014 | 31.6 | 45 | 43 | 89.6 |
| FR | 47°53′ 34″ N 09°31′ 55″ E |
575 | Wet depression | 12.2014 | 58.0 | 65 | 11 | 68.8 |
| SH1 | 47°37′ 43″ N 07°47′ 24″ E |
420 | Fresh depression | 05.2015 | 48.0 | 62 | 28 | 50.0 |
| SH2 | 47°38′ 06″ N 07°50′ 01″ E |
450 | Humid depression | 05.2015 | 20.0 | 23 | 39 | 78.0 |
| MS | 48°22′ 32″ N 09°33′ 53″ E |
762 | Moderately dry residual material from weathered limestone | 05.2015 | 19.6 | 32 | 26 | 52.0 |
| HS | 48°19′ 45″ N 09°19′ 48″ E |
750 | Moderately dry residual material from weathered limestone | 05.2015 | 15.5 | 24 | 48 | 96.0 |
| TR | 48°18′ 28″ N 09°14′ 26″ E |
720 | Moderately humid residual material from weathered limestone | 05.2015 | 46.2 | 49 | 5 | 25.0 |
Fig. 1 - Overview of differently developed collar necroses on investigated stem discs. (a): Stem disc with several young necroses; (b): advanced discoloration proximal to a bark necrosis; (c): partly brightening of discoloration due to wood decay and demarcation lines caused by Armillaria sp.; (d): severely affected cross section; (e): occasionally, successful wound closure was observed.
Tab. 2 - Number of first-time infections per site and year on 155 investigated trees.
| Site | Sum | 2007 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|---|---|
| AC | 19 | 0 | 0 | 4 | 8 | 7 | 0 | - |
| FR | 11 | 0 | 0 | 0 | 7 | 3 | 1 | 0 |
| HS | 20 | 0 | 0 | 1 | 6 | 8 | 4 | 1 |
| MS | 17 | 0 | 0 | 1 | 6 | 4 | 4 | 2 |
| SH1 | 4 | 0 | 1 | 2 | 0 | 0 | 1 | 0 |
| SH2 | 6 | 0 | 0 | 2 | 2 | 1 | 1 | 0 |
| SE | 46 | 0 | 0 | 9 | 19 | 6 | 4 | 8 |
| TR | 5 | 1 | 0 | 0 | 1 | 1 | 0 | 2 |
| WE | 27 | 0 | 2 | 9 | 8 | 7 | 1 | 0 |
| Sum | 155 | 1 | 3 | 28 | 57 | 37 | 16 | 13 |
| Percentage | 100 | 0.6 | 1.9 | 18.1 | 36.8 | 23.9 | 10.3 | 8.4 |
Dating of collar necroses and reconstruction of prevalence history
The bottom sides of the stem discs were either grinded or planed. The year of necrosis formation was ascertained by analysing tree ring structures under a dissecting microscope. The year of the youngest unaffected tree ring was considered as the year of necrosis initiation. This was done for every individual necrosis separately except for stem discs from stand AC, where only the age of the oldest necrosis was identified per disc. These data and the data of prevalence of collar necroses at the time of sampling (Tab. 1) were used to retrospectively reconstruct the development of collar necrosis infection rate in the stands. A logistic curve was fitted to the mean percentage of infected trees by year of necrosis formation using the none-linear least squares method (R package “car” - [9]). This model has the following form (eqn. 1):
where y is the percentage of infected trees, φ1 is the upper asymptote, φ2 and φ3 are growing parameters and x is the year. Logistic models are widely used to describe disease growth in the analyses of plant disease epidemics ([2], [35], [30]). The upper asymptote and the inflexion point of the sigmoid curve can be indicators for the final disease incidence ([14], [31]). For a highly susceptible host, the asymptote would be expected to be located at an infection rate of 100 % and the inflexion point at an infection rate of 50 %. The inflexion point of the curve was determined with the R package “inflection” ([4]).
Characterization of collar necroses and rot
Sapwood proximal to necroses usually changes to darker, brownish colour after necrosis formation (Fig. 1a, Fig. 1b), but partly brightens up again with increasing degree of wood decay (Fig. 1c). The activity of certain wood-destroying fungi, Armillaria spp. in particular, is also indicated by the presence of demarcation lines in the wood (Fig. 1c).
All stem discs except discs from stand AC were visually categorized according to the proportion of the cross-section area that demonstrated indications of rot by using a ruler. They were divided into the following classes of rot intensity: (0) no indications of rot; (1) rot on less than 20 % of the cross-section area; (2) rot on more than 20 % of the cross-section area. Successful wound closure (Fig. 1e) was also recorded for these stem discs. For all stem discs that originated from the sites HS, MS, SH1, SH2 and TR, the width of necrosis was measured along the cambium with a tape line.
Occurrence of Armillaria spp., H. fraxineus and other microbes
Necroses with characteristic rhizomorphs and white mycelial fans between bark and wood (Rhizomorpha subcorticalis) were classified as infected by Armillaria spp. ([10]). All individual necroses were inspected in this way, except for stem discs from AC and WE. Here, infections by Armillaria spp. were documented only on the level of trees. A total of 73 necroses from the sites of HS (37 necroses) and MS (36 necroses) were randomly chosen for isolations of microbes. Xylem not deeper than 2 cm below the cambial necroses was radially split in the lateral advancing zone of the discoloration with a rack and pinion press ([22], [23]). Four specimens per necrosis (292 in total) of about 12 mm³ were taken immediately from the new, untouched surfaces with a cork borer under sterile airflow. Two specimens per necrosis were placed on synthetic nutrient-poor agar (SNA - [29]) and ash leaf malt agar ([16]), respectively, and incubated for three weeks at room temperature. The two culture media were used for a comparison of isolation success. Eight of the resulting Armillaria isolates were further analysed by molecular means according to Tsykun et al. ([34]) in order to identify the Armillaria species.
Results
Development of symptom prevalence
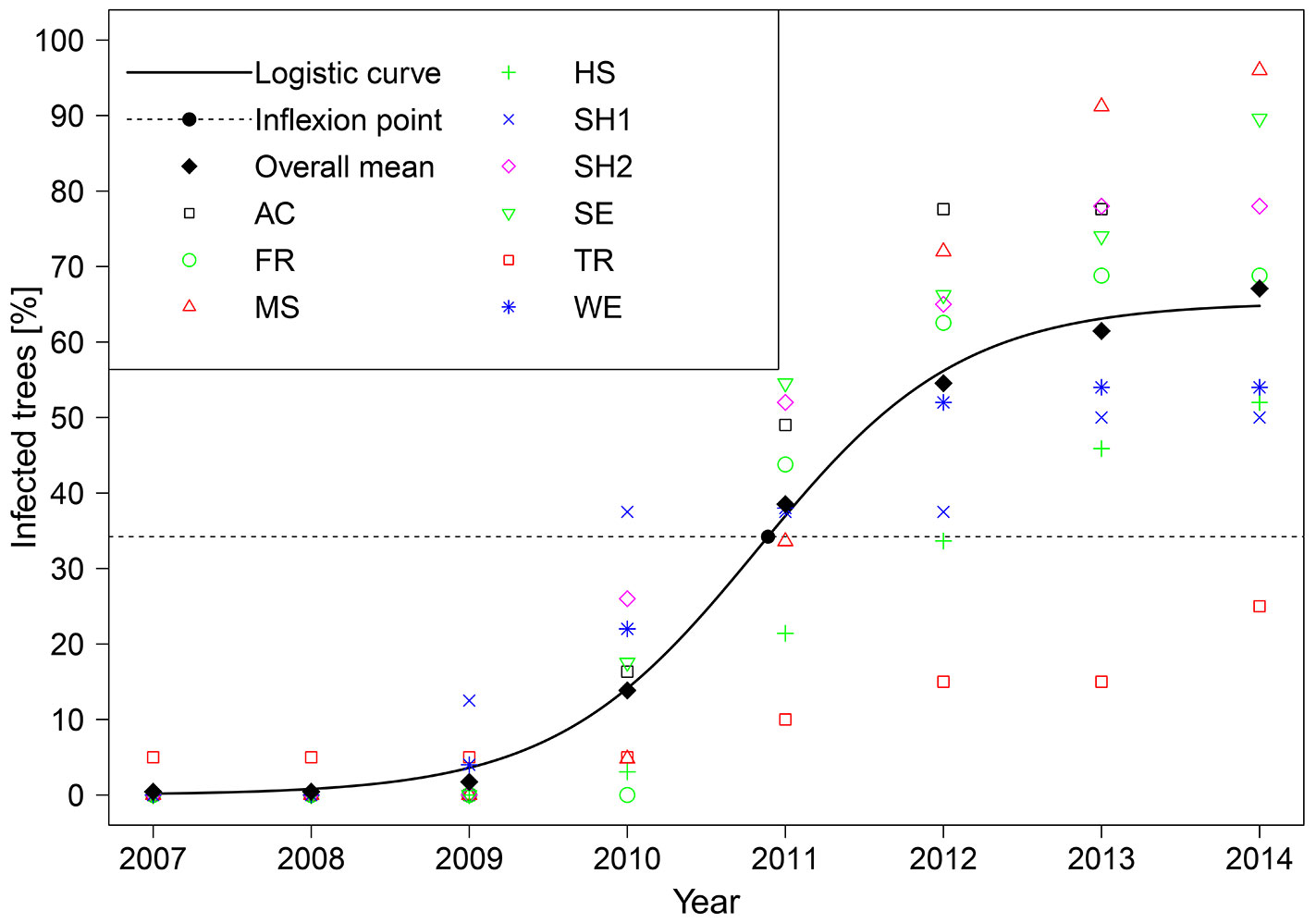
The first collar necrosis in the nine stands developed in 2007 in stand TR (Tab. 2). In stands WE and SH1, first necroses occurred in 2009, in the majority of stands in 2010. In 2011, collar necroses occurred in all stands. The majority of trees were infected in 2011 for the first time at the stem base. In subsequent three years, the number of first-time infected trees decreased. The asymptote of the logistic model was located at y = 65.3, the inflection point at y = 34.2 (Fig. 2), both indicating that final disease incidence will be less than 100 %.
Fig. 2 - Temporal development of the proportion of infected trees in total and per stand, calculated from the year of first infection per tree and the proportion of infected trees at the time of sampling (see Tab. 1). Data for the stand AC ends in 2013.
Initially, the temporal development of individual necroses followed a similar pattern and only few necroses occurred before 2010 (Tab. 3). In the years 2011 to 2013, about a fifth of the investigated necroses emerged, respectively. Even more individual necroses occurred in 2014, particularly in the site SE.
Tab. 3 - Number of individual necroses and year of their formation.
| Site | Sum | 2007 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | Mean no. per tree |
Maximum no. per tree |
|---|---|---|---|---|---|---|---|---|---|---|
| FR | 60 | 0 | 0 | 0 | 13 | 19 | 13 | 15 | 5.5 | 12 |
| HS | 61 | 0 | 0 | 1 | 10 | 18 | 14 | 18 | 3.1 | 7 |
| MS | 51 | 0 | 0 | 1 | 9 | 12 | 13 | 16 | 3.0 | 8 |
| SH1 | 19 | 0 | 1 | 3 | 0 | 1 | 12 | 2 | 4.8 | 8 |
| SH2 | 13 | 0 | 0 | 2 | 4 | 2 | 3 | 2 | 2.2 | 4 |
| SE | 232 | 0 | 0 | 16 | 49 | 33 | 31 | 103 | 5.0 | 16 |
| TR | 12 | 1 | 3 | 1 | 1 | 1 | 2 | 3 | 2.4 | 5 |
| WE | 59 | 0 | 2 | 14 | 12 | 20 | 10 | 1 | 2.2 | 5 |
| Sum | 507 | 1 | 6 | 38 | 98 | 106 | 98 | 160 | 3.3 | 12 |
| Percentage | 100 | 0.2 | 1.2 | 7.5 | 19.3 | 20.9 | 19.3 | 31.6 | - | - |
Isolates
The results of the isolation experiment are presented in Tab. 4. In total, H. fraxineus was isolated 94 times and detected in 58.9 % of the investigated necroses and 67.6 % of the stem discs. There was almost no difference in frequency of detection between the two stands (MS and HS) and the two culture media. Armillaria mycelium was isolated from 10 specimens. Bacteria (not further identified) emerged from 88 specimens. A total of 35 isolates were assigned to other fungal species: Fusarium sp. (three isolates), Phialophora sp., Cladosporium sp. and Acremonium sp. (two isolates, respectively), Trichoderma sp., Scytalidium lignicola, Exophiala sp. and Phoma sp. (one isolate, respectively) and 22 not identified fungal mycelia. No growth of microbes could be observed on 80 specimens (27.4 %).
Tab. 4 - Number of isolated microbes per site (ME and HS), culture medium and in total and number of individual necroses with respective microbe detections.
| Counts | H. fraxineus | A. gallica | Other fungi |
Bacteria | Without growth of microbes |
|---|---|---|---|---|---|
| Total no. of isolates | 94 (32.2 %) | 10 (3.4 %) | 35 (12.0 %) | 88 (30.1 %) | 80 (27.4 %) |
| No. isolates in ME | 42 (29.2 %) | 5 (3.5 %) | 24 (16.7 %) | 49 (34.0 %) | 32 (22.2 %) |
| No. isolates in HS | 52 (35.1 %) | 5 (3.4 %) | 11 (7.4 %) | 39 (26.4 %) | 48 (32.4 %) |
| No. isolates on ash leaf agar | 45 (30.8 %) | 7 (4.8 %) | 12 (8.2 %) | 44 (30.1 %) | 40 (27.4 %) |
| No. isolates on synthetic nutrient-poor agar | 49 (33.6 %) | 3 (2.1%) | 23 (15.8 %) | 44 (30.1 %) | 40 (27.4 %) |
| No. of necroses with isolates | 43 (58.9 %) | 7 (9.6 %) | 24 (32.9 %) | 50 (68.5 %) | 2 (2.7 %) |
| No. of stem discs with isolates | 25 (67.6 %) | 6 (16.2 %) | 17 (45.9 %) | 31 (83.8 %) | 1 (2.7 %) |
Characteristics of necroses of differing age
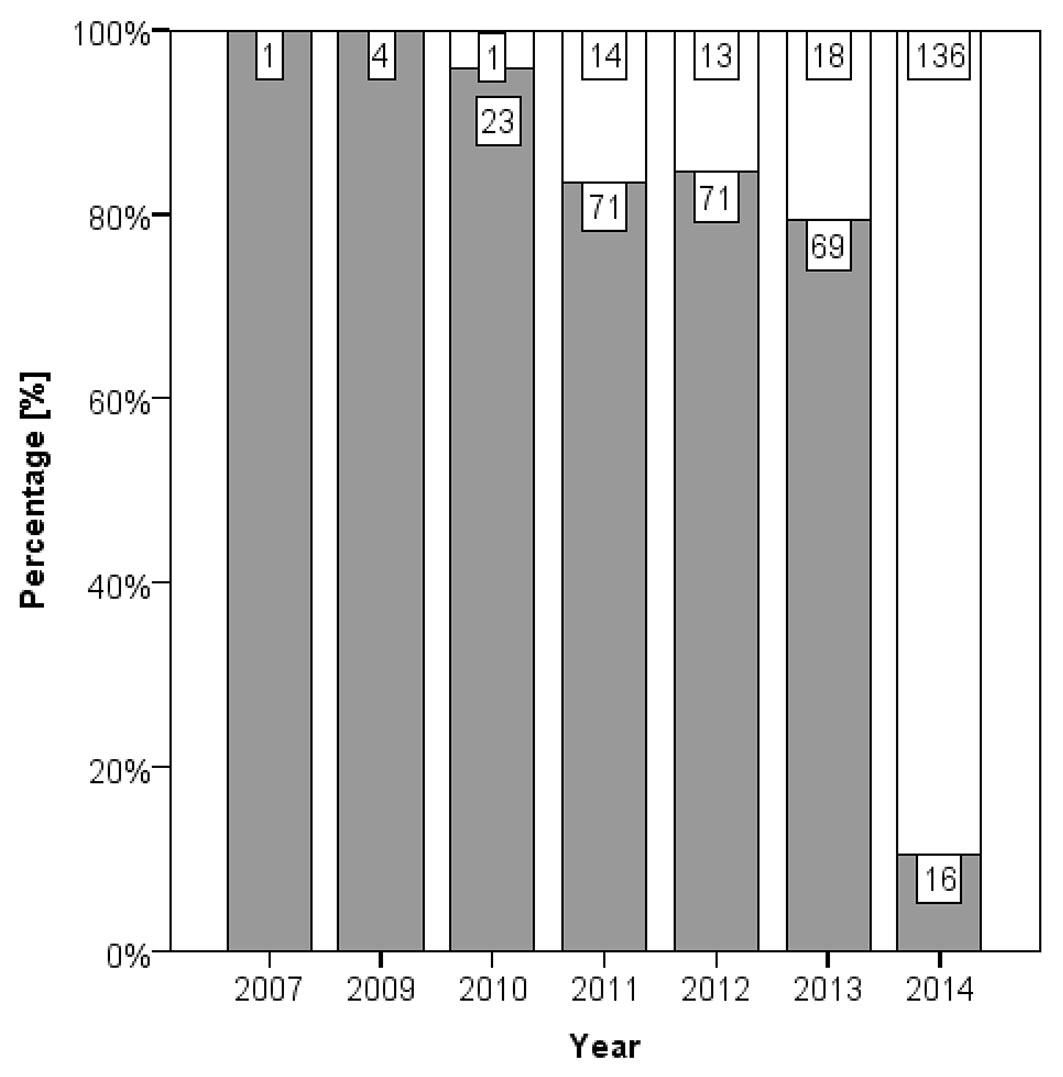
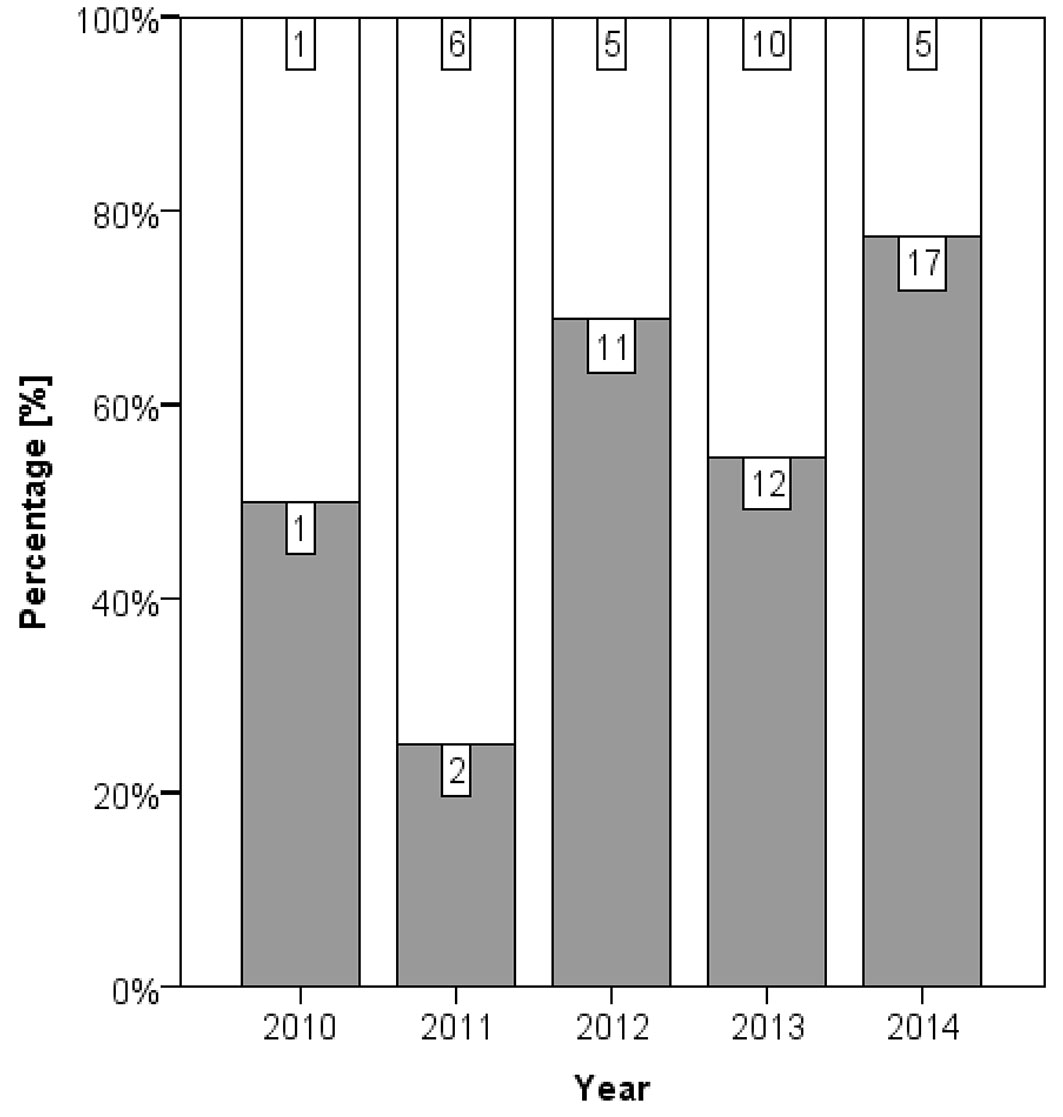
Infections by Armillaria were detected on 58.4 % of the 437 necroses that were tested either by isolation or by observation of characteristic mycelium or rhizomorphs. The temporal development of the colonisation by Armillaria spp. is presented in Fig. 3. Only 10.4 % of young necroses that experienced one complete growing season at maximum were infected by Armillaria. After two complete growing seasons 79.3 % of necroses were colonized by Armillaria and the fungus was observed on 95.8 % of necroses that experienced five complete growing seasons. A Mann-Whitney test for the year of necrosis formation between necroses infected and not infected by Armillaria was highly significant (p < 0.001). A contrary tendency was detected for the number of H. fraxineus isolates (Fig. 4), although the smaller sample number did not reveal significance in this case (Mann-Whitney test, p = 0.055). Successful isolation of H. fraxineus was particularly high for necroses that experienced only one complete growing season at maximum (78.3 %) and was smaller for older necroses. The pathogen could still be isolated from a necrosis that experienced five complete growing seasons.
Fig. 3 - Proportion of individual necroses colonized (grey bars) and not colonized (white bars) by Armillaria spp. per year of necrosis formation. The number of necroses per category is displayed, respectively. There was no data for necroses from stands AC and WE.
Fig. 4 - Proportion of necroses from stands HS and MS with detection by isolation of H. fraxineus (grey bars) and without detection (white bars) separated by year of necrosis formation. The number of necroses per category is displayed, respectively (70 in total).
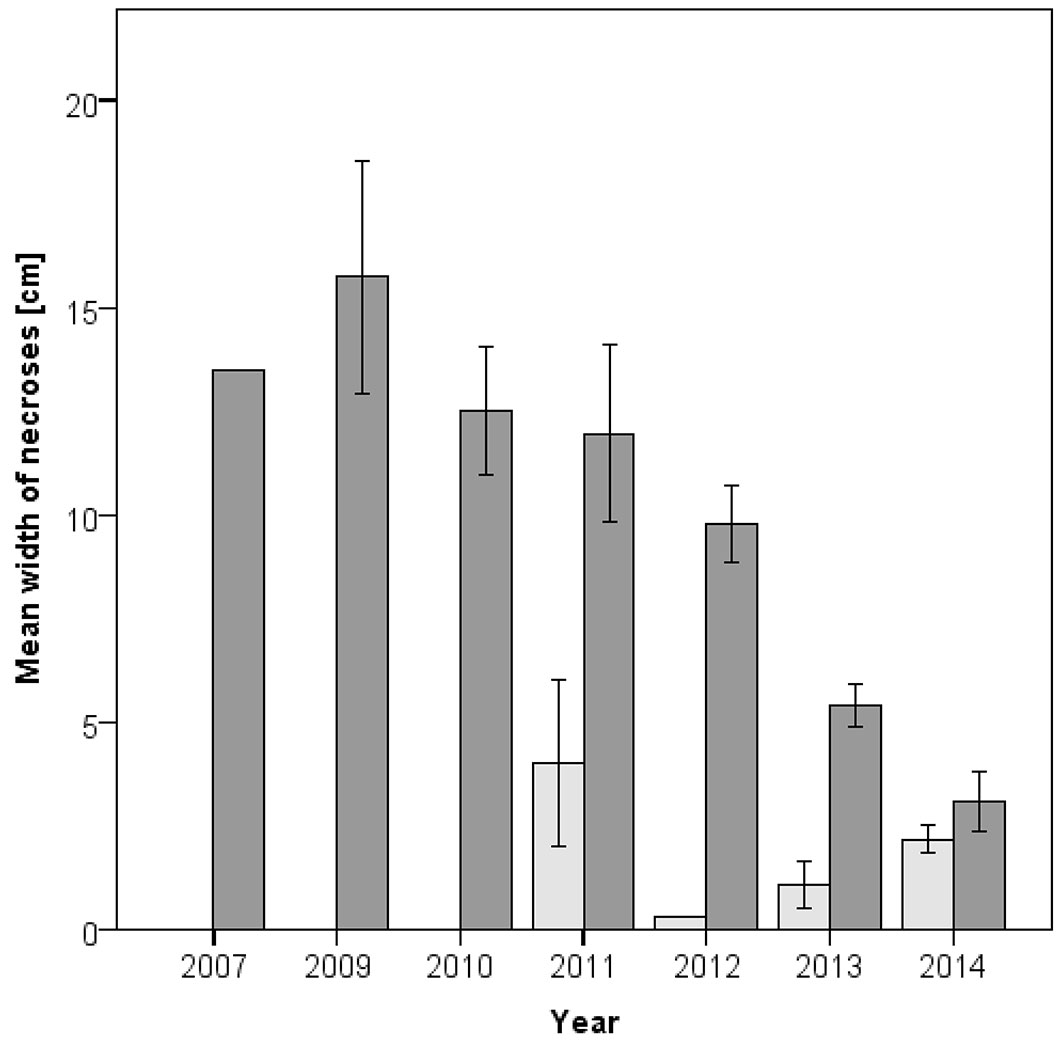
The necroses width was significantly correlated with the time of necrosis formation (Spearman correlation, ρ = -0.661; p < 0.001). On average, older necroses colonized by Armillaria spp. were considerably wider than younger necroses. In contrast, such relationship was not evident when only necroses without Armillaria infection were considered (Fig. 5). Successful wound closure (Fig. 1e) of individual necroses was observed on 21 of 137 stem discs (15.4%).
Fig. 5 - Mean width of 151 necroses of different age from stands HS, MS, SH and TR, separated by necroses with (dark grey) and without (light grey) indications of Armillaria colonization. Error bars (± standard error) are missing when only one necrosis was present in a category.
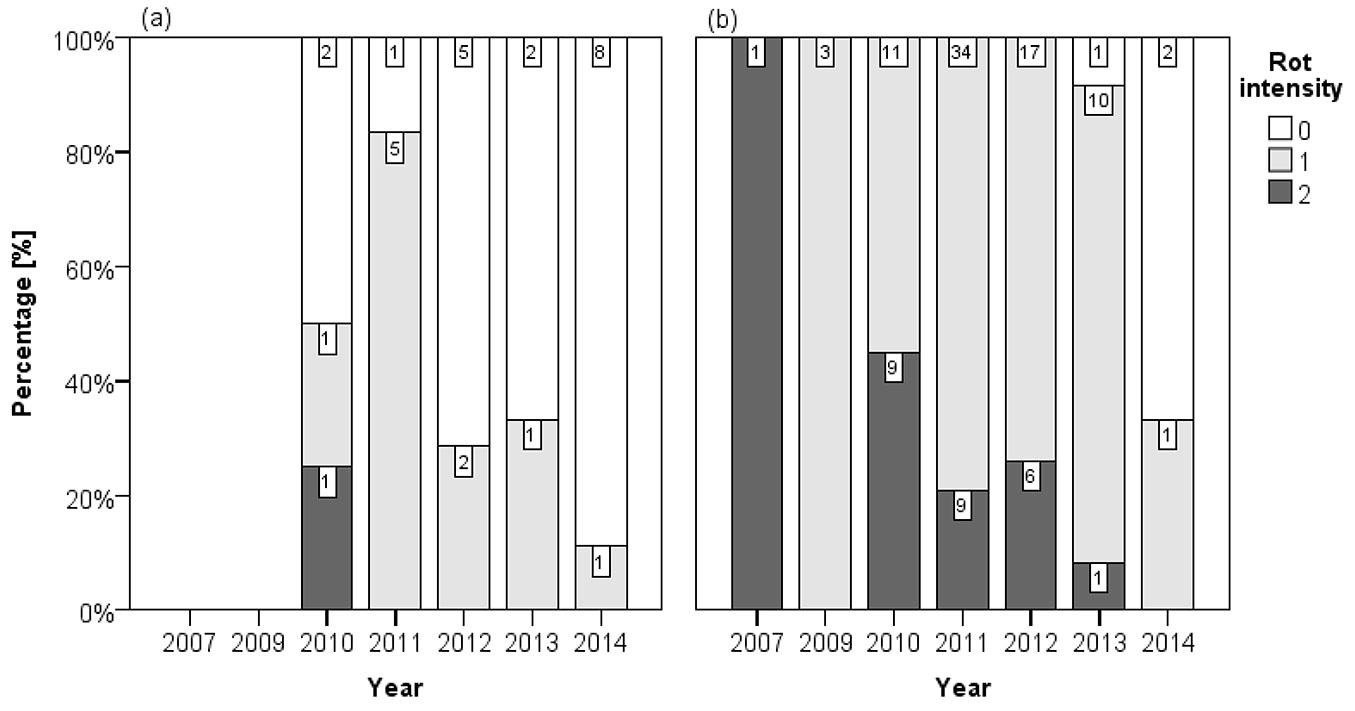
Almost one fifth (19.9 %) of the inspected stem discs were assigned to rot intensity class 2 (more than 20 % of the cross-section area). Less pronounced rot was observed on 64.7 % of stem discs (rot intensity 1), whereas 15.4 % of discs showed discoloration but no indications of rot (rot intensity 0). Rot intensity was significantly correlated with the time of first necrosis formation (Spearman, ρ = -0.420; p < 0.001). Indications for rot occurred on 23.1 % of the discs that did not become diseased before 2014, but were local and all of rot intensity class 1. 81.2 % of stem discs with oldest necroses from 2013 exhibited rot and one disc was even assigned to rot intensity class 2. The proportion of discs with rot intensity 2 increased with the time of infection and reached 41.7 % for stem discs that were infected in 2010 for the first time. Significantly stronger rot intensity (Mann-Whitney test, p < 0.001) was observed on stem discs that were colonized by Armillaria spp., whereas rot intensity class 2 occurred only once on a disc that appeared to be not infected by this fungus (Fig. 6).
Fig. 6 - Percentage in classes of rot intensity and year of first infection per stem discs without (a) and with (b) indications of colonization by Armillaria spp. Rot intensity: (0) no indications of rot; (1) rot on less than 20 % of the cross-section area; (2) rot on more than 20 % of the cross-section area. The number of stem disc per category is displayed, respectively.
Discussion
The retrospective approach of analysing tree ring structures revealed comprehensive information about the temporal development of collar necroses and butt rot including the colonization by the main pathogens H. fraxineus and Armillaria spp. New insights about the pathogenesis and recommendations for practitioners can be inferred from these results. In contrast to most other studies, we examined stumps of cut trees. This allowed investigating also young necroses that would not have been detectable on standing trees.
Prevalence of collar necroses and site conditions
The high prevalence of collar necroses in the investigated stands can be explained with their targeted selection. Moreover, it can be assumed that infection pressure by H. fraxineus and Armillaria spp. is higher in pure ash stands than in mixed stands. According to a representative survey for south-western Germany, ash trees with collar necroses accounted for only 17.5 % of the total ash stock, whereby infection rate decreased with tree age ([7]). In this survey on standing trees, however, young necroses were probably not yet detectable.
The locally high frequency of collar necroses suggests site effects for prevalence. Indeed, data of several studies indicate that humid, wet, waterlogging and floodplain sites are predisposing for collar necroses ([15], [6], [27], [20]). The majority of sites investigated in this study were humid, which supports the above cited findings. However, prevalence was also high in the sites ME and HS, which were categorised as moderately dry. Apparently, there are important factors other than site humidity that influence prevalence of collar necroses. Further studies that include, for example, the influence of tree age, are needed to clarify these very important interdependencies.
Fungal isolates
The method of sterile sampling used for isolation from fresh surfaces is advantageous over surface sterilisation, which can bias the isolation success. H. fraxineus was isolated with high frequency. Armillaria strains were isolated only scarcely, because specimens were sampled from the margins of discolorations, where the presence of Armillaria spp. is rather unlikely. Another study was able to detect H. fraxineus in 98 % and Armillaria spp. in 41 % of necroses by molecular methods ([3]). These necroses did not provide any visual indications for the presence of Armillaria spp., and the authors assumed that frequency of this fungus can be underestimated in visual surveys. The two tested culture media (SNA and ash leaf agar) were similar in effectiveness for the isolation of Armillaria spp. and H. fraxineus. The latter grew faster on ash leaf agar, but phialides, which are important for the morphological identification of C. fraxinea, were easier to identify in SNA.
Temporal development of collar necroses and rots
According to the data presented, collar necroses started to become problematic in south-western Germany in 2010, which is four years after the first indications of the presence of H. fraxineus ([24]). Also in France, the first collar necroses occurred later than crown symptoms and several years after the arrival of the pathogen ([15], [20]). The year 2011 was an inflexion point, as the number of first-time infected trees decreased in subsequent years (Fig. 2, Tab. 2). In theory, an inflexion point would be expected at an infection rate of 50 % ([30]) and an asymptote for disease progression at an infection rate of 100% ([28]), under the presumption that all individuals will become diseased. Inflexion points at lower infection rates suggest a lower final incidence of the disease ([14]). A similar assumption can be done for the asymptote ([31]). The observed development suggests that a fraction of trees will remain in healthy conditions. This can be expected when assuming that currently uninfected individuals are less susceptible towards the symptom ([27]). Another possible explanation is that the remaining ash trees are growing in micro locations that are not suitable for collar infections. Of course, further monitoring is needed, as the presented time series comprises only few years and is too short for a reliable prediction of future infection development. However, general conditions in the years after 2011 apparently have been favourable to infection, as many collar necroses occurred at already infected trees (Tab. 3). Since most infections likely occur during the main time of sporulation, one may speculate that the high number of emerging individual necroses in 2014 is connected to the extraordinarily high precipitation during summer 2014 in the region: the mean precipitation in the Federal State of Baden-Württemberg was 191.6 mm in July and 115.6 mm in August, according to DWD (the German Meteorological Service).
The more frequent detections of Armillaria spp. on older necroses (Fig. 3) and the opposite pattern for H. fraxineus (Fig. 4) indicate that the necroses are primarily caused by the latter pathogen ([3]). Nevertheless, Armillaria spp. play an important aggravating role in this pathosystem. Obviously, necroses infected by Armillaria strongly enlarge over time (Fig. 5) and increasingly girdle the root collar, which is crucial for tree mortality. Collar girdling can also occur because of the accumulation of necroses ([20]). Although other wood decaying fungi can cause rot in ash collar necroses ([13], [18]), our data demonstrate that extensive rots rarely occur in absence of Armillaria spp.
Implications for forest management
In forest management, collar necroses are relevant for two main reasons: (i) they dramatically increase tree mortality; and (ii) they reduce the stability of trees, which can be crucial for the safety of forestry workers, forest visitors and traffic. For timber marketing, collar necroses are of moderate importance, as they affect usually less than 1 m of the basal trunk. The wood quality of the remaining trunk is not compromised, if trees are harvested in time, i.e., before trees have lost more than 70% of the canopy or even have died ([25]).
In the majority of cases, the anticipated mortality of the next years dictates the need for action in management of ash dieback affected stands. For the assessment of future mortality it is recommended to rate not only crown symptoms. At least on a random sample of trees, root collars should be inspected for the presence of necroses and butt rot, as described by Skovsgaard et al. ([33]). Prevalence of these symptoms can change drastically within few years and operational decisions should not be made on a basis of older observations. Rot on more than 20 % of the cross-section area was detected on a necrosis that was only two years old. We estimate that such extent of rot can already be a risk for forest workers and traffic.
Most trees affected by collar necroses are expected to die within a few year and/ or their growth performances to be strongly reduced. Such trees should be removed during thinnings similar to trees with strongly defoliated crowns (exceeding 70 % - [25], [33]) in order to minimize risk of accidents. Exceptions may be trees with small, already closed necroses. Trees appearing resistant to the disease, also regarding collar necroses, should be conserved and their regeneration promoted.
The presence of Armillaria spp. is not to be expected in afforestation sites where thinnings were not yet conducted. The same likely holds for solitary trees, e.g., in parks or alongside roads. Under these circumstances, the situation of collar necroses can be considered much less severe. In the absence of Armillaria spp., rot was observed only rarely and only on several years old necroses.
Conclusions
Dating the time of collar necrosis formation by tree ring analyses revealed a typical sigmoid disease progression in a set of 155 trees from nine south-western German severely infested stands. Modelling of disease progression suggests that comparatively few trees will become first-time infected in the future and a fraction of trees will not become affected by collar necroses in the long term. However, future monitoring of disease incidence is necessary to confirm these results. The data supports the hypothesis that H. fraxineus is the major agent of collar necroses in ash, although Armillaria spp. play an important aggravating role. Ash trees colonized by Armillaria spp. begin to pose risks to forestry staff and forest visitors within two years after necrosis formation. Five years after necrosis formation, 41.7 % of the affected root collars exhibited decay to a degree which we assume to be potentially hazardous.
Acknowledgements
We would like to thank the local forestry authorities of Emmendingen, Lörrach, Ortenaukreis, Ravensburg and Reutlingen for providing study sites and assistance in sampling. Further, we thank Gudrun Seiffert for the competent work on isolations and the identification of the Armillaria spp. and Niklas Ohlmann for the skilful assistance in R application. The study was funded by ForstBW.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Felicitas Sander
Berthold Metzler
Department of Forest Health, Forest Research Institute of Baden-Württemberg, Wonnhaldestrasse 4, 79100 Freiburg (Germany)
Corresponding author
Paper Info
Citation
Enderle R, Sander F, Metzler B (2017). Temporal development of collar necroses and butt rot in association with ash dieback. iForest 10: 529-536. - doi: 10.3832/ifor2407-010
Academic Editor
Alberto Santini
Paper history
Received: Feb 13, 2017
Accepted: Apr 14, 2017
First online: May 05, 2017
Publication Date: Jun 30, 2017
Publication Time: 0.70 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2017
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 51056
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 42751
Abstract Page Views: 3327
PDF Downloads: 3869
Citation/Reference Downloads: 22
XML Downloads: 1087
Web Metrics
Days since publication: 3202
Overall contacts: 51056
Avg. contacts per week: 111.62
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2017): 23
Average cites per year: 2.56
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Assessment of presence and distribution of Armillaria and Heterobasidion root rot fungi in the forest of Vallombrosa (Apennines Mountains, Italy) after severe windstorm damage
vol. 12, pp. 118-124 (online: 11 February 2019)
Research Articles
Lenticel infection in Fraxinus excelsior shoots in the context of ash dieback
vol. 12, pp. 160-165 (online: 04 March 2019)
Research Articles
Secondary metabolites of six Siberian and Crimean Armillaria species and their in vitro phytotoxicity to pine, larch and poplar
vol. 15, pp. 38-46 (online: 04 February 2022)
Research Articles
Genetic variation of Fraxinus excelsior half-sib families in response to ash dieback disease following simulated spring frost and summer drought treatments
vol. 9, pp. 12-22 (online: 08 September 2015)
Review Papers
Dutch elm disease and elm bark beetles: a century of association
vol. 8, pp. 126-134 (online: 07 August 2014)
Research Articles
Application of fungicides and urea for control of ash dieback
vol. 8, pp. 165-171 (online: 13 August 2014)
Review Papers
Genomics of the Dutch elm disease pathosystem: are we there yet?
vol. 8, pp. 149-157 (online: 07 August 2014)
Review Papers
Avoidance by early flushing: a new perspective on Dutch elm disease research
vol. 2, pp. 143-153 (online: 30 July 2009)
Research Articles
Monitoring of the incidence of Dutch Elm Disease and mortality in experimental plantations of French Ulmus minor clones
vol. 15, pp. 289-298 (online: 29 July 2022)
Review Papers
Linking patterns of forest dieback to triggering climatic and weather events: an overview on Mediterranean forests
vol. 17, pp. 309-316 (online: 30 September 2024)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword