Photosynthetic parameters of urban greening trees growing on paved land
iForest - Biogeosciences and Forestry, Volume 12, Issue 4, Pages 403-410 (2019)
doi: https://doi.org/10.3832/ifor2939-012
Published: Aug 13, 2019 - Copyright © 2019 SISEF
Research Articles
Abstract
Two common urban greening trees, ash (Fraxinus chinensis Roxb.) and maple (Acer truncatum Bunge.), were planted in arranged pervious and impervious land pavements to clarify the response in the photosynthetic processes of the urban tree under different types of pavement. Leaf light and CO2 response curves of the net photosynthetic rate were constructed based on in situ measurements in the 4th year after planting, and additional photosynthetic parameters were obtained. The surface temperature and soil temperature significantly increased while the soil moisture significantly decreased in the land pavement, and these changes varied with types of pavement. The light-saturated net photosynthetic rates of both ash and maple, the saturated intercellular CO2 concentration of ash, and the light saturation point, CO2-saturated net photosynthetic rate and maximum carboxylation rate of maple significantly decreased in impervious pavement, indicating that both the capacity of leaf photosynthesis and utilization of high light and CO2 concentrations were significantly reduced by land pavement. The down-regulation of photosynthesis in the impervious pavement was mainly due to the reduction of available soil water. Photosynthetic parameters of maple showed more sensitivity to the land pavement than those of ash. There was less impact from pervious pavement than impervious pavement on the photosynthetic parameters of ash and maple.
Keywords
Impervious Pavement, Pervious Pavement, Photosynthesis, Photosynthetic Parameter, Urban Tree
Introduction
Urban trees can help ameliorate urban ecology and environments and can provide a wide range of ecosystem services for residents ([44]). Urban trees can mitigate urban waterlogging ([18]), alleviate heat island effects ([30]), improve air quality ([22]), reduce noise pollution ([28]), increase carbon sequestration ([14]), and enhance the aesthetic quality of urban landscape ([3]). However, because of the expanding impervious surface within cities, urban trees are surrounded by artificial land pavements, including roads, squares, and parking lots ([43]), which can provide hostile microenvironments for trees via reducing water infiltration ([17]), increasing surface and soil temperature ([13]), restricting nutrient input ([47]), aggravating soil compaction ([25]), and inhibiting soil-air gas exchange ([24]). Growing in even adjacent sites bordering this unfavorable habitat, urban trees suffer from restricted growth and are even more vulnerable to death, which eventually limits their ecological benefits ([6], [25]).
Previous studies have shown that land pavements can lead to drought stress, heat stress, and nutrient stress in urban trees ([1], [12], [23]) and can affect plant growth and leaf photosynthesis; specifically, photosynthetic rates, transpiration rates and stomatal conductance ([32], [41]). The effects of land pavement on the physiology of urban trees change with different covering materials and whether the materials are pervious or not ([20], [21]), and the responses of various species and/or years of urban trees on the same land pavement are also different ([6], [41]). In particular, studies on the effects of pervious materials which are currently widely used for land pavement on the growth of urban trees and their eco-physiological factors are still very limited and have produced inconsistent results ([19], [36]). Furthermore, in terms of research methods, a few in situ experiments have been directly carried out on different types of pavement within cities ([32]); these studies are subject to unavoidable problems, such as inconsistent experimental conditions and surroundings, widely varying soil physicochemical properties, and increased vulnerability to human disturbance.
Photosynthesis is influenced by many ecological factors, including temperature, light intensity, CO2 concentration, soil moisture, and air humidity ([46]). Light and CO2 are the indispensable energy source and basic materials for photosynthesis, respectively ([49]). Studies on the photosynthetic response characteristics to light and CO2 can be very instrumental in elucidating the adaptability of plant photosynthetic ecophysiological parameters in changing environments ([7]). These studies could be very suitable for studying the responses and adaptability of urban tree-related photosynthetic parameters in land pavement environments; however, studies related to these phenomena are scarce ([4]). Fortunately, advanced instruments allow more photosynthetic parameters to be measured in the field; especially, light and CO2 response curves of the net photosynthetic rate can be fitted by various models to calculate related parameters, which could help to further study the influence of environmental changes on plant photosynthesis ([7], [49]).
For this study, a field-simulated experiment with three pavement treatments was designed and established in the urban environment of Beijing, China. The treatments included the control (non-paved, natural land), pervious pavement, and impervious pavement, and two common greening trees, ash (Fraxinus chinensis Roxb.) and maple (Acer truncatum Bunge.), were planted. In this experimental field, Chen et al. ([4]) reported on the growth and photosynthetic characteristics of these seedlings in the 2nd year after planting, but continuous studies for longer durations are not carried out. Wang et al. ([40]) reported on the leaf net photosynthetic rate, stomatal conductance, and chlorophyll fluorescence of these trees in the 4th year after planting and analyzed the influence of the main environmental factors, including soil moisture and temperature, but studies on the responses of leaf photosynthetic parameters to light and CO2 under land pavement are lacking, which fails to elucidate the underlying mechanism of the impacts of environmental factors on photosynthesis. In this study, we measured leaf light and CO2 response curves of the net photosynthetic rate of ash and maple in situ in the 4th year after planting. We aimed to investigate the various responses of the photosynthetic parameters in different urban tree species to the pervious pavement and impervious pavement and to further explore the biochemical factors and the underlying mechanism in order to provide references for improving the growth conditions and ecological functions of urban trees.
Materials and methods
Experimental design
A field experiment was conducted in a seed test base at Zhangtou Village, Changping District, in a suburb of Beijing, China (40° 12′ N, 116° 08′ E). The area has a typical temperate continental monsoon climate, with four distinct seasons. The mean annual temperature is 12.1 °C, and the mean annual precipitation is 542 mm; the rainfall mostly occurs from June to September. The soil texture at the study site is a sandy loam, the bulk density is 1.5 g cm-3, the mean soil organic matter content is 16.4 g kg-1, the total nitrogen is 0.9 g kg-1, the rapidly available phosphorus is 38.1 mg kg-1, the rapidly available potassium is 102.1 mg kg-1, and the pH value is 8.3 ([5]).
In April 2012, three types of pavement treatments were established: the control (non-paved, natural land), pervious pavement, and impervious pavement. The pervious pavement and impervious pavement were laid using permeable bricks (permeability > 0.4 mm s-1, 20 × 10 × 6 cm) and impermeable bricks (permeability ≈ 0, 20 × 10 × 6 cm), respectively. Three blocks were arranged for each type of pavement, which constituted three replicates; each block had the same area of 22.8 m × 17 m. One-year-old ash and maple seedlings with consistent height and basal diameter were transplanted. In each block, 18 seedlings of each species were planted in 4 parallel dislocation rows in an east-west direction with a density of 2 × 2 m. A 20 × 20 cm square tree pool was reserved on the corresponding plant point. In August 2015, plant height and basal diameter were measured using a telescopic standard measuring rod (10M, Zhongbao, Shijiazhuang, China) and a vernier caliper (E0551, Endura, Shanghai, China), respectively. The plant height showed pervious pavement > control > impervious pavement (P<0.05) and control > pervious pavement > impervious pavement (P<0.05) for ash and maple, respectively. The basal diameter of maple significantly decreased in pervious and impervious pavements as compared with the control (P<0.05) but not for ash ([40]). In September 2015, the canopy leaf area index (LAI) values were measured by a plant canopy analyzer (LAI-2000®, LI-COR, Lincoln, NE, USA). The LAI showed pervious pavement > control > impervious pavement (P<0.05) for ash and the LAI of maple significantly decreased in pervious and impervious pavements as compared with the control (P<0.05 - [40]).
Photosynthetic rate measurements in response to changes in light and CO2 exposure
Net photosynthetic rates were measured using a portable photosynthesis system (LI-6400®, LI-COR, Lincoln, NE, USA) equipped with a fluorescent leaf chamber on sunny windless mornings from 8:30 to 11:30 in 10-23 August, 10-23 September, and 8-21 October 2015. Two healthy plants were selected per block of each species; one mature healthy leaf exposed to the sun at a height of approximately 3 m for each selected plant was subsequently measured. During the measurements, the temperature and relative humidity of the leaf chamber was set to 25 ± 0.5 °C and 50 ± 5%, respectively. To obtain leaf light response curves of the net photosynthetic rate, the leaves were induced with saturation light intensity before the measurements, the reference CO2 concentration was controlled at 400 μmol CO2 mol-1 ([7]) by using CO2 supplied from a small CO2 cylinder, the photosynthetic photon flux density (PPFD) gradient consisted of 1800, 1500, 1200, 900, 600, 250, 150, 75, and 0 μmol photon m-2 s-1, and the data acquisition time at each PPFD gradient was 3 min. To obtain leaf CO2 response curves of the net photosynthetic rate, the PPFD was set to 1200 μmol photon m-2 s-1 ([48]), and the leaves were induced by the set PPFD for approximately 5 min before measurement. The CO2 gradient consisted of 400, 300, 200, 100, 50, 400, 600, 800, 1000, 1200, and 1500 μmol CO2 mol-1 by using CO2 supplied from a small CO2 cylinder. The data acquisition time at each CO2 concentration was 3 min.
Measurement of environmental factors
The pavement surface temperature (Tp, °C) was measured by the infrared temperature sensor (Optris CS, Optris GmbH, Germany) which was installed vertically on a bracket at the height of 1 m, and a transparent plastic cover was installed at the top of the sensor to prevent the influence of precipitation. The soil temperature (Ts, °C) at the depth of 20 cm was measured by the thermocouple wires. The data of Tp and Ts were recorded every 10 min by using a data acquisition device (CR1000®, Campbell, USA). The soil volume water content (VWCS, %) was measured by the ECH2O monitoring system (Decagon Devices Inc., Pullman, WA, USA) which is composed of an EC-5 soil water content sensor and an EM50 data collector. The EC-5 sensor was buried in the soil at the depth of 20 cm, and the data were recorded every 10 min by the EM50 data collector. Each sensor was installed in the center of each type of pavement.
Data analysis
Modified rectangular hyperbolic models (see Appendix 1 in Supplementary material) were used to fit the correlations between net photosynthetic rates and PPFD and CO2 concentrations ([49]). Numerous studies have confirmed that the modified rectangular hyperbolic models are very successful for modeling the responses of photosynthetic rates to PPFD and CO2 concentrations ([49], [50]) and for accurately estimating photosynthetic parameters ([38]). From the light response model, we estimated the following parameters: light-saturated net photosynthetic rate (PNmax, μmol CO2 m-2 s-1), light saturation point (Isat, μmol photon m-2 s-1), light compensation point (Ic, μmol photon m-2 s-1), and dark respiration rate (RD, μmol CO2 m-2 s-1). In addition, from the CO2 response model, we estimated the following parameters: CO2-saturated net photosynthetic rate (Amax, μmol CO2 m-2 s-1), saturated intercellular CO2 concentration (Cisat, μmol CO2 mol-1), CO2 compensation point (Γ, μmol CO2 mol-1), and photorespiration rate (Rp, μmol CO2 m-2 s-1). Furthermore, biochemical parameters were estimated from the biochemical model (see Appendix 1 - [8], [9]); these parameters included the maximum carboxylation rate (Vcmax, μmol CO2 m-2 s-1), maximum electron transport rate (Jmax, μmol CO2 m-2 s-1), and triose phosphate utilization rate (TPU, μmol CO2 m-2 s-1). The ratio Jmax/Vcmax was also calculated. The modified rectangular hyperbolic model for light and CO2 concentration responses and the biochemical model for CO2 concentration responses were both parameterized by a software developed by Jianjun Liu and Li Peng ([49]).
The data were analyzed via normal distribution tests (Shapiro-Wilk test) and homogeneity of variance tests (Levene test) before statistical analyses. Post-hoc least significant difference (LSD) tests were performed only when significant differences were detected by ANOVA. The interaction of pavement and time on photosynthetic parameters was determined using repeated measures of analysis of variance (ANOVA). The differences in environmental factors and photosynthetic parameters among different types of pavement were determined by the paired sample t-test and the LSD test, respectively. Data preparation, statistical analysis, and drawings were carried out using Excel™ 2016 (Microsoft, Redmond, WA, USA), SPSS® ver. 17.0 (SPSS, Chicago, IL, USA), and Origin® ver. 8.0 (Origin Lab, Hampton, MA, USA) software programs, respectively.
Results
Environmental factors in the three types of pavement
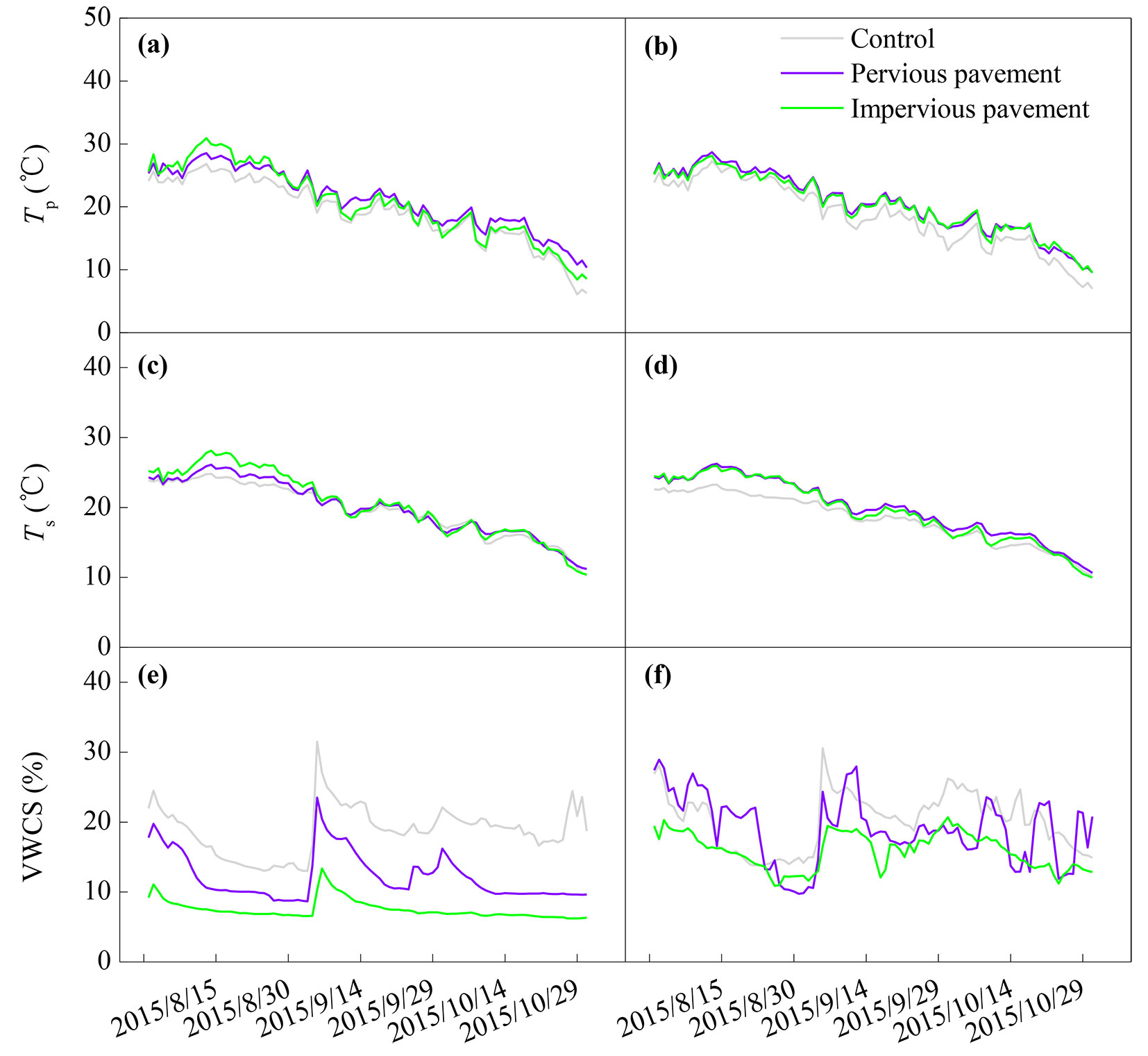
The daily mean values of Tp, Ts, and VWCS in the three types of pavement for ash and maple from 1 August to 31 October 2015 are shown in Fig. 1. There were consistent influences of the pavements on Tp for ash and maple (Fig. 1a, Fig. 1b). Compared with that on the control pavement, the Tp significantly increased on the pervious pavement and the impervious pavement by 1.97 °C and 1.50 °C for ash (P<0.05) and by 1.93 °C and 1.69 °C for maple (P<0.05), respectively. The Tp on the pervious pavement significantly increased by 0.46 and 0.23 °C for ash and maple, respectively (P<0.05), as compared with that on the impervious pavement. The influences of the pavements on Ts for ash and maple were different (Fig. 1c, Fig. 1d). For ash, the Ts was 0.87 and 0.36 °C higher in the impervious pavement and the pervious pavement than in the control and was 0.51 °C higher in the impervious pavement than in the pervious pavement (P<0.05). For maple, the Ts was 1.60 and 1.19 °C higher in the pervious pavement and the impervious pavement than in the control, and was 0.40 °C higher in the pervious pavement than in the impervious pavement (P<0.05). Because of the low permeability, the VWCS in the impervious pavement was lowest in the three types of pavement for both ash and maple (P<0.05) (Fig. 1e, Fig. 1f). Compared with that in the control pavement, the VWCS significantly decreased in the impervious pavement and the pervious pavement by 11.3% and 6.6% for ash (P<0.05) and by 4.3% and 1.3% for maple (P<0.05), respectively. The VWCS in the impervious pavement significantly decreased by 4.7% and 3.1% for ash and maple, respectively (P<0.05), as compared with that in the pervious pavement.
Fig. 1 - Daily mean values of surface temperature (Tp, °C), soil temperature (Ts, °C), and soil volume water content (VWCS, %) in the three types of pavement for ash (a, c, and e) and maple (b, d, and f).
Parameters from light response curves
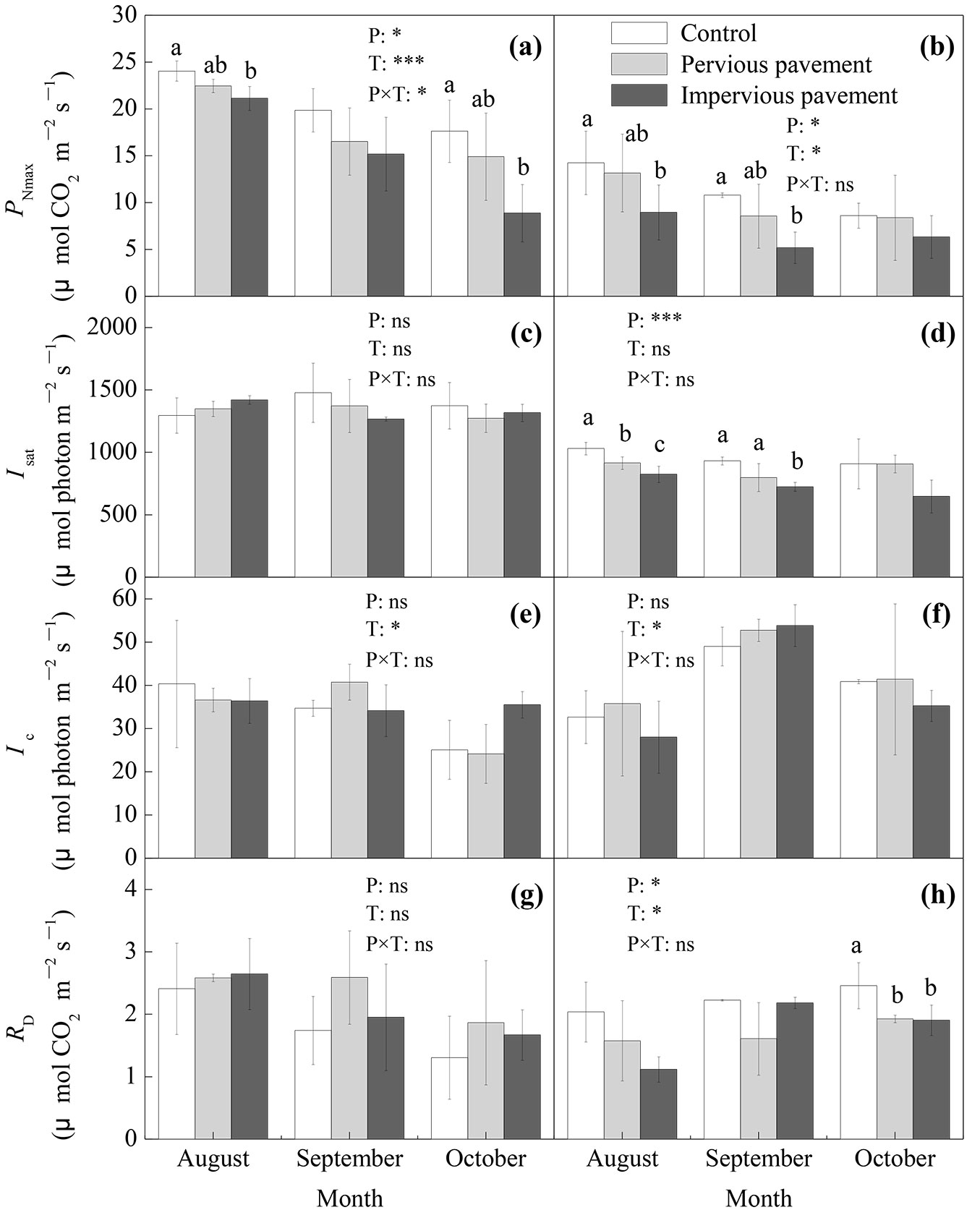
The coefficients of determination (R2) that fit the leaf light response curve of the net photosynthetic rate by the modified rectangular hyperbolic model were all greater than 0.95 (P<0.01). The pavement and time showed significant effects on the PNmax of both ash and maple (P<0.05 - Fig. 2a, Fig. 2b). Compared with that on the control pavement, the PNmax on impervious pavement significantly decreased by 12.1% and 49.6% for ash in August and October, respectively (P<0.05) and by 37.2% and 52.0% for maple in August and September, respectively (P<0.05 - Fig. 2a, Fig. 2b). There were significantly positive relationships between PNmax and VWCS for ash in August and October and maple in September (P<0.05) but no significant relationship was found between PNmax and Tp, and Ts for both ash and maple (Tab. 1). The pavement exerted significant influence on the Isat of maple (P<0.05), the Isat of maple was significantly lower on the impervious pavement than both on the pervious and control pavements in August (P<0.05) and September (P<0.05) and was significantly lower on the pervious pavement than on the control pavement in August (P<0.05); however, there was no significant difference in Isat between different pavements in any month for ash (Fig. 2c, Fig. 2d). The Ic of ash and maple exhibited no significant differences between different pavements for each measurement (Fig. 2e, Fig. 2f). The RD of maple on the control pavement was significantly higher than that on the other pavements in October (P<0.05), while the RD of ash showed no significant difference between different pavements in any month (Fig. 2g, Fig. 2h).
Fig. 2 - Light-saturated net photosynthetic rate (PNmax), light saturation point (Isat), light compensation point (Ic), and dark respiration rate (RD) of ash (a, c, e, and g) and maple (b, d, f, and h) estimated from photosynthetic light response curves under different types of pavement. The data are the averages ± SD (n = 6); different lowercase letters indicate significant differences among the different types of pavement in the same month (LSD test, P<0.05). The results of the repeated measurements ANOVA are also reported; asterisks show the significance of factors (P: pavement; T: time) and their interaction. (***): P<0.001; (*): P<0.05; (ns): P>0.05.
Tab. 1 - Pearson’s correlation coefficient between environmental factors and PNmax, and Amax of ash and maple (n = 9). (**): P<0.01; (*): P<0.05. No asterisks indicate the absence of any significant relationship.
| Parameter | Month | Ash | Maple | ||||
|---|---|---|---|---|---|---|---|
| T p | T s | VWCS | T p | T s | VWCS | ||
| P Nmax | August | -0.659 | -0.664 | 0.669* | -0.179 | -0.337 | 0.418 |
| September | -0.422 | -0.451 | 0.573 | -0.571 | -0.434 | 0.777* | |
| October | -0.082 | 0.233 | 0.699* | -0.233 | 0.050 | 0.336 | |
| A max | August | -0.395 | -0.284 | 0.341 | 0.197 | -0.099 | 0.841** |
| September | -0.485 | -0.383 | 0.547 | -0.511 | -0.311 | 0.885** | |
| October | -0.790* | -0.676* | 0.499 | 0.034 | 0.411 | 0.191 | |
Parameters from CO2 response curves
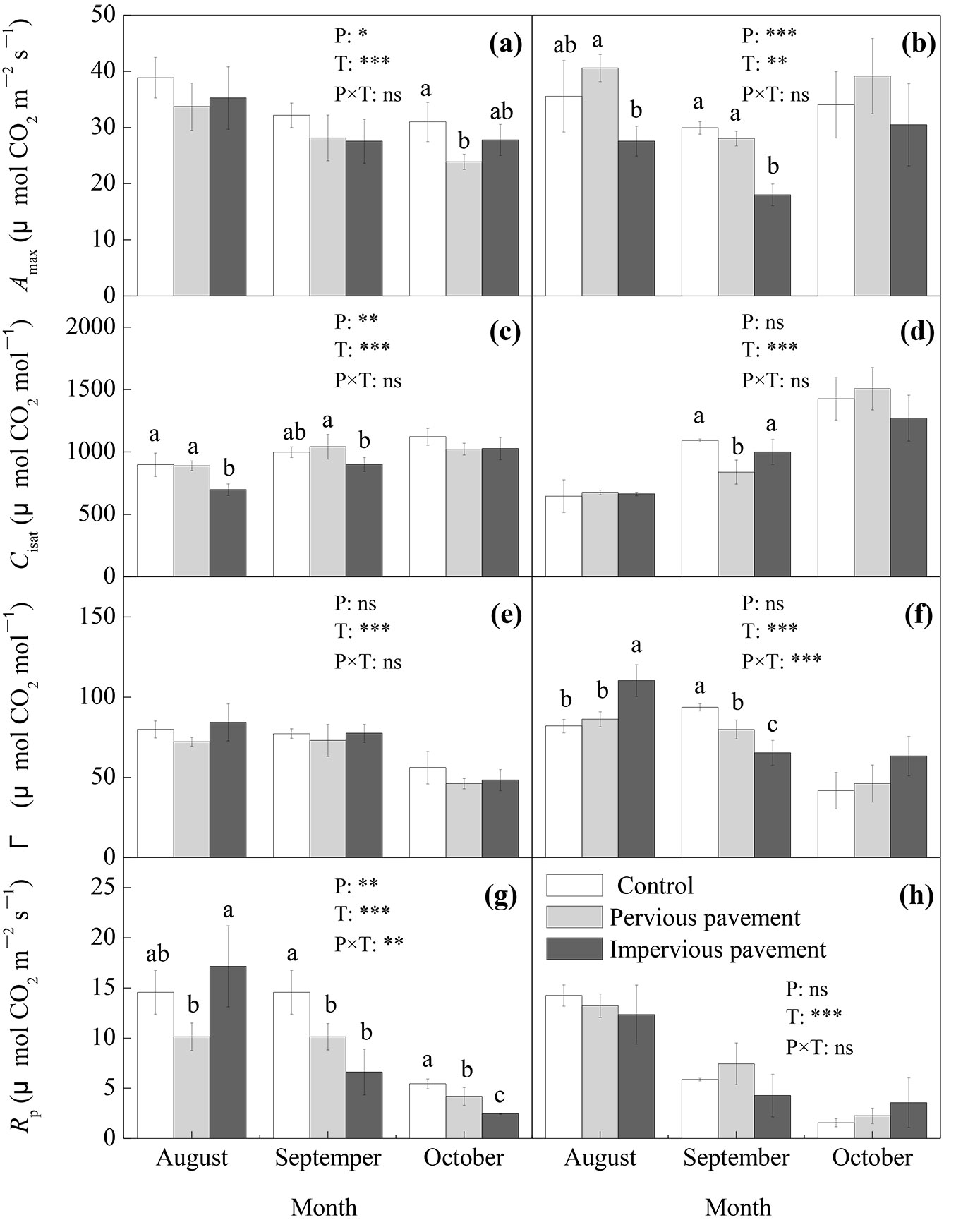
The coefficients of determination (R2) in response to fitting the net photosynthetic rate response curves to CO2 were all greater than 0.95 (P<0.01). The pavement and time but not their interactions showed significant effects on the Amax of both ash and maple (P<0.05 - Fig. 3a, Fig. 3b). Compared with that on the control pavement, the Amax on the pervious pavement significantly decreased by 23.0% for ash in October (P<0.05) and on the impervious pavement decreased by 39.8% for maple in September (P<0.05). Compared with that on the pervious pavement, Amax on the impervious pavement significantly decreased by 32.1% and 35.8% for maple in August (P<0.05) and September (P<0.05), respectively (Fig. 3a, Fig. 3b). There were significant negative relationships between Amax and Tp, and Ts for ash in October (P<0.05) and positive relationships between Amax and VWCS for maple in August and September (P<0.05 - Tab. 1). There were significant influences of pavement and time but not their interactions on the Cisat of ash (P<0.05), the Cisat of ash was significantly lower on the impervious pavement than on the other pavements in August and September (P<0.05), and the Cisat of maple was lower on the pervious pavement than on the other pavements in September (P<0.05 - Fig. 3c, Fig. 3d). The differences in Γ between pavement treatments varied with measurements for maple, while there was no significant difference for ash (Fig. 3e, Fig. 3f). The pavement and time and their interactions significantly impacted the Rp of ash (P<0.05), the Rp of ash was significantly lower on the pervious pavement than on the impervious pavement in August (P<0.05), but the opposite case occurred in September (P<0.05) and October (P<0.05). The Rp of ash was higher on the control pavement than on the other pavements in September and October (P<0.05). There was no significant difference in Rp between different pavements in any month for maple (Fig. 3g, Fig. 3h).
Fig. 3 - CO2-saturated net photosynthetic rate (Amax), saturated intercellular CO2 concentration (Cisat), CO2 compensation point (Γ), and photorespiration rate (Rp) of ash (a, c, e, and g) and maple (b, d, f, and h) estimated from photosynthetic CO2 response curves under different types of pavement. The data are the averages ± SD (n = 6); different lowercase letters indicate significant differences among the different types of pavement in the same month (LSD test, P<0.05). The results of the repeated measurements ANOVA are also reported; asterisks show the significance of factors (P: pavement; T: time) and their interaction. (***): P<0.001; (**): P<0.01; (*): P<0.05; (ns): P>0.05.
Biochemical parameters from CO2 response curves
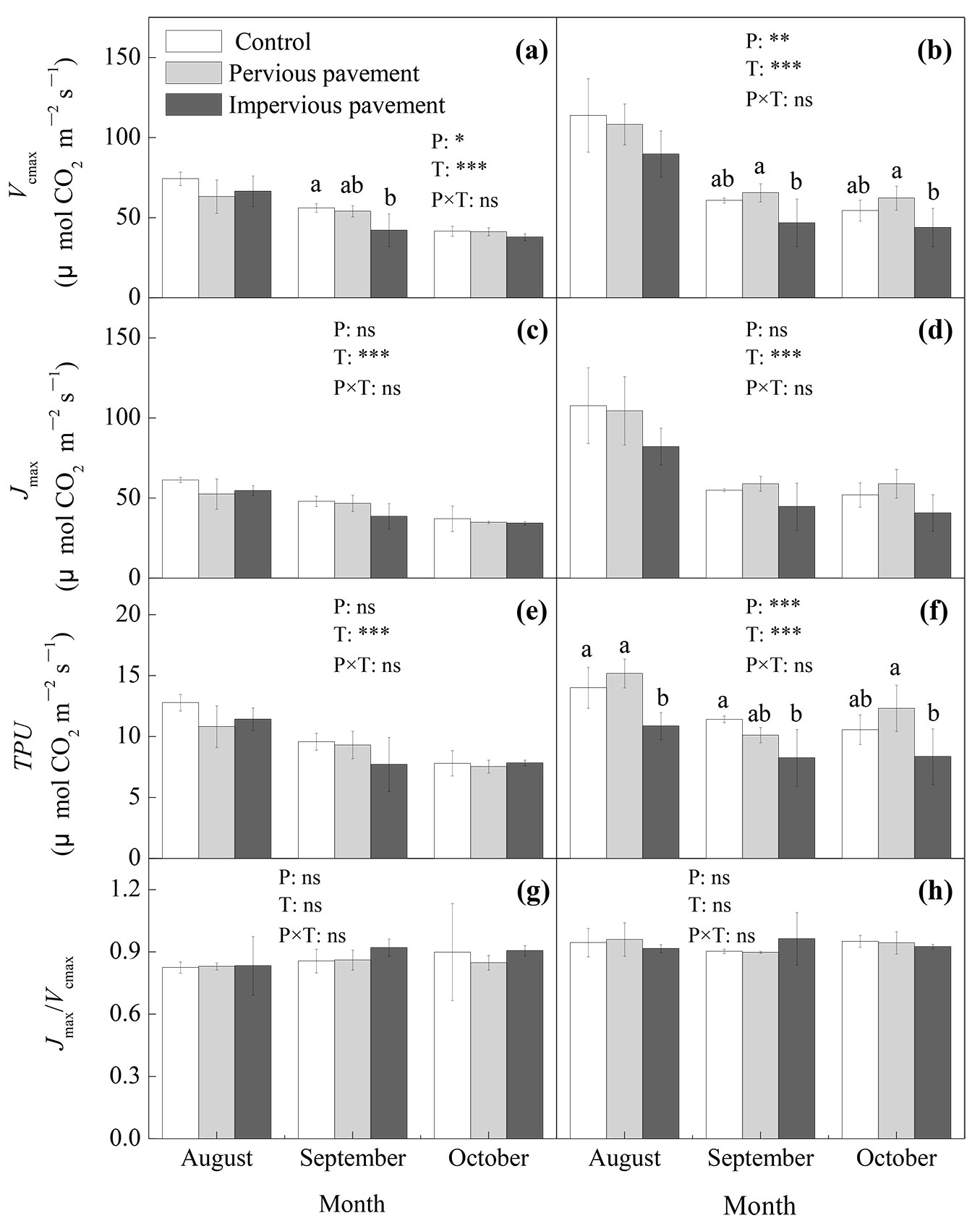
The pavement and time but not their interactions showed significant effects on the Vcmax of both ash and maple (P<0.05) (Fig. 4a, Fig. 4b). The Vcmax was significantly lower on the impervious pavement than on the control pavement for ash in September (P<0.05) and was significantly lower on the impervious pavement than on the pervious pavement for maple in both September and October (P<0.05 - Fig. 4a, Fig. 4b). The pavement and time but not their interactions significantly impacted on the TPU of maple (P<0.05), the TPU of maple was significantly lower on the impervious pavement than on the control pavement in August (P<0.05) and September (P<0.05) and was significantly lower on the impervious pavement than on the pervious pavement in October (P<0.05). The TPU of ash exhibited no significant difference between any of the pavements (Fig. 4e, Fig. 4f). There was no significant difference in the Jmax or Jmax/Vcmax ratio between any of the pavement treatments for either ash or maple (Fig. 4c, Fig. 4d, Fig. 4g, Fig. 4h).
Fig. 4 - Maximum carboxylation rate (Vcmax), maximum electron transport rate (Jmax), triose phosphate utilization rate (TPU), and Jmax/Vcmax of ash (a, c, e, and g) and maple (b, d, f, and h) estimated from photosynthetic CO2 response curves under different types of pavement. The data are the averages ± SD (n = 6); different lowercase letters indicate significant differences among the different types of pavement in the same month (LSD test, P<0.05). The results of the repeated measurements ANOVA are also reported; asterisks show the significance of factors (P: pavement; T: time) and their interaction. (***): P<0.001; (**): P<0.01; (*): P<0.05; (ns): P>0.05.
Discussion
Impacts of land pavement on the photosynthesis of plants
The pavement-induced decreases in photosynthesis are mainly attributed to the increased air and soil temperature and the decreased air relative humidity and soil moisture content ([32], [41]). We found on the impervious pavement the Tp and Ts significantly increased and the VWCS significantly decreased for both ash and maple (Fig. 1), which could be credibly deduced that the effects of higher temperature and less available soil water on photosynthesis occurred in the impervious pavement. There were significantly positive relationships between PNmax and VWCS but not Tp, and Ts for both ash and maple (Tab. 1), indicating that PNmax decreased under pavement treatments due to the reduction in soil moisture but not temperatures. The possible reason was that temperature was not significantly changed by pavement due to the strong shading effects of high leaf area index of ash and maple after their canopy closed ([40]).
Photosynthesis is regulated by both stomatal and biochemical factors ([15]). The decrease of stomatal conductance is one of the reasons that the decline of photosynthesis caused by land pavement ([32], [41]). Impervious pavement decreased water amount available for trees, and leaf stomatal conductance was reduced ([40]). In the biochemical aspect, plant photosynthesis is a complex physiological process and in general mainly consists of three steps: (1) primary reactions, including light absorption, transmission and conversion; (2) electron transport, photophosphorylation, and the formation of biological chemical energy (ATP and NADPH); and (3) carbon assimilation, which is the conversion of active chemical energy into stable chemical energy (fixed CO2, formation of sugars - [27]). The photosynthesis/photosynthetic rate would be inevitably altered if one step is affected. Intensive measurements of the physiological process of photosynthesis could help in understanding the relationships between environmental changes and photosynthesis. A series of parameters can be obtained from the light and CO2 response curves of the photosynthetic rates curves for characterizing photosynthetic process ([7], [49]).
In the first step of the photosynthetic process, leaf response to light intensity is critical. We have assessed the initial slope of the light-response curve and did not find any significant difference between pavement treatments, indicating under low light intensity the photosynthesis was not significantly influenced by pavement ([50]). But we found the Isat of maple significantly decreased on the impervious pavement (Fig. 2), implying that the utilization ability of high light intensity was reduced ([38]) and the leaf may be more susceptible to photoinhibition, which was supported by Wang et al. ([41]) based on chlorophyll fluorescence measurements. Meanwhile, the Ic of maple showed no significant change on the impervious pavement (Fig. 2), indicating that in the daytime the period of effective net photosynthesis would be reduced ([45]), which would ultimately restrict the light harvesting.
In the second step of photosynthetic processes, electron transport plays a key role and can be characterized by the Jmax in the present study. However, there was no significant difference of Jmax between pavement treatments for both ash and maple (Fig. 4). Even some previous studies have found that the Jmax was significantly inhibited by land pavement and/or drought ([16], [41]).
In the third step of the photosynthetic process, the carbon assimilation pathway of C3 plants can be divided into three stages: carboxylation, reduction, and regeneration stages. The Vcmax is the maximum carboxylation reaction rate catalyzed by the ribulose-1.5-diphosphate carboxylase/oxygenase (Rubisco - [9]). The Vcmax and Amax of maple significantly decreased on the impervious pavement (Fig. 3, Fig. 4), indicating that the photosynthetic enzyme activity and the utilization ability of high CO2 concentrations of maple might be reduced. The Vcmax is primarily affected by Rubisco content and activity, which is positively related to the leaf nitrogen (N) content and the amount of leaf N invested in the Rubisco ([37]). Under land pavements litter return to the soil is prevented, thereby decreasing nutrient supply to leaf N. Moreover, the decreased available soil water and enhanced soil compaction inhibit root nutritional uptake, all result in a smaller allocation of leaf N to Rubisco ([42]). Triose phosphates, including glyceraldehyde 3-phosphate (PGAld), are products of the reduction stage and serve as the renewable material for ribulose-1.5-diphosphate (RuBP) in the regeneration phase ([27]). The TPU of maple decreased significantly on the impervious pavement (Fig. 4). Meanwhile, there was no significant difference of Jmax and the Jmax/Vcmax ratio between pavement treatments for both ash and maple (Fig. 4), indicating that the functional balance between the electron transport and Rubisco ability of leaves was not broken. From these results, carbon assimilation rate seems to be limited by TPU rather than the rate of RuBP regeneration by Jmax. This may be because of the phosphorus (P) limitation and concomitant decrement of mycorrhizal activity under drought stress and nutrient scarcity ([11], [31]) that may be caused by land pavement. Another possible explanation is that decreasing leaf P substantially decreases the sensitivity of Vcmax to leaf N ([37]), implying that lower leaf P limits RuBP regeneration ([29]). Some previous studies have found a decrement of soil P and leaf P concentration in trees under urban environment or land pavement ([2], [10]).
Apart from the three steps of the photosynthetic process, photorespiration (Rp) is an important process influencing the net photosynthetic rate of plants. Our results showed that the Rp of ash decreased significantly in both pervious pavement and impervious pavement except in August (Fig. 3). Rubisco activity is closely related to the capacity of photorespiration ([9]) and also mediates the response of oxygen resulting in a release of CO2 as oxygenation. In the sense of decreased Vcmax in the impervious pavement for ash, the Rp should decrease. The simultaneous decrease in Vcmax and Rp in urban tree saplings under drought and pavement environment was also found in our previous study ([41]). The decrease of photorespiration could inhibit its protection mechanism for photosynthesis under high light and temperature conditions, that is using excess energy to avoid the damage of photosynthetic apparatus and decreases in the photosynthetic electron transport rate and light phosphorylation ([26]). In addition to consuming excess energy, photorespiration can release phosphate (Pi) groups that could be combined with PGAld; this phenomenon could mitigate the restrictions of the electron transport and photophosphorylation caused by the temporary deficit of Pi ([39]).
Different impacts between pervious and impervious pavements
Generally, pervious pavement can alleviate impervious pavement-induced decreases in water infiltration through the surface into the subsoil. Former studies reported higher infiltration rates and higher soil water contents under pervious pavement than under impervious pavement ([4]). Our results also showed that the VWCS was significantly higher under pervious pavement than under impervious pavement for both ash and maple (Fig. 1). However, pervious pavement contains more pores filled with air, which prevents heat from transferring downward; as such, the surface temperature increases quickly, resulting in higher surface temperatures on pervious pavement than on impervious pavement ([6]), this phenomenon was also observed in the present study (Fig. 1). Due to the complex effects of pervious pavement on the hydrothermal environment for trees, related studies reported inconsistent results such as pervious pavements have adverse or no significant ameliorative effects on tree growth ([19], [35]). Field measurements showed that, compared with unpaved land, pervious pavement, as well as impervious pavement, inhibits leaf net photosynthetic rates ([4]). Those inhibitions may occur when the trees are young and could not provide significant shading effects on paved land; in return, the trees suffer from a harsh, hotter environment. In the present study where tree canopy was approaching to closure, the PNmax and Amax of ash and maple did not significantly reduce on the pervious pavement but significantly reduced on the impervious pavement (Fig. 2, Fig. 3). This may be due to the difference in available soil water between pavement treatments, since the limited decrement of VWCS in pervious pavement did not decline below the tolerance of these two tree species and thus could not exert significant effects on their photosynthesis. Although there were significant impacts of the pervious pavement on some photosynthetic parameters (e.g., Rp for ash - Fig. 3), more parameters were impacted by the impervious pavement than by the pervious pavement because of less available soil water under the former.
Influences of tree species
In our study, the impacts of land pavement on photosynthesis/photosynthetic parameters varied with the two tree species. Photosynthetic parameters such as Isat, Amax, Vcmax, and TPU were significantly influenced by land pavement (especially impervious pavement) for maple more than for ash (Fig. 2, Fig. 3, Fig. 4). This finding indicated that maple showed more sensitivity than did ash on the pavement. This phenomenon may be due to the interaction between environmental factors and tree species and the different physiological features of ash and maple. We documented that the Ts was highest in the impervious pavement for ash while in the pervious pavement for maple (Fig. 1), which was also documented in the study of Chen et al. ([6]), and this interaction might make the effects of land pavement on photosynthetic processes for different tree species appear more complex. Apart from the different thermal environments, there is a noticeable difference in physiological feature between the ash and maple, the former is ring-porous wood and the latter is diffuse-porous wood. Ring-porous wood has a stronger maximum water transport capacity than does diffuse-porous wood; this ability can ensure water supply better ([33]). The latter study pointed out that ash has higher stomatal conductance and leaf transpiration and more obvious variations between pavement treatments than maple ([40]), which seems to be the main cause of much more difference in VWCS between pavement treatments in ash in comparison to maple. Although ring-porous wood is more susceptible to producing cavitation and embolisms under water stress, compared with diffuse-porous wood, this type of wood species have a stronger stomatal regulatory ability that can reduce more effectively the threat of embolisms ([34]). Thus, ash trees can ensure better water supplies than maple, even under unfavorable soil water conditions caused by impervious pavement. Overall, land pavement-induced soil moisture decreases could impact maple more than ash.
Conclusions
Urban trees growing on paved land experience hostile environments, e.g., hot surfaces, soil moisture deficit, and soil compaction. Plant responses to stress initially manifest in physiological characteristics. The results of this study showed that temperature significantly increased and the soil moisture significantly decreased in the land pavement, and these changes varied between impervious pavement and pervious pavement. The PNmax of both ash and maple significantly decreased on the impervious pavement, mainly due to the reduction of available soil water. Different changes in photosynthetic parameters were found in different tree species. For ash, Cisat decreased significantly, indicating that the utilization ability for high concentrations of CO2 decreased, Rp significantly decreased, which inhibited the protection mechanism. For maple, Isat decreased significantly, such that photoinhibition would occur more easily; Amax decreased significantly, inhibiting the photosynthetic electron transport and phosphorylation activity; Vcmax was significantly reduced, implying that the Rubisco activity was influenced; and the TPU significantly decreased, which inhibited RuBP regeneration. The significant decreases in the Isat, Amax, and Vcmax of maple and in the Cisat of ash on the impervious pavement indicate that the capacity of leaf photosynthesis and the utilization of high light and CO2 concentrations were significantly reduced by land pavement. Overall, land pavement affected the photosynthetic parameters of maple more than those of ash, implying that maple showed more sensitivity. Compared with that from the impervious pavement, there was less influence from the pervious pavement on the photosynthetic parameters of ash and maple, suggesting pervious materials are more suitable for land pavement where trees will be planted.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (41571053, 71533005), the National Key R&D Program of China (2016YFC0503004), and the State Scholarship Fund of China Scholarship Council (201804910455).
References
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Xiaoke Wang
Yuanyuan Chen
State Key Laboratory of Urban and Regional Ecology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085 (China)
University of Chinese Academy of Sciences, Beijing 100049 (China)
School of Forestry and Environmental Studies, Yale University, New Haven CT 06511 (USA)
Corresponding author
Paper Info
Citation
Wang X, Wang X, Chen Y, Berlyn GP (2019). Photosynthetic parameters of urban greening trees growing on paved land. iForest 12: 403-410. - doi: 10.3832/ifor2939-012
Academic Editor
Silvano Fares
Paper history
Received: Aug 02, 2018
Accepted: May 26, 2019
First online: Aug 13, 2019
Publication Date: Aug 31, 2019
Publication Time: 2.63 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2019
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 44698
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 37777
Abstract Page Views: 3298
PDF Downloads: 2816
Citation/Reference Downloads: 1
XML Downloads: 806
Web Metrics
Days since publication: 2382
Overall contacts: 44698
Avg. contacts per week: 131.35
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2019): 4
Average cites per year: 0.57
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Photosynthesis of three evergreen broad-leaved tree species, Castanopsis sieboldii, Quercus glauca, and Q. myrsinaefolia, under elevated ozone
vol. 11, pp. 360-366 (online: 04 May 2018)
Research Articles
Magnolia grandiflora L. shows better responses to drought than Magnolia × soulangeana in urban environment
vol. 13, pp. 575-583 (online: 07 December 2020)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Short Communications
Variation in growth, photosynthesis and water-soluble polysaccharide of Cyclocarya paliurus under different light regimes
vol. 10, pp. 468-474 (online: 04 April 2017)
Research Articles
Wintertime photosynthesis and spring recovery of Ilex aquifolium L.
vol. 12, pp. 389-396 (online: 31 July 2019)
Research Articles
Adjustment of photosynthetic carbon assimilation to higher growth irradiance in three-year-old seedlings of two Tunisian provenances of Cork Oak (Quercus suber L.)
vol. 10, pp. 618-624 (online: 17 May 2017)
Research Articles
The use of branch enclosures to assess direct and indirect effects of elevated CO2 on photosynthesis, respiration and isoprene emission of Populus alba leaves
vol. 1, pp. 49-54 (online: 28 February 2008)
Research Articles
Response of Chinese sea buckthorn clonal growth and photosynthetic physiological mechanisms toward a soil moisture gradient
vol. 14, pp. 337-343 (online: 15 July 2021)
Research Articles
Light acclimation of leaf gas exchange in two Tunisian cork oak populations from contrasting environmental conditions
vol. 8, pp. 700-706 (online: 08 January 2015)
Research Articles
Green oriented urban development for urban ecosystem services provision in a medium sized city in southern Italy
vol. 7, pp. 385-395 (online: 19 May 2014)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword