Population genetic structure of Platanus orientalis L. in Bulgaria
iForest - Biogeosciences and Forestry, Volume 4, Issue 4, Pages 186-189 (2011)
doi: https://doi.org/10.3832/ifor0580-004
Published: Aug 11, 2011 - Copyright © 2011 SISEF
Technical Reports
Collection/Special Issue: IUFRO RG 7.01 2010 - Antalya (Turkey)
Adaptation of Forest Ecosystems to Air Pollution and Climate Change
Guest Editors: Elena Paoletti, Yusuf Serengil
Abstract
This paper reports the results of a genetic survey on population structure of Platanus orientalis L. in Bulgaria. Nine populations from southern Bulgaria were investigated by using isozyme gene markers. Nine of the enzyme systems were polymorphic. The populations revealed minor polymorphism, which indicates that the predominant allele was the same for all populations and its frequencies were higher than 0.5. The average number of alleles varied from 2.2 to 2.3, and the effective number of alleles ranged from 1.294 to 1.406. The percent of polymorphic loci ranged from 53.8% to 76.9%. Heterozygosity in the populations (average: 0.242; range: 0.229-0.289) was higher than the mean values reported for broad-leaved species (0.183). The expected and observed heterozygosities had similar values. The results showed that genetic diversity among populations measured by FST (0.077) and genetic distances (mean 0.029) was within the range of the values for Angiosperm tree species. The information could be used for designing proper gene conservation strategies.
Keywords
Introduction
Eastern plane (Platanus orientalis L.) occurs in Europe only in the eastern part of Mediterranean region, including some remnants in Calabria and Sicily (southern Italy - [23]). Its natural range spreads eastward through Caucasus and Asia Minor to Iran ([12], [15]). P. orientalis is a fast growing species having both economical and ornamental importance. A limitation for a wider cultivation of the species in Europe is its frost sensitivity and therefore in the other parts of central and western Europe hybrid plane (London plane, Platanus × acerifolia Willd.) is preferred ([18], [14]).
In Bulgaria P. orientalis appears southwards of the 42nd latitudinal parallel and occupies mainly river banks. In contrast to other riparian species, it is very tolerant to soil conditions and can grow on very limited soil layers and on gravel along the rivers, thus depending mostly on soil humidity. Even though it is rather abundant along the rivers in the southern part of the Balkan peninsula and in the Aegean islands, the species is considered rare in Bulgaria and was included in the Red Data Book of Bulgaria ([24]). The habitats of P. orientalis (Palaearctic code 44.711) are included in the Appendix 1 of the resolution no. 4/1996 of the Permanent Committee of the Bern convention. Therefore, this species requires measures for conservation and sustainable management.
Populations of this species in Bulgaria are located in three regions over four river basins. Since river basins are separated by relatively high mountain ridges and some of the populations are also rather distant geographically, it is of particular interest to study the genetic variation and the differentiation between and within populations of P. orientalis. Both biochemical and morphological quantitative markers could be useful for such a study, since the molecular markers and quantitative traits tend to express different levels and patterns of variation. Although the use of both types of traits for population structure and differentiation stu-dies is controversial ([10], [6]), there are some examples of successful application of such an approach ([9]).
The objective of the present study was to assess the genetic variation and population structure of Platanus orientalis in Bulgaria, where it reaches the northernmost limit of its natural distribution. The knowledge of the distribution of genetic variation within and among populations could be useful for better understanding the species evolution and for designing gene conservation strategies.
Material and methods
Plant material
The material for the study was collected from nine populations, representing the whole area of distribution of the species in Bulgaria (Tab. 1). Fifty to sixty randomly chosen trees per population were sampled. Dormant winter buds, sometimes starting to flush, were used for the analysis.
Tab. 1 - Bulgarian populations of Platanus orientalis from which samplings were collected.
| Population (abbreviation) | Geographic coordinates | Altitude (m a.s.l.) |
|---|---|---|
| Kresna (KR) | 41º 44’ N 23º 08’ E |
300 |
| Sandanski (SA) | 41º 36’ N 23º 20’ E |
200 |
| Slavyanka (SL) | 41º 26’ N 23º 33’ E |
500 |
| Petrich (PE) | 41º 24’ N 23º 03’ E |
400 |
| Melnik (ME) | 41º 30’ N 23º 24’ E |
250 |
| Goce Delchev (GD) | 41º 37’ N 23º 52’ E |
300 |
| Assenovgrad (AS) | 41º 58’ N 24º 52’ E |
150 |
| Topolovo (TO) | 41º 54’ N 25º 00’ E |
150 |
| Ivaylovgrad (IV) | 41º 35’ N 26º 06’ E |
300 |
Isozyme analysis
Enzymes extraction was done after grinding the bud tissue in Tris-HCl extraction buffer pH 7.3. Ten milligrams of Polyvinil-polypyrrholidone (PVPP-40) were added as a stabilizing agent in the plastic vessels, where the extraction took place. Before extraction, 15 mg dithiothreitol (DTT), 500 mg saccharose, 150 mg Polyvinil-pyrrholidone (PVP-40) and 5 mg Na2EDTA were dissolved in 15 ml extraction buffer, and 100 μl β-mercaptoethanol were also added to the solution.
Standard 12 % starch gel electrophoresis was applied to separate the isoenzyme variants, in two buffer systems: Lithium-borate - Tris-citrate pH 8.1 discontinuous buffer system ([1]) and Tris-citrate pH 7.0 continuous buffer system ([19]). The enzyme systems and loci scored are listed in Tab. 2.
Tab. 2 - Investigated enzyme systems. (A): Lithium borate (pH 8.1) Tris Citrate (pH 8.1) discontinuous buffer system ([1]). (TC): Tris Citrate (pH 7.0) continuous buffer system ([19]).
| Enzyme system (abbreviation and EC code) | No of loci scored | Buffer system |
|---|---|---|
| Glutamate dehydrogenase (GDH, 1.4.1.2) | 1 | A |
| Glutamate-oxaloacetate transaminase (GOT, 2.6.1.1 ) | 2 | A |
| Leucine aminopeptidase (LAP, 3.4.11.1) | 2 | A |
| Malate dehydrogenase (MDH, 1.1.1.37) | 4 | TC |
| Menadione reductase (MNR, 1.6.99.2) | 1 | A |
| Phosphoglucomutase (PGM, 5.4.2.2) | 1 | TC |
| Phosphpoglucose isomerase (PGI, 5.3.1.9) | 2 | A |
| Shikimate dehydrogenase (SkDH,1.1.1.25) | 2 | TC |
| Alcohol dehydrogenase (ADH, 1.1.1.1) | 2 | TC |
The loci were numbered according to their position from the anode, i.e., the fastest one was numbered 1 and so forth, and the alleles were numbered according to their relative mobility relative to the most common allele (allele 100).
Data analysis
Allele frequencies were calculated from diploid genotypes. Genetic diversity within populations was characterized by the following parameters: mean number of alleles (Na); effective number of alleles (Ne = 1/Σi p i2, where p i is the frequency of the i-th allele) for a locus and harmonic mean of all loci; percent of polymorphic loci, applying 0.05 criterion, which means that a locus is considered polymorphic if the frequency of the predominant allele does not exceed 0.95; expected heterozygosity H e = 1 - Σi (1 - p i2 ), where p i is the frequency of the i-th allele ([13]); observed (direct-count) heterozygosity (Ho); inbreeding coefficient F = 1 - Ho /He. The software Biosys-1 ([22]) was used. Shannon index of diversity (I’) was used as an additional criterion. Genetic distances between population pairs were computed according to Nei ([13]). The differentiation was tested by means of F-statistics ([26]) following the method of Weir & Cockerham ([25]) and using the software GENEPOP v.3.1c. ([17]). While F ST was used to measure differentiation over populations, F IS and F IT were used to test the deviation from Hardy-Weinberg expectations within populations and in the total population, respectively. Multidimensional scaling (Principal Coordinate Analysis - [7]) was applied for better interpretation of genetic distances by means of the software SYN-TAX ([16]).
Results and discussion
Polymorphism and diversity
Nine of the enzyme systems studied were polymorphic - LAP, GOT, GDH, PGI, PGM, MNR, ADH, MDH, and SKDH. The allele frequencies are available from authors upon request. The predominant allele was the same in all populations, which corresponds to the so-called “minor polymorphism” ([11]). The polymorphism and diversity parameters (Tab. 3) indicate that the level of intrapopulation genetic diversity in Platanus orientalis is well within the range of the figures reported for cross-pollinating, long-lived broadleaved species ([8]). The mean allele number per locus varied within the narrow range 2.2 to 2.3. Maximum number of alleles (5) was found in the Got-2 locus. The effective allele number (or the reciprocal of homozygosity - [2]) ranged from 1.294 (Asenovgrad) to 1.406 (Slavyanka). The Shannon’s index of diversity, borrowed from the information theory and numerical ecology ([3]) is sometimes used as an additional measure of diversity. The Got-2 locus had the highest value, with the highest allele number (Tab. 4).
Tab. 3 - Characteristics of genetic diversity and polymorphism. (N): mean number of individuals studied; (Na): mean number of alleles/locus; (Ne): effective allele number (harmonic mean); (P): percent of polymorphic loci (0.05 criterion); (Ho): observed heterozygosity; (He): expected heterozygosity; (SE): standard error; (F): inbreeding coefficient (F = 1 - Ho/He); (*): significantly different from zero (p≤0.05).
| Population | N (SE) |
Na (SE) |
N e | P | Ho (SE) |
He (SE) |
F |
|---|---|---|---|---|---|---|---|
| Kresna | 52.7 (1.9) |
2.3 (0 .2) |
1.314 | 61.5 | 0.239 (0.072) |
0.237 (0.059) |
-0.008 |
| Sandanski | 49.2 (1.9) |
2.2 (0 .1) |
1.312 | 61.5 | 0.238 (0.060) |
0.260 (0 .053) |
0.085* |
| Slavyanka | 53.8 (2.7) |
2.5 (0 .3) |
1.406 | 69.2 | 0.289 (0.066) |
0.289 (0.055) |
0.000 |
| Petrich | 56.0 (0.5) |
2.3 (0.2) |
1.331 | 61.5 | 0.249 (0.066) |
0.245 (0.055) |
-0.016 |
| Melnik | 67.5 (1.6) |
2.2 (0.2) |
1.297 | 53.8 | 0.229 (0.064) |
0.229 (0.058) |
0.000 |
| Goce Delchev | 63.5 (0.3) |
2.2 (0.3) |
1.349 | 76.9 | 0.259 (0.079) |
0.241 (0.057) |
-0.075 |
| Asenovgrad | 38.5 (1.4) |
2.2 (0.2) |
1.294 | 61.5 | 0.227 (0.063) |
0.249 (0.056) |
0.088* |
| Topolovo | 51.3 (2.4) |
2.3 (0.2) |
1.295 | 76.9 | 0.228 (0.070) |
0.283 (0.053) |
0.194* |
| Ivaylovgrad | 49.6 (1.0) |
2.2 (0.3) |
1.337 | 69.2 | 0.252 (0.069) |
0.242 (0.055) |
-0.041 |
Tab. 4 - Shannon index of diversity (I’).
| Locus | I’ |
|---|---|
| Lap-1 | 0.2201 |
| Lap-2 | 0.1930 |
| Got-2 | 1.1594 |
| Gdh | 0.6158 |
| Pgi-1 | 0.2655 |
| Pgi-2 | 0.8955 |
| Mnr | 0.1372 |
| Adh-1 | 0.6392 |
| Adh-2 | 0.6121 |
| Pgm | 0.2390 |
| Mdh | 0.4290 |
| Skdh | 0.3049 |
| Mean | 0.4545 |
The percent of polymorphic loci ranged from 53.8 (Melnik) to 76.9 % (Topolovo - 0.05 criterion). These results are somewhat higher than the average values summarized in Hamrick et al. ([8]) for tree species (59.5 %). Both observed and expected heterozygosities were high and the differences between them for the respective populations were low, as indicated by the inbreeding coefficient (Tab. 3). The highest heterozygosity was found in the population of Slavyanka (0.289) and the lowest one was in the population of Melnik (0.229).
Genetic differentiation among populations
Usually the genetic differentiation is measured by comparing the pairwise genetic distances ([13]) and the average indicator of the differentiation F ST (or G ST). The overall genetic differentiation (F ST) was 0.077 (Tab. 5), fairly similar to values summarized by Hamrick et al. ([8]) for species with similar life-history characteristics. High level of inbreeding was found at MNR locus, which differed from the remaining loci. The populations were most differentiated at locus Lap-2, which could be due to occurrence of silent alleles, a relatively frequent phenomenon in LAP isozymes, but undetectable when diploid tissue is analyzed.
Tab. 5 - F-statistics and putative migration. (Nm): number of migrants per generation calculated as Nm = 0.25 (1 - FST)/ ST.
| Locus | F IS | F IT | F ST | Nm |
|---|---|---|---|---|
| Lap-1 | 0.306 | 0.324 | 0.026 | 9.1949 |
| Lap-2 | 0.351 | 0.429 | 0.121 | 1.8230 |
| Got-1 | 0.328 | 0.366 | 0.057 | 4.1396 |
| Got-2 | -0.206 | -0.168 | 0.031 | 7.7110 |
| Gdh | -0.161 | -0.059 | 0.088 | 2.5750 |
| Pgi-1 | 0.450 | 0.473 | 0.042 | 5.6915 |
| Pgi-2 | 0.385 | 0.533 | 0.240 | 0.7901 |
| Mnr | 0.379 | 0.388 | 0.015 | 16.6564 |
| Adh-1 | -0.031 | 0.010 | 0.040 | 6.0737 |
| Adh-2 | -0.323 | -0.281 | 0.032 | 7.5851 |
| Pgm | -0.056 | 0.022 | 0.073 | 3.1635 |
| Mdh | 0.140 | 0.168 | 0.032 | 7.4486 |
| Skdh | 0.154 | 0.189 | 0.040 | 5.9241 |
| Mean | 0.019 | 0.094 | 0.077 | 2.9710 |
The putative number of migrants calculated by using the F ST values varied from 2 to 16 for the different loci (mean = 2.97). The average number of migrant calculated by the Private allele method ([20]) was 7.49. The differences could be due to the specific approaches for calculation or to violations of the assumptions, but in both cases this number was below 10. This fact indicates that there were mechanisms that make the isolation ineffective. This could be due to the light pollen grains of the species, and also to the fact that its fruits could be transported by the water stream at long distances. We hypothesize that even a small number of migrants is sufficient to overpower the differences among populations that could appear solely by genetic drift.
Locus MNR again showed different patterns of variation. It was the least differentiating locus, and with high inbreeding. As its visualization and interpretation was the easiest one, we can exclude possible misinterpretation of the electrophoregram as an explanation; we thus hypothesize that there were some mechanisms of selection most affecting this locus.
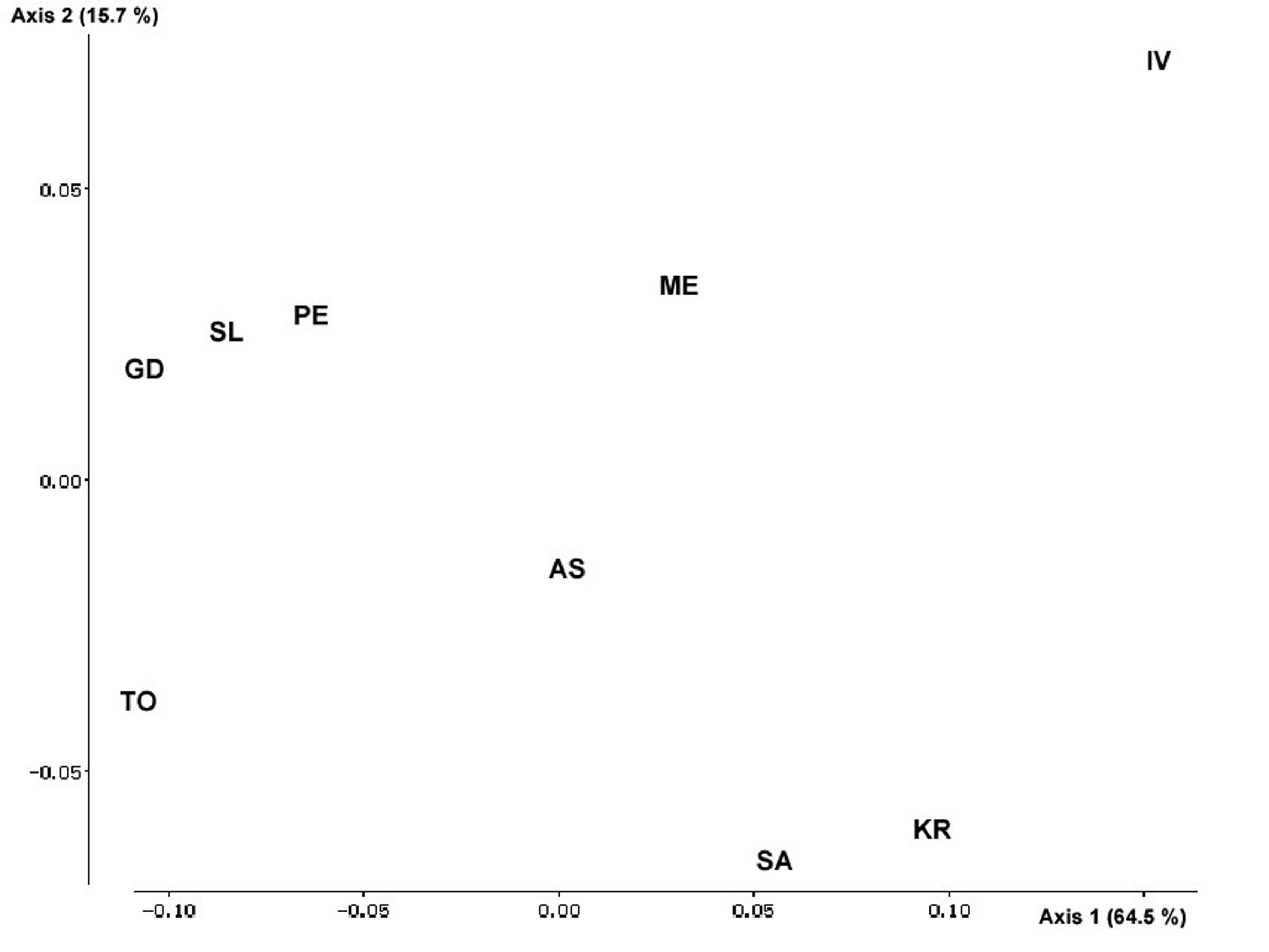
El-Kassaby & Yanchuk ([5]) and Davidson & El-Kassaby ([4]) modified the approach used by Slatkin ([21]) for assessing the genetic uniqueness of a population by removing it from the dataset and recalculating the overall level of differentiation among remaining populations. The procedure is repeated n times, where n is the number of populations (see the references above for details). Obviously, when a genetically most different population is out of the data set, the overall differentiation will be the lowest of all cases. In our study this population was Ivaylovgrad (results not shown).
The genetic distances were of magnitude 0.005 to 0.085, and the most different population again appeared to be Ivaylovgrad, which was also the most distant and isolated one (Tab. 6). Ivaylovgrad showed the highest genetic distances toward the other populations, while highest genetic distances among them were 0.078 (Ivaylovgrad - Goce Delchev) and 0.069 (Ivaylovgrad - Slavyanka).
Tab. 6 - Genetic distances between the population pairs.
| Population | SA | SL | PE | ME | GD | AS | TO | IV |
|---|---|---|---|---|---|---|---|---|
| Kresna (KR) | 0.012 | 0.050 | 0.035 | 0.017 | 0.052 | 0.019 | 0.052 | 0.028 |
| Sandanski (SA) | - | 0.034 | 0.033 | 0.014 | 0.042 | 0.012 | 0.040 | 0.033 |
| Slavyanka (SL) | - | - | 0.008 | 0.019 | 0.008 | 0.017 | 0.018 | 0.069 |
| Petrich (PE) | - | - | - | 0.010 | 0.005 | 0.013 | 0.010 | 0.055 |
| Melnik (ME) | - | - | - | - | 0.018 | 0.005 | 0.029 | 0.022 |
| Goce Delchev (GD) | - | - | - | - | - | 0.014 | 0.013 | 0.078 |
| Asenovgrad (AS) | - | - | - | - | - | - | 0.023 | 0.038 |
| Topolovo (TO) | - | - | - | - | - | - | - | 0.085 |
| Ivaylovgrad (IV) | - | - | - | - | - | - | - | - |
The Multidimensional Scaling (Fig. 1) confirms these patterns of variation and differentiation and clearly shows the outstanding position of Ivaylovgrad, followed by Topolovo, Goce Delchev and Kresna.
The analysis of the genetic variation and population structure of Platanus orientalis in Bulgaria showed a good level of genetic diversity, both within and among populations. This fact facilitates the selection of populations for conservation. Although the decision requires deeper studies and applying different approaches, this study demonstrated the usefulness of genetic information in designing gene conservation strategies.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Corresponding author
Paper Info
Citation
Grueva M, Zhelev P (2011). Population genetic structure of Platanus orientalis L. in Bulgaria. iForest 4: 186-189. - doi: 10.3832/ifor0580-004
Paper history
Received: May 04, 2011
Accepted: May 19, 2011
First online: Aug 11, 2011
Publication Date: Aug 11, 2011
Publication Time: 2.80 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2011
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 58367
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 48272
Abstract Page Views: 3430
PDF Downloads: 4976
Citation/Reference Downloads: 17
XML Downloads: 1672
Web Metrics
Days since publication: 5257
Overall contacts: 58367
Avg. contacts per week: 77.72
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2011): 3
Average cites per year: 0.20
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Technical Reports
Conservation and use of elm genetic resources in France: results and perspectives
vol. 13, pp. 41-47 (online: 03 February 2020)
Research Articles
Comparison of genetic parameters between optimal and marginal populations of oriental sweet gum on adaptive traits
vol. 11, pp. 510-516 (online: 18 July 2018)
Research Articles
Networking sampling of Araucaria araucana (Mol.) K. Koch in Chile and the bordering zone of Argentina: implications for the genetic resources and the sustainable management
vol. 2, pp. 207-212 (online: 22 December 2009)
Commentaries & Perspectives
The genetic consequences of habitat fragmentation: the case of forests
vol. 2, pp. 75-76 (online: 10 June 2009)
Review Papers
Implementing the dynamic conservation of elm genetic resources in Europe: case studies and perspectives
vol. 8, pp. 143-148 (online: 07 August 2014)
Research Articles
Fine-scale spatial genetic structure in a multi-oak-species (Quercus spp.) forest
vol. 8, pp. 324-332 (online: 05 September 2014)
Editorials
Workshop COST E52 “Evaluation of beech genetic resources for sustainable forestry”
vol. 2, pp. 104 (online: 10 June 2009)
Research Articles
Delineation of seed collection zones based on environmental and genetic characteristics for Quercus suber L. in Sardinia, Italy
vol. 11, pp. 651-659 (online: 04 October 2018)
Research Articles
Patterns of genetic diversity in European beech (Fagus sylvatica L.) at the eastern margins of its distribution range
vol. 10, pp. 916-922 (online: 10 December 2017)
Research Articles
A comparative fluctuating asymmetry study between two walnut (Juglans regia L.) populations may contribute as an early signal for bio-monitoring
vol. 3, pp. 150-152 (online: 15 November 2010)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword