Ulmus laevis in the Iberian Peninsula: a review of its ecology and conservation
iForest - Biogeosciences and Forestry, Volume 8, Issue 2, Pages 135-142 (2015)
doi: https://doi.org/10.3832/ifor1201-008
Published: Aug 07, 2014 - Copyright © 2015 SISEF
Review Papers
Collection/Special Issue: 3rd International Elm Conference, Florence (Italy - 2013)
The elms after 100 years of Dutch Elm disease
Guest Editors: A. Santini, L. Ghelardini, E. Collin, A. Solla, J. Brunet, M. Faccoli, A. Scala, S. De Vries, J. Buiteveld
Abstract
European white elm (Ulmus laevis Pallas) populations are scarce, small and fragmented in the Iberian Peninsula. Due to these characteristics the indigenous status of the species in the region has been questioned, whilst the species’ role in Iberian riparian forest ecology has been neglected. Herein we review past studies regarding this species’ distribution and ecology in the Iberian Peninsula, with special emphasis on the establishment of conservation priorities. We first present a collection of palaeogeographic, historic and genetic data suggesting that the Iberian Peninsula was a glacial refuge for U. laevis. Secondly, we analyse U. laevis distribution in relation to soil physico- chemical properties and water availability in Spain. Following this, we focus on the reproductive biology of the species, and investigate the effect of masting and empty seed production on predation and regeneration establishment. Finally, based on this knowledge, we propose conservation policies for U. laevis in the Iberian Peninsula.
Keywords
Elm Conservation, Drought-stress Vulnerability, Root Iron Uptake, Population Genetics, Seed Dispersal, Seed Predation, Ulmus laevis’ Distribution
Introduction
The European white elm (Ulmus laevis Pallas) is a hardwood deciduous tree which grows in river margins and damp bottomland forests, tolerating flooding for some periods of the year ([17]). Ulmus laevis belongs to section Blepharocarpus ([107]), in contrast to the other two native European elms, U. minor Mill. and U. glabra Huds., which belong to the Ulmus section. Ulmus laevis has been considered to be naturally distributed across Europe, from Ural Mountains to eastern France, and from southern Finland to the Caucasus, Balkans and southern France ([16]). The loss of suitable habitats due to human-induced changes in riparian forests, combined with the effect of Dutch elm disease (DED), has compromised the survival of many U. laevis populations ([16]). It is estimated that only 1 % of the elms still remain alive in Germany ([57]), whereas the white elm is considered an endangered species in northern Belgium ([95]), southern France ([91]), and Finland ([18], [93]). Moreover, many small isolated populations throughout its distribution range are at risk of genetic drift ([16]). As such, this species has been identified as needing specific conservation measures in Europe ([18]).
According to Flora Ibérica ([65]), U. laevis is an established alien species in Spain which was introduced as an ornamental; a conclusion based on its small population sizes and scarcity. This is the opinion which has prevailed among botanists, and thus, limited efforts were initially undertaken towards white elm conservation in Spain. However, the presence of U. laevis in Spain was cited for the first time by Lapeyrouse ([47]) in the Pyrenees. Following this, it was also observed in Asturias ([73]), and as a result, was included in the first Iberian Floras ([108], [4]). Nevertheless, other botanical studies failed to include this species ([19], [49], [46], [12], [79]) or considered it as an introduced species ([11]). More recently, attending to several white elm stand characteristics, certain authors considered that U. laevis could be native to the Iberian Peninsula ([81], [2]).
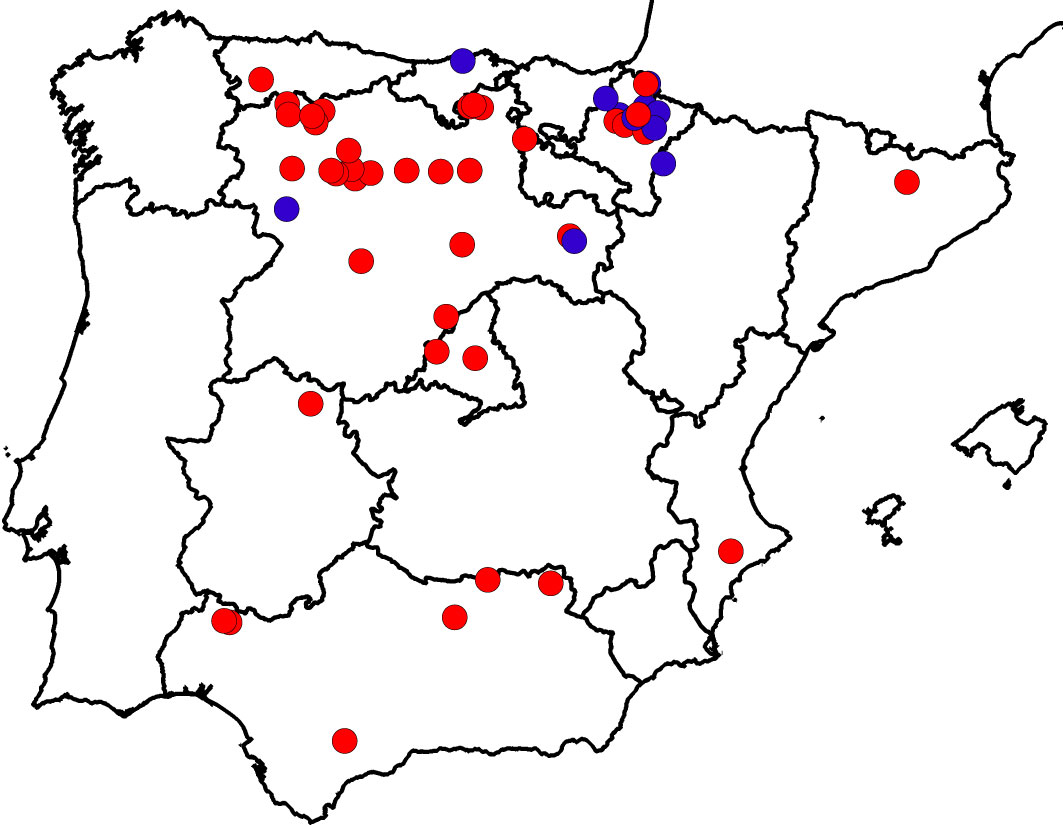
Following the second DED pandemic ([10]), several small, scattered, previously unknown populations of U. laevis were detected during the field surveys carried out by the Spanish Elm Conservation and Breeding Programme (SECBP, Technical University of Madrid, Spanish Environmental Ministry - Fig. 1). The location and characteristics of some of these newly located populations indicated that human introduction was highly unlikely. The fact that 41 of these 52 stands had not been previously detected was quite surprising, but can be explained by: (i) some stands growing in difficult access areas; (ii) difficulty in distinguishing U. laevis from field elm (U. minor) or wych elm (U. glabra) morphologically, unless the tree has flowers or fruits; and (iii) lack of interest in reporting its presence, given that it was considered an alien species. In addition, U. laevis trees were more easily detected after DED pandemics because they remained alive, whereas other elms died. Despite white elms being highly susceptible to DED ([77], [82]) they can survive the disease by an escape mechanism: bark beetles (Scolytus spp.), the propagation vectors of DED, prefer feeding on U. minor and U. pumila L. ([102], [103]) due to the chemical composition of their bark ([71], [60]).
Fig. 1 - Distribution of Ulmus laevis populations in Spain. Populations previously cited in the literature (blue dots) are differentiated from those detected by the Spanish Elm Conservation and Breeding Programme (red dots). Modified from Venturas et al. ([98]).
Determining whether or not small marginal populations of a species are native is complicated, especially in Mediterranean riparian forests, as they have been deeply transformed by humans over the last 4000 years ([33], [94]). Indeed, all elm species were initially considered to be introduced in the Iberian Peninsula due to the lack of evidence for their presence in the first fossil records ([37]). Following this, and based on taxonomic traits, Richens & Jeffers ([78]) established that U. glabra was native to northern Spain, and considered that if U. minor was to be indigenous, it would only be so in the eastern half of the Iberian Peninsula. When the number of palaeobotanic records increased and Ulmus spp. pollen was found all over Spain ([25], [50]), fossil remains were assumed to belong to U. glabra in the mountain areas, and to U. minor in the lowlands. Contrary to the hypothesis put forth by Richens & Jeffers ([78]), palaeobotanic remains which appeared in non-mountainous areas of western Spain were assumed to be from U. minor, despite the fact that pollen and wood from European elms cannot be morphologically distinguished at species level ([80], [86]). Therefore, after the discovery of new U. laevis populations, the following question arose: could part of the pollen from western Spain belong to U. laevis?
Herein, we review the process followed by SECBP to demonstrate the nativeness of U. laevis in Spain, and the species’ natural distribution, ecology, conservation status and recovery possibilities.
Nativeness of U. laevis in the Iberian Peninsula
Neutral genetic markers have proved to be useful when it comes to determining the status of populations of uncertain origin. Iberian native tree populations are usually genetically differentiated from central and eastern European populations, due to isolation and limited gene flow between Pleistocene discrete glacial refugia (e.g., [41], [75], [32], [58]). In contrast, introduced populations normally show lower genetic diversity than the populations from which they originate, and lack private alleles ([88]).
Ulmus laevis genetic diversity is relatively low compared to those of other tree species ([55], [56], [104], [93], [66]). Only three chloroplast haplotypes have been identified across Europe ([104]): haplotype A, the high frequency one which extends all over Europe, and two rare ones, haplotype B, restricted to southern France, and haplotype C, located in the Balkans and southwest Russia. Whiteley ([104]) argued that this haplotype distribution was congruent with white elm expansion from a core glacial refugium in Russia, as proposed by Huntley & Birks ([37]), but that this could also indicate the existence of additional refugia in southern European peninsulas.
Building on previously existing information ([105], [104]), 20 populations from central Europe, southern France and Spain were sampled in order to determine whether Iberian populations of U. laevis were native ([22], [23]). Chloroplast (cpDNA) restriction fragment length polymorphism (RFLP) and nuclear (nDNA) microsatellite markers were analyzed. Genetic diversity indexes were calculated, and Bayesian clustering and demographic analyses were performed.
The native status of U. laevis in the Iberian Peninsula was supported by: (i) diversity levels, which were low but similar to other European populations; (ii) the presence of haplotypes A and B, and of nDNA private alleles in Spain; (iii) genetic differentiation and spatial genetic structure of Spanish populations; and (iv) demographic analyses which showed signs of an ancestral bottleneck ([22], [23]). Moreover, there is no historic information supporting U. laevis introduction, nor its extensive use in Spain, contrary to what happens with U. minor ([26]) or U. pumila ([15]). Therefore, the Iberian Peninsula is considered to have contained some glacial refugia for U. laevis ([22], [23]), as also shown for other European tree taxa ([75], [58]). This interpretation is also consistent with the climatic modeling of the species’ distribution, which identifies parts of the Iberian Peninsula suitable for U. laevis under both the Last Glacial Maximum and present day climates ([89]).
Edaphic factors and distribution
Calcicole species can grow normally on both calcareous and siliceous soils ([112]). However, calcifuge species growing on calcareous soils suffer nutrient deficiencies due to a limited absorption of phosphorous ([92], [111]), manganese ([61], [90]) and chiefly iron ([109], [110], [112]). Iron is of great importance for many metabolic and enzymatic processes ([42]). Iron deficiency compromises plant growth and establishment because it causes reductions in photosynthetic rates ([48]). Plants have developed specialized mechanisms for increasing root iron availability and facilitating its uptake and transport within the plant ([59]). Elms are classified as Strategy I plants ([59]), and as such, their root iron uptake mechanisms are based on: (i) the production of root ferric reductase for transforming Fe3+ into Fe2+ compounds; (ii) the induction of Fe2+ membrane transporters; (iii) increasing iron solubility by acidifying the rizhosphere excreting protons (H+); and (iv) increasing iron availability by secreting organic compounds ([1]).
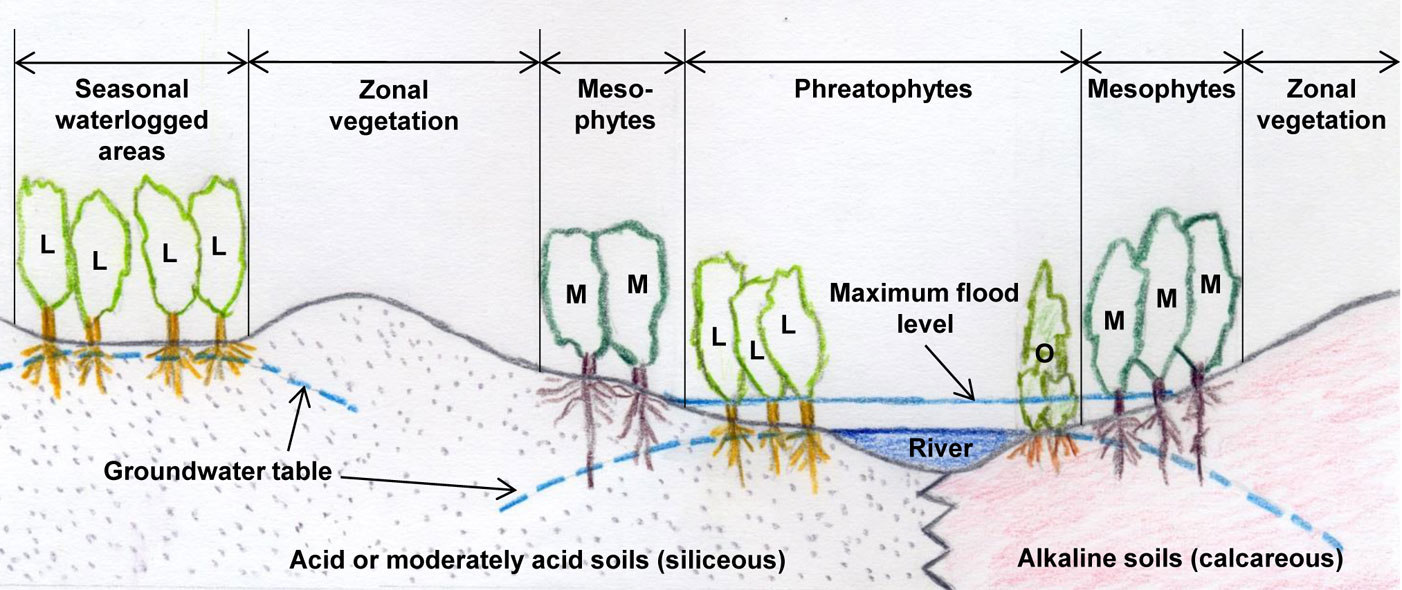
Ulmus laevis populations in Spain grow on siliceous moderately acid and acid soils, thus suggesting that this species has a calcifuge character within the region (Fig. 2). Ulmus minor can grow both on acid and alkaline soils, but is thought to be native to calcareous soils ([78]). In light of this, we tested whether differences in the efficiency of root iron uptake mechanisms may account for the differential distribution of these two species in Spain ([97], [100]).
Fig. 2 - Ulmus laevis and U. minor distribution sketch in relation to soil nature and water availability. Ulmus laevis trees (L); Ulmus minor trees (M); other phreatophytes (O).
Seedlings from both species were grown in hydroponic culture under iron uptake limiting conditions. Their growth, degree of lime-induced chlorosis symptoms, root proton extrusion capability, and root ferric reductase activity were evaluated. Results showed that both species suffered severe lime-induced chlorosis if grown with no iron. However, re-supply of iron in U. minor led to a better mobilization of iron over the complete plant and leaf surface, whereas this was confined to young leaves and along the leaf nerves in U. laevis. Moreover, U. laevis seedlings showed a lower root proton extrusion capacity and root ferric reductase activity than U. minor. Therefore, differences in the iron acquisition mechanisms may be at least partly responsible for these species’ distribution in the Iberian Peninsula, enabling U. minor to grow on calcareous soils where iron absorption is more limiting for U. laevis ([97], [100]).
Water availability and U. laevis
Ulmus laevis is a riparian tree which thrives in damp soils ([17], Fig. 2). An association study between species composition and soil types conducted in Estonia showed that this species grows mainly in embankments where the predominant soils are Eutric Gleysols or Gleyic Fluvisols ([70]). It has also been observed that U. laevis has better survival and growth rates than U. minor in heavy clay soils with prolonged waterlogging ([14]). This indicates the high water demand and waterlogging tolerance of this elm species.
Wetland and flood-tolerant plants survive waterlogging due to complex anatomical, physiological, morphological, and life-history adaptations ([9], [43], [76]). Xylem cavitation, for example, might help stem and root oxygen supply in flooded U. laevis plants ([99]). In a recent study, seedlings of U. laevis subjected to experimental waterlogging exhibited 40% loss of root hydraulic conductivity relative to control, well-watered plants. Most likely in relation to this, stomata partly closed and net photosynthesis was reduced. Respiration rates of leaves, stems and roots increased soon after waterlogging started, so that net carbon gain at the plant level was severely diminished. However, seedlings survived two months of waterlogging and recovered normal water-transport capacity and net photosynthesis afterward. This indicated that U. laevis is sensitive but considerably resistant to waterlogging at the seedling stage (Li et al., unpublished data).
Drought-induced hydraulic failure affects plant productivity and survival ([84], [51], [6]), and the European white elm has been shown to be highly susceptible to drought-induced xylem cavitation ([99]). Large xylem vessels confer this species’ high water transport capacity but counter its resistance to drought, thus supporting the trade-off between waterlogging and drought tolerance observed across species ([67]). Therefore, the expected aridification of the Iberian Peninsula ([83]) could further compromise the survival of U. laevis populations ([3], [31]), as this will probably increase water demand for irrigation, causing further depletion of water tables, and increased drought episodes. A drought-stress experiment carried out with plants from France, Sweden and Germany showed that there is substantial additive genetic variation for drought adaptation in U. laevis populations ([8]). This has also been observed for other adaptive traits such as growth and leaf phenology ([106]). Therefore, there is a genetic basis for U. laevis adaptation to future water availability changes ([8], [31]), and Mediterranean populations may well be an important resource for breeding programmes.
Reproductive ecology
Ulmus laevis is an anemophilous, self-incompatible ([63]), and highly outcrossing species ([66]). Its fruits are samaras (winged nuts) with ciliated margins, which are dispersed by both wind (anemochory) and water (hydrochory - [16]). Dispersal by two or more agents (diplochory) increases dispersal benefits and decreases seed mortality probabilities ([96]). Wind disperses 95 % of U. laevis seeds at short distances (less than 30 m), thus enabling them to reach suitable microhabitats close to mother trees, or landing on a water surface for secondary transport. In contrast, hydrochory allows long-distance gene exchange and the colorization of new sites ([101]). In some U. laevis stands, a marked spatial genetic structure can be found due to the low wind dispersal distance ([66], [98]) and the lack of secondary seed movement ([101]).
In riparian species, seed release timing is very important for seed dispersal and seedling establishment ([20], [69]). In the Iberian Peninsula samara abscission occurs from mid-April to the end of June. Therefore, samara dispersal usually occurs just after spring floods, when the conditions are optimal for seed germination and seedling establishment as the pre-existing vegetation is eliminated with the floods, and mud is deposited as water returns to the main channels ([53], [69]). Samara release rates mainly depend on maturity (phenology) and increase with strong winds, but not with rain ([101]). White elm seeds germinate soon after they are dispersed and show high germination rates ([13], SECBP, unpublished data).
Ulmus laevis is a masting species. A three year study performed in a population from central Spain showed that, in a mast year, seed production could be 24 times higher than during a non-mast year. As a result, the mast year maximum seed rain was 9020 seeds m-2, whereas in the lowest seed production year, this value only reached 8 seeds m-2 ([101]). Increased pollination efficiency in anemophilous species, and satiation of seed predators, are two factors which often favor the development of masting ([40]). Taking into consideration that the spatial distribution of U. laevis has never been large, it is unlikely that this species could control seed predator populations by masting, especially not those of highly mobile seed predators such as birds, which are attracted by large seed crops ([74]). In light of this, masting probably evolved as a result of increased pollination efficiency in U. laevis ([101]).
Seed predation is an important selective pressure which drives the evolution of seed characteristics ([39]). Parthenocarpy and empty fruit formation, which are common in elms ([52], [54]), act as a mechanism that enhances plant fitness by reducing pre- and post-dispersal seed predation ([24], [74]). Birds are the main pre-dispersal predators, consuming up to 98 % of full seeds in non-mast years ([101]). Rodents are the main post-dispersal predators, and can cause local seed extinction, especially under shrub cover, which is their favorite microhabitat ([35], [36], [74]). Therefore, open microhabitats created by floods are also of great importance to recruitment from a seed survival perspective ([101]).
Vegetative propagation mechanisms (root suckers and stool-shoots) may be important for U. laevis regeneration and colonization of new sites after flooding disturbance in riparian formations ([16], [20]). No root-suckers were observed in two Spanish wetland stands, but stool-shoots could have helped these populations to maintain genetic diversity levels after tree felling ([98]).
Conservation of Spanish populations
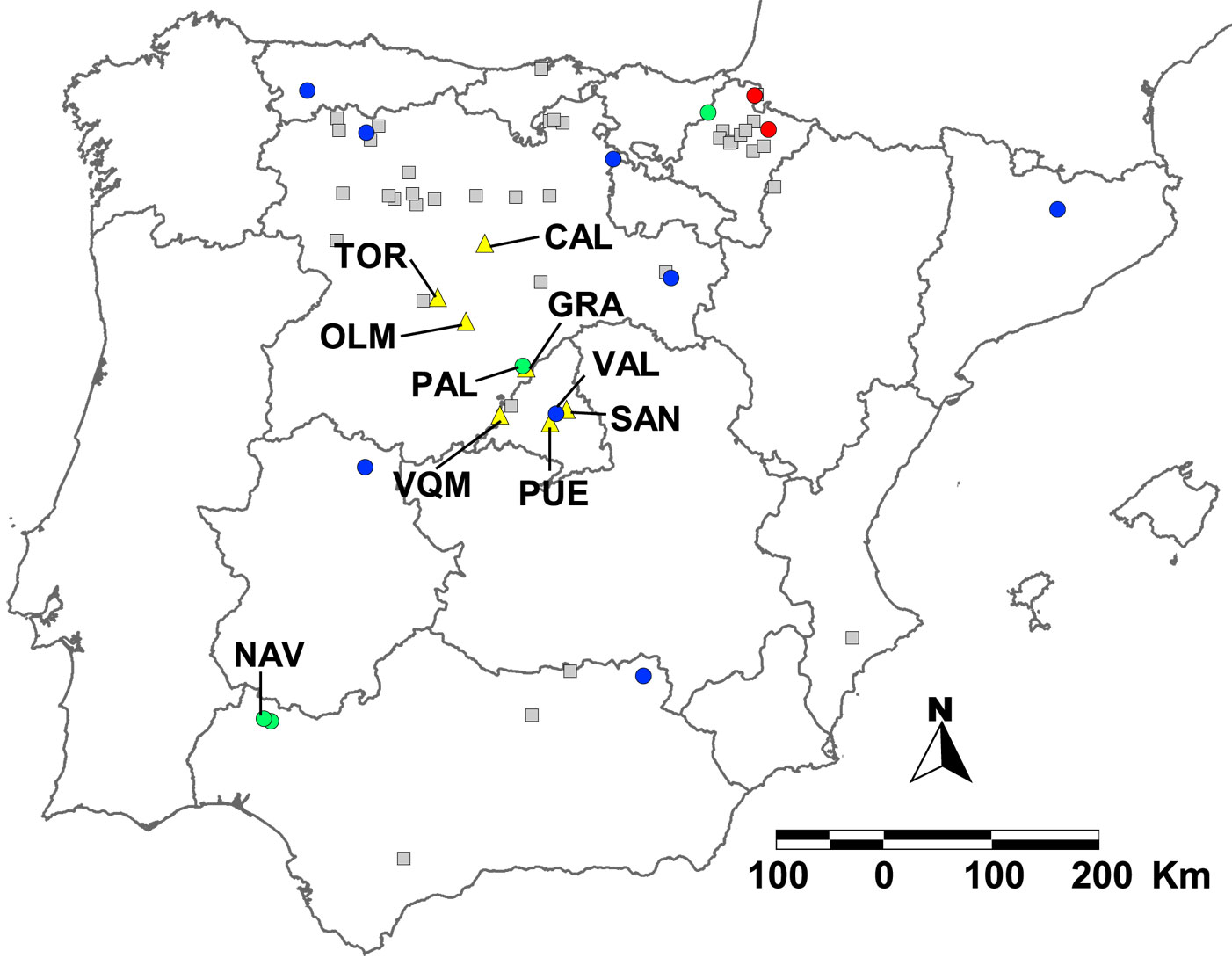
Spanish U. laevis populations are vulnerable to genetic drift, bottlenecks and stochastic events due to their small size and fragmentation ([23]). However, the greatest risk for these populations is human-induced habitat transformation. For example, the population of Palazuelos de Eresma (Segovia - PAL in Fig. 3) lost 22 % of its mature elms and cannot naturally regenerate because it was transformed into a golf course and housing complex ([98], [23]). Valdelatas stand (Madrid - VAL in Fig. 3) lacks sufficient recruitment due to underground water-table loss caused by overexploitation of Madrid’s aquifer ([98], [101]). Flow regulation of Mediterranean rivers also negatively affects the dispersal, recruitment and establishment of hardwoods due to decreased water discharge and increased flood control after damming ([68], [69], [7]). Long term conservation of U. laevis requires restoration of hydrological regimes, and the re-establishment of ecological patterns and processes where they have been destroyed by humans ([34]), taking into consideration landscape ecology concepts ([45]).
Fig. 3 - Spanish Ulmus laevis populations’ genetic knowledge and ex situ conservation sites. Populations on which genetic studies have been performed (circles), populations on which genetic knowledge is lacking (grey squares) and ex situ conservation sites (yellow triangles). Populations with chloroplast DNA (cpDNA) haplotype A (blue circles); populations with cpDNA haplotype B (red circles); populations containing both A and B cpDNA haplotypes (green circles). Data: Fuentes-Utrilla et al. ([23]), Venturas et al. ([98]), and SECBP unpublished data.
According to the International Union for Conservation of Nature’s (IUCN) Red List Criteria ([38]) U. laevis should be considered as “critically endangered” in the Iberian Peninsula because: (i) it is native; (ii) its populations cover an area smaller than 10 km2; and (iii) it is suffering a decline in habitat quality and number of adult individuals ([23]). In light of this, SECBP is currently working for the inclusion of U. laevis in the Red List of Spanish Vascular Flora ([5]), thus meaning it would be protected by national laws. Moreover, Mediterranean riparian forests with U. laevis should be included as a “natural habitat type of community interest”, under Section 92 of the Habitat and Species Directive (Council Directive 92/43/EEC), in order to strengthen U. laevis conservation within Natura 2000 Network ([23]).
The first steps towards conservation of Spanish U. laevis populations have already been initiated by the SECBP. An inventory of the occurrence of the species is the first prerequisite for establishing any gene conservation programme ([21]). SECBP already has an inventory of the populations, but it is still gathering more information on U. laevis occurrence, abundance and stand characteristics. Acquiring genetic information is of great importance when it comes to ensuring genetic variation is withheld in conservation programmes ([21], [28]). It is also necessary for defining evolutionary significant units, and management units within these, upon which conservation measures should be based ([64]). Genetic knowledge regarding certain Spanish white elm populations has served well to define management units ([23]). However, it would be interesting to complete the analyses with populations which have not yet been sampled (Fig. 3). For example, a recent survey on the population of Las Navas (Huelva - NAV in Fig. 3) has shown that it is constituted by trees with both haplotypes A and B, extending further south the presence of haplotype B (Diego Maya & SECBP, unpublished data).
Several in situ and ex situ conservation measures have been proposed for elms ([18]). Whenever possible, in situ conservation should be carried out as it enables dynamic adaptation. To avoid the potential loss of additive variance, the effective population size (Ne) to preserve should be larger than 50. Conversely, in situ stands should contain 150-200 individuals ([21]). In situ conservation is currently only being carried out in Valdelatas (Madrid). As Ne has been estimated at approximately 30 individuals ([98]), the first measure taken was to increase population size (53 mature trees) using seedlings grown from seeds collected in the stand. Ex situ conservation of Valdelatas is also being implemented by planting seedlings from this provenance in the Viñuelas stream (350 individuals; San Sebastián de los Reyes, Madrid - SAN in Fig. 3), and in the gardens (90 individuals) of Valdequemada municipality (Madrid - VQM in Fig. 3). SECBP has already established three seed-orchards with approximately 300 seedlings, each belonging to 65 families from Palazuelos de Eresma (Segovia - PAL in Fig. 3). These are located in Calabazanos (Palencia - CAL in Fig. 3), Tordesillas (Valladolid - TOR in Fig. 3) and La Granja de San Ildefonso (Segovia - GRA in Fig. 3). A re-forestation scheme with 2220 individuals from 18 families of Palazuelos de Eresma has also been carried out at the confluence of Adaja and Eresma rivers (Olmedo, Valladolid - OLM in Fig. 3), in order to allow for dynamic ex situ conservation. A few genotypes (37) from nine Spanish provenances have also been planted in the conservation orchard of Puerta de Hierro (Madrid - PUE in Fig. 3). Furthermore, in situ conservation activities should also be carried out to increase the size of other populations. Optimizing ca- nopy irradiation by thinning has been proposed as an appropriate method to increase elm stand vitality in the Czech Republic ([85]). Despite Iberian white elm stands having a different structure, the felling of competing vegetation might help increase elms’ vitality, and create clearings in the riparian forest where elm seedlings can establish themselves.
Cryopreservation of seeds is a relatively cheap and easy static way in which to preserve the genetic resources of U. laevis ([18]). Whilst there is a European cryobank with Ulmus spp. resources ([30]), it does not contain U. laevis from Spain because at the time when the bank was established the taxon was not yet considered an indigenous species. Future work should focus on cryopreserving seeds from a wide range of Spanish U. laevis populations.
Finally, the conservation of U. laevis would produce extra benefits beyond the species’ conservation itself, particularly when it occurs in a multifunctional landscape. Ulmus laevis has been identified as a good choice for floodplain reforestations ([57]) because it is less likely to be affected by DED than U. minor ([103]), and due to its ability to survive long flooding periods ([16]). The latter is an important issue for forest planning as extreme flooding events are expected to occur more frequently in central and western Europe as a consequence of climate change ([27], [44]). Restoration of river banks with U. laevis could also be beneficial for the conservation of granivorous fauna due to the large seed-crops it produces even in non-mast years ([74], [101]). White elm has been shown to be important for the conservation of epiphytic bryophyte and lichen communities in Latvia ([62]), as well as for millipede (Diplopoda) communities in Slovakia ([87]). Moreover, extracts from U. laevis may also be useful for developing drugs against cancer ([72], [29]). Conservation activities are now more likely to occur and succeed since all of the research work outlined above has led to confirmation of U. laevis’ autochthonous status and made it possible to delineate its ecology in the Iberian Peninsula.
Acknowledgements
We would like to thank Eudaldo González for the elm surveys he has carried out all over Spain, and Diego Maya for collecting samples in Huelva. We are also grateful to Pablo Sanjuanbenito, and Cuenca Alta del Manzanares, park managers, for their support. This study was funded by the Ministerio de Agricultura, Alimentación y Medio Ambiente (MAGRAMA), and by the Comunidad de Madrid (project S2009AMB-1668). M.V. was sponsored by a Technical University of Madrid “PIF” Pre-Doctoral Fellowship.
In memoriam of Margarita Burón. She was the first member of SECBP to consider that U. laevis could be native to Spain.
References
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Rosana López
Ramón Perea
Victoria Fernández
Paula Guzmán
Meng Li
Jesús Rodríguez-Calcerrada
Eva Miranda
Jorge Domínguez
Guillermo González-Gordaliza
Elena Zafra
Miriam Fajardo-Alcántara
Juan A Martín
Nikos Nanos
Carmen Collada
Luis Gil
GENFOR, Grupo de Investigación en Genética y Fisiología Forestal, E.T.S.I. Montes, Universidad Politécnica de Madrid, Ciudad Universitaria s/n, E-28040 Madrid (Spain)
ARK-Genomics, The Roslin Institute, University of Edinburgh, Easter Bush, EH25 9RG Edinburgh (UK)
IE University, Cardenal Zúñiga 12, E-40003 Segovia (Spain)
Institute of Evolutionary Biology, University of Edinburgh, West Mains Rd., EH9 9JT Edinburgh (UK)
Departamento de Química Agrícola, Facultad de Ciencias, Universidad Autónoma de Madrid, E-28049 Madrid (Spain)
Dirección General Desarrollo Rural y Política Forestal, Ministerio de Agricultura, Alimentación y Medio Ambiente, Madrid (Spain)
Corresponding author
Paper Info
Citation
Venturas M, Fuentes-Utrilla P, López R, Perea R, Fernández V, Gascó A, Guzmán P, Li M, Rodríguez-Calcerrada J, Miranda E, Domínguez J, González-Gordaliza G, Zafra E, Fajardo-Alcántara M, Martín JA, Ennos R, Nanos N, Lucena JJ, Iglesias S, Collada C, Gil L (2015). Ulmus laevis in the Iberian Peninsula: a review of its ecology and conservation. iForest 8: 135-142. - doi: 10.3832/ifor1201-008
Academic Editor
Alberto Santini
Paper history
Received: Dec 13, 2013
Accepted: Mar 01, 2014
First online: Aug 07, 2014
Publication Date: Apr 01, 2015
Publication Time: 5.30 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 60614
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 49309
Abstract Page Views: 4841
PDF Downloads: 4924
Citation/Reference Downloads: 26
XML Downloads: 1514
Web Metrics
Days since publication: 4224
Overall contacts: 60614
Avg. contacts per week: 100.45
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 16
Average cites per year: 1.45
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Seed trait and rodent species determine seed dispersal and predation: evidences from semi-natural enclosures
vol. 8, pp. 207-213 (online: 28 August 2014)
Research Articles
The effect of seed size on seed fate in a subtropical forest, southwest of China
vol. 9, pp. 652-657 (online: 04 April 2016)
Short Communications
Evidence of Alectoris chukar (Aves, Galliformes) as seed dispersal and germinating agent for Pistacia khinjuk in Balochistan, Pakistan
vol. 14, pp. 378-382 (online: 22 August 2021)
Research Articles
Seed germination traits of Pinus heldreichii in two Greek populations and implications for conservation
vol. 15, pp. 331-338 (online: 24 August 2022)
Review Papers
Implementing the dynamic conservation of elm genetic resources in Europe: case studies and perspectives
vol. 8, pp. 143-148 (online: 07 August 2014)
Research Articles
Inter- and intra-annual patterns of seed rain in the black spruce stands of Quebec, Canada
vol. 10, pp. 189-195 (online: 13 December 2016)
Research Articles
Dispersal and hoarding of sympatric forest seeds by rodents in a temperate forest from northern China
vol. 7, pp. 70-74 (online: 18 November 2013)
Short Communications
Towards an optimal sampling effort for paternity analysis in forest trees: what do the raw numbers tell us?
vol. 5, pp. 18-25 (online: 27 February 2012)
Research Articles
Moderate wildfire severity favors seed removal by granivores in a Mexican pine forest
vol. 18, pp. 121-127 (online: 24 May 2025)
Review Papers
Soil seed banks of pioneer tree species in European temperate forests: a review
vol. 11, pp. 48-57 (online: 25 January 2018)
iForest Database Search
Search By Author
- M Venturas
- P Fuentes-Utrilla
- R López
- R Perea
- V Fernández
- A Gascó
- P Guzmán
- M Li
- J Rodríguez-Calcerrada
- E Miranda
- J Domínguez
- G González-Gordaliza
- E Zafra
- M Fajardo-Alcántara
- JA Martín
- R Ennos
- N Nanos
- JJ Lucena
- S Iglesias
- C Collada
- L Gil
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
- M Venturas
- P Fuentes-Utrilla
- R López
- R Perea
- V Fernández
- A Gascó
- P Guzmán
- M Li
- J Rodríguez-Calcerrada
- E Miranda
- J Domínguez
- G González-Gordaliza
- E Zafra
- M Fajardo-Alcántara
- JA Martín
- R Ennos
- N Nanos
- JJ Lucena
- S Iglesias
- C Collada
- L Gil
Search By Keywords
PubMed Search
Search By Author
- M Venturas
- P Fuentes-Utrilla
- R López
- R Perea
- V Fernández
- A Gascó
- P Guzmán
- M Li
- J Rodríguez-Calcerrada
- E Miranda
- J Domínguez
- G González-Gordaliza
- E Zafra
- M Fajardo-Alcántara
- JA Martín
- R Ennos
- N Nanos
- JJ Lucena
- S Iglesias
- C Collada
- L Gil
Search By Keyword