Networking sampling of Araucaria araucana (Mol.) K. Koch in Chile and the bordering zone of Argentina: implications for the genetic resources and the sustainable management
iForest - Biogeosciences and Forestry, Volume 2, Issue 6, Pages 207-212 (2009)
doi: https://doi.org/10.3832/ifor0524-002
Published: Dec 22, 2009 - Copyright © 2009 SISEF
Research Articles
Collection/Special Issue: EFI 2008 Annual Conference Week - Orvieto (Italy)
Adaptation of Forest Landscape to Environmental Changes
Guest Editors: Giuseppe Scarascia Mugnozza (CRA - Rome, Italy)
Abstract
Araucaria araucana, a southern South American tree species, is considered a representative symbol of Chilean forest biodiversity due to its endemicity and longevity. An expedition to Chile and its border zone with Argentina was carried out with the aim of determining the current state of this species and to establish a tree network to study its genetic variability. Eight locations were selected across the range of distribution of the species and several experimental plots were established based on the heterogeneity within each location. Field trips revealed a high fragmentation in Araucaria forests showing low or non-existent regeneration in most of its distribution area. Experimental plots allowed the identification of severely altered areas. Moreover, 371 trees were catalogued, 193 males and 178 females. The tree network established will be very useful in future evaluations of both the ecological status of the species and its genetic resources, allowing the development of conservation strategies.
Keywords
Araucaria araucana, Biodiversity, Ecological attributes, Genetic resources
Introduction
The Chilean native forest and that in the Argentinean border zone include more than one hundred species and constitute one of the forest ecosystems richest in biodiversity in the world ([23]). Within forest formations Araucaria araucana (Mol.) K. Koch stands out as the dominant species given its particular characteristics. Indeed, it is considered a representative symbol of the Chilean forest biodiversity due to its endemicity and its longevity ([18]).
Araucaria distribution is fragmented and restricted to some specific areas in Chile and Argentina. In Chile, it covers over 261 000 ha representing about 65% of the total area in which it is found. The principal location is situated in the Andean Cordillera at latitudes between 37º30’ and 39º30’ S, in Bio-Bio and Araucania Regions and there are some isolated stands in the north Andean limit in the Lakes Region. The second area is found in the coastal cordillera (Nahuelbuta Cordillera) between 37º20’ and 38º40’ S ([33]). In Argentina the species covers a restricted area at latitudes between 37º45’ and 40º20’ S.
A. araucana occurs in either pure or mixed species stands along with Nothofagus spp. at an elevation of between 1000 and 1600 m. It is associated with coigüe (Nothofagus dombeyi Mirb. Oerst.) and seldom with oak (Nothofagus obliqua Mirb. Oerst. var.) in the lower altitudes, with coigüe and lenga (Nothofagus pumilio Poepp. et Endel. Krasser) in medium altitudes and only with lenga at upper forest limits ([15], [9], [34]).
A. araucana is dioecious, but it can occasionally be monoecious with pollen predominantly dispersed by wind and gravity seed ([24]; [17]). It also regenerates vegetatively by sprouting and root suckering ([30], [33], [10]).
The araucaria’s current distribution is a remnant of a more extensive former distribution that was severely diminished by the intense exploitation of the species in the past. Other factors which have a great impact are domestic herbivores and seed harvest ([31], [33], [1], [6]) and forces of nature as wind, volcanism and fire ([33], [8]). As a result, conservation of the species is a subject of great concern given its restricted distribution, slow growth and poor regeneration capacity which make it particularly susceptible to external pressures ([22], [26], [27]). It is included in Appendix I of the Convention on International Trade of Endangered Species of Wild Flora and Fauna (CITES) and is listed as a “vulnerable” species on the Red Data list published by the International Union for Conservation of Nature ([19]). Furthermore, in Chile in the Supreme Decree 43, araucaria is classed as a national monument and is officially protected.
Although this species is not under imminent risk of extinction, its structure and natural forest dynamic show deep perturbations ([7], [14], [11]). In order to assess whether ongoing patterns of habitat fragmentation could threaten its genetic resources and its evolutionary potential, it is necessary to explore the genetic diversity and ecological attributes across the range of the species. Even more, the correlation of genetic data with ecological and geographic variables may show more complete information about the state of the species. In fact, Turner ([32]) mentioned that by combining all these aspects areas of potential conservation sites may be identified.
Studies based on the spatial distribution of the araucaria’s genetic variation have been conducted using different markers as gas liquid chromatography ([29]), isozymes ([16]) and RAPDs ([4], [5]). These studies indicated that genetic diversity is high and unaffected by the ecological heterogeneity of the species. Nevertheless, in recent years other DNA markers have proved to be more suitable for studying population genetic diversity as microsatellite or simple sequence repeats (SSRs). In fact, the identification of markers linked to genes involved in the adaptive response to environmental changes could contribute to define criteria to plan conservation strategies as is the case of EST-based SSRs. Moreover, there is no record relating to the temporal distribution of araucaria genetic diversity, that is, if the decrease in the surface area occupied by the species has caused changes in the genetic composition of young trees in relation to the older ones. In fact, in other woody plants as Olea europaea L., it has been demonstrated that older individuals include allelic variants that are not present in new stands ([25]). It is therefore of particular importance the establishment of a tree network which will allow the monitoring of the state of the genetic resources and the role of each stand in the conservation of the species.
In this study an expedition to the areas of A. araucana distribution was conducted to determine the current state of the species and to establish a tree network for future evaluation of its genetic variability and its spatial and temporal distribution. Specifically, further evaluations of ecological and germplasm data may allow the detection of possible threats to the genetic resources of the species and the development of conservation strategies to restore these degraded areas.
Material and methods
Study area
Aerial photographs and satellite imagery were used to select the study area. These fulfilled two objectives, the stratification and the initial plot measurement, both carried out in Concepción University (Chile). Geographic Information System (GIS) software was also used to provide information on the status and condition of the araucaria forests. Coverage included all forested lands in Chile: private and public.
Permanent sample plots have been recognised as an interesting approach in determining changes in forests. This type of sampling has two objectives: to provide a description of the stand and to determine areas to be repeatedly measured over time. Accordingly, this permanent method was used for establishing the tree network to detect changes in araucaria regeneration.
With this basic information, field trips were carried out from March to April 2007 across the species range. Field trips were based on one field sample site of 261 000 ha. The tree network was designed by an expert panel (DELPHI method) covering as many different habitats as possible.
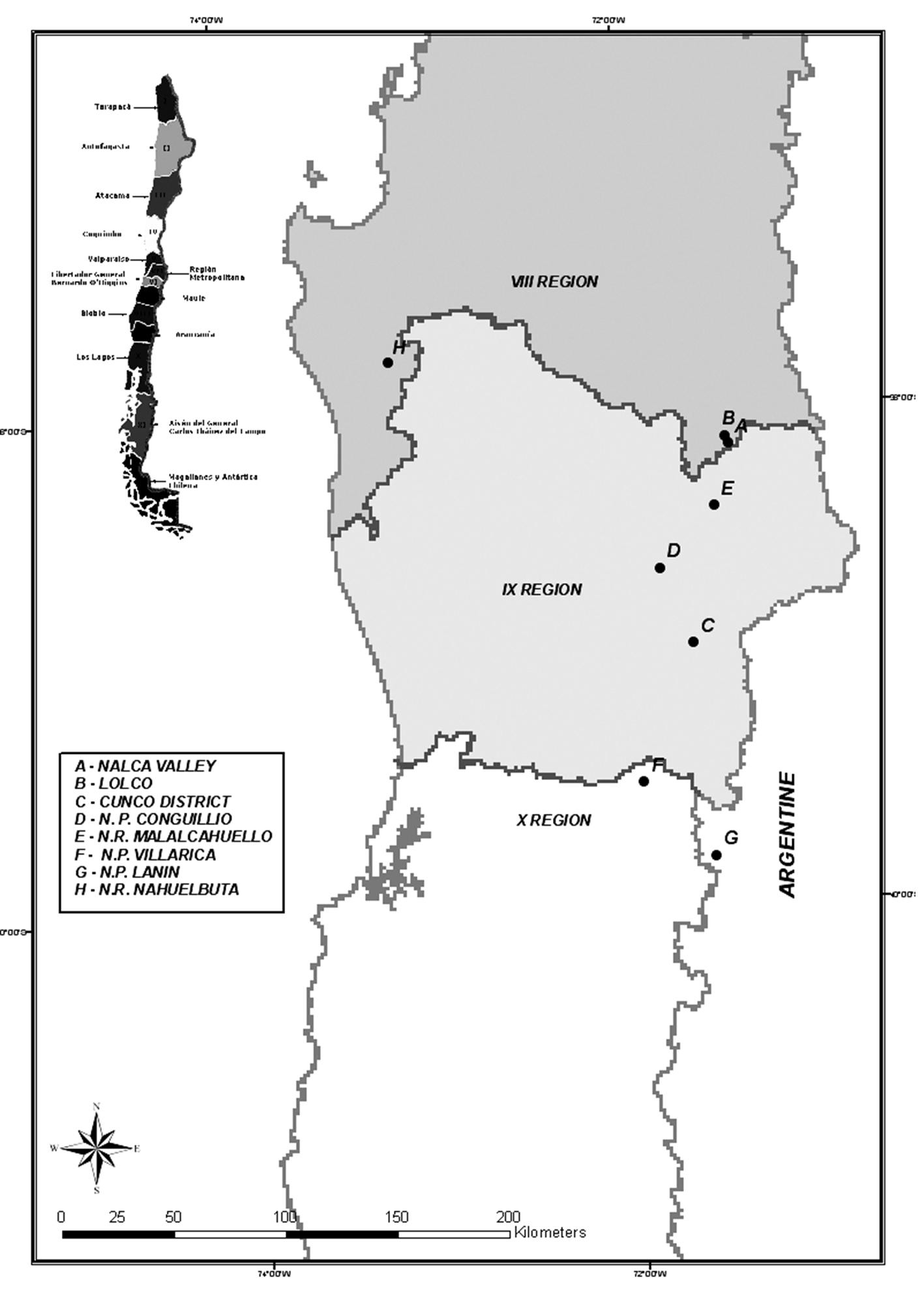
The locations chosen for this study included areas from both the Chilean and Argentinean sides of the Andean Cordillera and the coastal range of Chile (Fig. 1). According to climatic, ecological and geographical attributes, eight locations were identified and catalogued, seven in the Andean Cordillera and one in the Nahuelbuta Cordillera (Tab. 1). Depending on the ecosystems heterogeneity, between one and three circular plots (1000 m2) were installed within each location. Specifically, three plots were identified in Cunco District and Malalcahuello N.R, two plots in Conguillio N.P., Lanin N.P. and Nahuelbuta N.R. and one plot in Lolco, Nalca Valley and Villarrica (Tab. 2).
Tab. 1 - Data of latitude, longitude and altitude measured in each location in Chile and Argentina.
| Area | Region | Location | Latitude (S) | Longitude (W) | Altitude (m) | Country |
|---|---|---|---|---|---|---|
| Andean Cordillera | Bio-Bio (VIII) | Nalca Valley | 38º 17’ | 71º 27’ | 1200-1500 | Chile |
| Lolco | 38º 16’ | 71º 28’ | 990-1000 | Chile | ||
| Araucania (IX) | Cunco District | 38º 57’ | 71º 40’ | 1200-1450 | Chile | |
| Conguillio N.P. | 38º 39’ | 71º 50’ | 1100-1170 | Chile | ||
| Malalcahuello N.R. | 38º 25’ | 71º 32’ | 1350-1430 | Chile | ||
| Lakes (X) | Villarrica N.P. | 39º 32’ | 71º 57’ | 950-1000 | Chile | |
| Lanin N.P. | 39º 47’ | 71º 40’ | 1000-1100 | Argentina | ||
| Coastal Cordillera | (IX) | Nahuelbuta N.R. | 37º 48’ | 71º 57’ | 1200-1400 | Chile |
Tab. 2 - Ecological attributes measured in the eight locations in Chile and Argentina.
| Plot | Name | Stand composition |
Understory composition |
Slope (%) | Aspect | Regeneration | Stand development |
Observations | |
|---|---|---|---|---|---|---|---|---|---|
| full shade zone | open zone | ||||||||
| 1 | Nalca Valley | Nothofagus antartica (20%) | Chusquea quila | 5-15 | South-Eastern | Low | High | Even aged | Volcanic soil |
| 2 | Lolco | - | Nothofagus antartica; Chusquea quila | 0-5 | North-Western | None | None | Old grown stage | Dense shrub load |
| 3 | Cunco District |
Nothofagus dombeyi (50%) | Drymis winteri; Chusquea culeu; Nothofagus dombeyi | 15-30 | South | Low | None | Even aged | - |
| 4 | Cunco District |
Nothofagus dombeyi (90%) | Drymis winteri; Chusquea culeu; Nothofagus dombeyi | 5-15 | Flat | Low | None | Even aged | - |
| 5 | Cunco District |
Nothofagus pumilio (70%) | Nothofagus pumilio firewood | 5-15 | North | High | High | Uneven aged | Cattle |
| 6 | Conguillio N. P. |
Nothofagus dombeyi (40%) | Chusquea quila | 0-5 | Flat | Medium | None | Uneven aged | Lava cone |
| 7 | Conguillio N. P. |
Nothofagus dombeyi (5%) | Chusquea quila; Persea lingue regeneration | 0-5 | Flat | Low | None | Uneven aged | Lava descendent way |
| 8 | Malalcahuello N. R. | - | Nothofagus antartica; Podocarpus saligna | 5-15 | North-Eastern | High | Medium | Even aged | Presence of recent volcanic ash |
| 9 | Malalcahuello N. R. | - | Grassland | 0-5 | Flat | High | High | Uneven-age | Stand altitude limit |
| 10 | Malalcahuello N. R. | - | Grassland | 5-15 | South-Eastern | Medium | None | Uneven-age | Stand altitude limit |
| 11 | Villarica N. P. |
Nothofagus dombeyi (10%) | Nothofagus antartica; Persea lingue; Chusquea quila | 0-5 | North-Eastern | None | None | Even aged | Sparce regeneration. Thalweg |
| 12 | Lanin N. P. | Nothofagus dombeyi (10%) | Nothofagus antartica; Persea lingue; Chusquea quila | 0-5 | South | Low | None | Even aged | - |
| 13 | Lanin N. P. | - | Grassland | 0-5 | Flat | None | None | Old grown | Steppe |
| 14 | Nahuelbuta N. R. |
Nothofagus dombeyi (50%) | Drymis winteri; Persea lingue; Chusquea quila | 5-15 | South | Medium | Medium | Even aged | Rocky surface |
| 15 | Nahuelbuta N. R. |
Nothofagus dombeyi (5%) | Nothofagus antartica; Chusquea quila | 5-15 | North | Low | Medium | Even aged | Recreational area. Old surface fire |
Assessments
The position of all trees in each plot was mapped and data of their altitude, latitude and longitude were measured using the Geographical Positioning Satellite system (GPS). Selected trees were located in a latitude range from 37º48’ S to 39º47’ S and an altitude range from 950 to 1500 m.
In each site, tree diameter, understory species, natural regeneration and topographic attributes were measured within each plot (Tab. 2). Stands were evaluated according to their composition and development. For the stand composition, the percentage of associated species was calculated according to the total density of Araucaria and Nothofagus trees (Tab. 2). The araucaria structure was classified into three categories: even-aged, uneven-aged and old growth. The understory was described in terms of composition and plant cover and topographical conditions expressed through measurements of slope and aspect. Gap-phase regeneration is considered to be the common way in which forests are renewed due to the suitable conditions for the establishment of seedlings. Araucaria seedling growth is slow until the canopy opening is created. For this, araucaria regeneration was classified into two types depending on the possibility of seedling establishment: full shade zone and open zone. Open zone regeneration is the cornerstone of the species natural dynamics because of its slow growth and the competition from undergrowth species. Natural regeneration in each plot was established through visual estimation into four qualitative levels according to the seedling number: none (> 50 seedlings per ha), low (50-300 seedlings per ha), medium (300-600 seedlings per ha) and high (< 600 seedlings per ha).
To estimate the genetic diversity, the criterion was to catalogue 30 female and 30 male trees in each location, given that araucaria is a dioecious species. In the case of female individuals, seeds from each tree were collected for future genetic analyses using three age classes: thicket (< 50 years), polewood (100-200 years) and all growth (> 400 years). The assignation of each tree to its age class was conducted according to the index site and the diameter class equation proposed by Drake et al. ([12]) and modified specifically for araucaria by Peraza ([28]) (eqn. 1):
being DBH the diameter measured at breast height. In total, five index sites were calculated according to climatic and forestry attributes (Tab. 3).
Tab. 3 - Ecological attributes measured in the eight locations - DBH: diameter at breast height.
| Index site | DBH (cm) |
Height (m) |
Temperature range (Cº) |
Precipitation (mm) |
Location |
|---|---|---|---|---|---|
| 1 | 54.04 | 37 | 4.6/13.9 | 2900-3300 | Villarrica N.P, Lanin N.P. |
| 2 | 40.39 | 30 | -0.9/13.9 | 3200-3500 | Cunco District |
| 3 | 57.86 | 25 | 5.4/26 | 1300-1600 | Nahuelbuta N.R |
| 4 | 56.77 | 27 | -3 to 2/12 | 1800-2050 | Nalca Valley, Lolco, Conguillio N.P. |
| 5 | 44.79 | 15 | -3/12 | 1850-2100 | Malalcahuello N.R |
Results and Discussion
Description of Araucaria stands
Concerning the different locations studied, the stand composition associated with Araucaria-Nothofagus was verified in six of the eight locations studied (Tab. 2), while pure stands were found in Malacahuello N.R., Lolco and one of the two plots in Lanin N.P. In this latter site, the stand was composed of wooded steppe with a low canopy and conical shaped trees typical of growth without competition (Fig. 2F).
Fig. 2 - A representative sample of the A. araucana different habitats catalogued in Chile and Argentina. A) Cunco District (Chile); B) Malalcahuello N.R. (Chile); C) Conguillio N.P. (Chile); D) Lolco (Chile); E) Nahuelbuta N.R. (Chile) and F) Lanin N.P. (Argentina).
Lolco and Lanin N.R. were the most fragmented areas (Fig. 2D). In Lolco, the low density of the species made plot establishment difficult (<50 trees/ha). Likewise, it was stated that this area was severely altered since no signs of regeneration were detected, and the remaining individuals showed old grown state. According to Drake ([13]), this might indicate that araucaria stands are in decline phase and will be lost in a short period of time. In Villarrica N.P. araucaria stands showed a good height growth, but without regeneration and with a low ratio female/male trees (Tab. 2 and 4). Nalca Valley and one of the plots in Conguillio N.P. showed medium levels of regeneration. Nevertheless, in Nalca Valley regeneration was found to be better in open zones while in Conguillio N.P. regeneration was found in full shade zones (Fig. 2A, 2B and 2C).
In Nahuelbuta Cordillera, araucaria showed medium levels of natural regeneration forming even aged stands associated with N. dombeyi (Tab. 2, Fig. 2E). Furthermore, due to the high forest density in these stands a higher proportion of trees were found with umbrella form. In Malalcahuello N.R. araucaria stands grew in different physiographical conditions (slope and aspect). In Cunco District, the stand (N. Dombeyi and N. Pumilo) and the canopy composition (percentage of associated species) displayed irregular natural regeneration (Tab. 2).
Establishment of a tree network for the genetic diversity analysis
Altogether, 371 trees were catalogued, 193 males and 178 females. Nevertheless, the different characteristics of the evaluated stands made our initial objective feasible in only two locations, Cunco District and Malalcahuello N.R. The target number of female trees was also achieved in two other sampling sites, Nalca Valley and Lanin N.R. and the target male in one more, Nahuelbuta N.R. (Tab. 4). The expected number of sampled trees was not attained in all locations due to the different states of the evaluated stands. The situation found in Villarrica N.R. and Lolco was more complicated than the aforementioned since the number of female trees was very low (8 and 3, respectively) and in the latter this was also the case with respect to the number of male trees (Tab. 4). In fact, in Lolco the ecological situation was highly altered and with alarming density levels (Tab. 2). These results reveal that the trees listed in this study are the only remaining of the species in these areas (Tab. 4). Given all this, the impossibility to attain the sampling target is clear proof of the threat to its genetic diversity.
Tab. 4 - Number of female and male trees sampled in the collecting mission.
| Location | Nº Female trees | Nº Male trees | Nº Total trees |
|---|---|---|---|
| Nalca Valley | 30 | 25 | 55 |
| Lolco | 3 | 5 | 8 |
| Cunco District | 30 | 37 | 67 |
| Conguillio N.P. | 25 | 22 | 47 |
| Malalcahuello N.R. | 30 | 39 | 69 |
| Villarrica N.P. | 8 | 20 | 28 |
| Lanin N.P. | 31 | 0 | 31 |
| Nahuelbuta N.R. | 21 | 45 | 66 |
| Total | 178 | 193 | 371 |
Nevertheless, we consider that not only the number of catalogued trees but also their distribution (spatial, sexes and age classes) is an excellent basis for genetic analysis, providing for a first round examination of the species from a broad distribution range and allowing the identification of further areas of interest.
Our future goal, whenever funding will be available, would be to address the above studies using genetic markers as seed storage proteins and SSRs. Indeed, the former have been used as an estimator of the genetic diversity in forest species ([2], [3], [20], [21]) and in the case of conifers this technique is particularly favourable, given the haploid nature of the reserve tissue.
In fact, as the collecting mission was carried out in the fruit ripening period, an additional activity of sampling seeds from all female trees was undertaken. We consider that the use of both kinds of the above markers will provide good information to set up lines of action aimed to ensure the preservation of the species genetic resources and its survival for future generations.
Conclusions
The expedition showed that the natural regeneration of A. araucana is low or nonexistent in most of its distribution range, and this is the most evident sign of araucaria forests degradation. Furthermore, the state of degradation is such that the aim of establishing a tree network to be used in genetic diversity studies was only partially achieved.
Taking into account both regeneration ability and the total number of catalogued trees in each site, the best conserved areas were Malalcahuello N.R., Cunco District and Nahuelbuta N.R. Conversely, a severely altered situation was detected in Villarrica N.R., Lanin N.R. and Lolco. In fact, preliminary results allowed areas of dramatic landscape alteration to be identified as Lolco, which according to the available information has experienced an extensive loss of populations throughout its distribution range. This should be a priority area for conservation because if the current will persist, the above araucaria population will be lost, as it happened with other habitats.
To sum up, the complex structure and distribution range of the species makes a strategic approach necessary to conserve the genetic resources of the species and guarantee its future. In fact, the genetic studies that can be carried out on the basis of this tree network will be of great utility for the development of this strategy.
Acknowledgments
This research was partially supported by grant No. 207.141.018-1.0 from the Research Service of Concepción University (Chile) and by grant B/6585/06 of the Spanish Agency of International Cooperation (AECI) from the Ministry for Foreign Affairs and Cooperation (Spain).
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Departamento de Manejo de Bosques y Medioambiente, Facultad de Ciencias Forestales, Universidad de Concepción, Casilla 160-C, Concepción (Chile)
JR Molina
Departamento de Ingeniería Forestal, Escuela Técnica Superior de Ingenieros Agrónomos y de Montes, Edificio Leonardo Da Vinci, Universidad de Córdoba, ES-14071 Córdoba (Spain)
Private Company, León Gallo 0548, Temuco (Chile)
Corresponding author
Paper Info
Citation
Drake F, Martín MA, Herrera MA, Molina JR, Drake-Martin F, Martín LM (2009). Networking sampling of Araucaria araucana (Mol.) K. Koch in Chile and the bordering zone of Argentina: implications for the genetic resources and the sustainable management. iForest 2: 207-212. - doi: 10.3832/ifor0524-002
Academic Editor
Marco Borghetti
Paper history
Received: Apr 08, 2009
Accepted: Nov 09, 2009
First online: Dec 22, 2009
Publication Date: Dec 22, 2009
Publication Time: 1.43 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2009
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 55564
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 46152
Abstract Page Views: 3747
PDF Downloads: 4424
Citation/Reference Downloads: 18
XML Downloads: 1223
Web Metrics
Days since publication: 5915
Overall contacts: 55564
Avg. contacts per week: 65.76
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2009): 6
Average cites per year: 0.35
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Technical Reports
Conservation and use of elm genetic resources in France: results and perspectives
vol. 13, pp. 41-47 (online: 03 February 2020)
Editorials
Workshop COST E52 “Evaluation of beech genetic resources for sustainable forestry”
vol. 2, pp. 104 (online: 10 June 2009)
Commentaries & Perspectives
The genetic consequences of habitat fragmentation: the case of forests
vol. 2, pp. 75-76 (online: 10 June 2009)
Research Articles
Delineation of seed collection zones based on environmental and genetic characteristics for Quercus suber L. in Sardinia, Italy
vol. 11, pp. 651-659 (online: 04 October 2018)
Research Articles
Patterns of genetic diversity in European beech (Fagus sylvatica L.) at the eastern margins of its distribution range
vol. 10, pp. 916-922 (online: 10 December 2017)
Technical Reports
Population genetic structure of Platanus orientalis L. in Bulgaria
vol. 4, pp. 186-189 (online: 11 August 2011)
Research Articles
Seedling emergence capacity and morphological traits are under strong genetic control in the resin tree Pinus oocarpa
vol. 17, pp. 245-251 (online: 16 August 2024)
Research Articles
Comparison of genetic parameters between optimal and marginal populations of oriental sweet gum on adaptive traits
vol. 11, pp. 510-516 (online: 18 July 2018)
Research Articles
Fragmentation of Araucaria araucana forests in Chile: quantification and correlation with structural variables
vol. 9, pp. 244-252 (online: 28 August 2015)
Research Articles
Patterns of genetic variation in bud flushing of Abies alba populations
vol. 11, pp. 284-290 (online: 13 April 2018)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword